Abstract
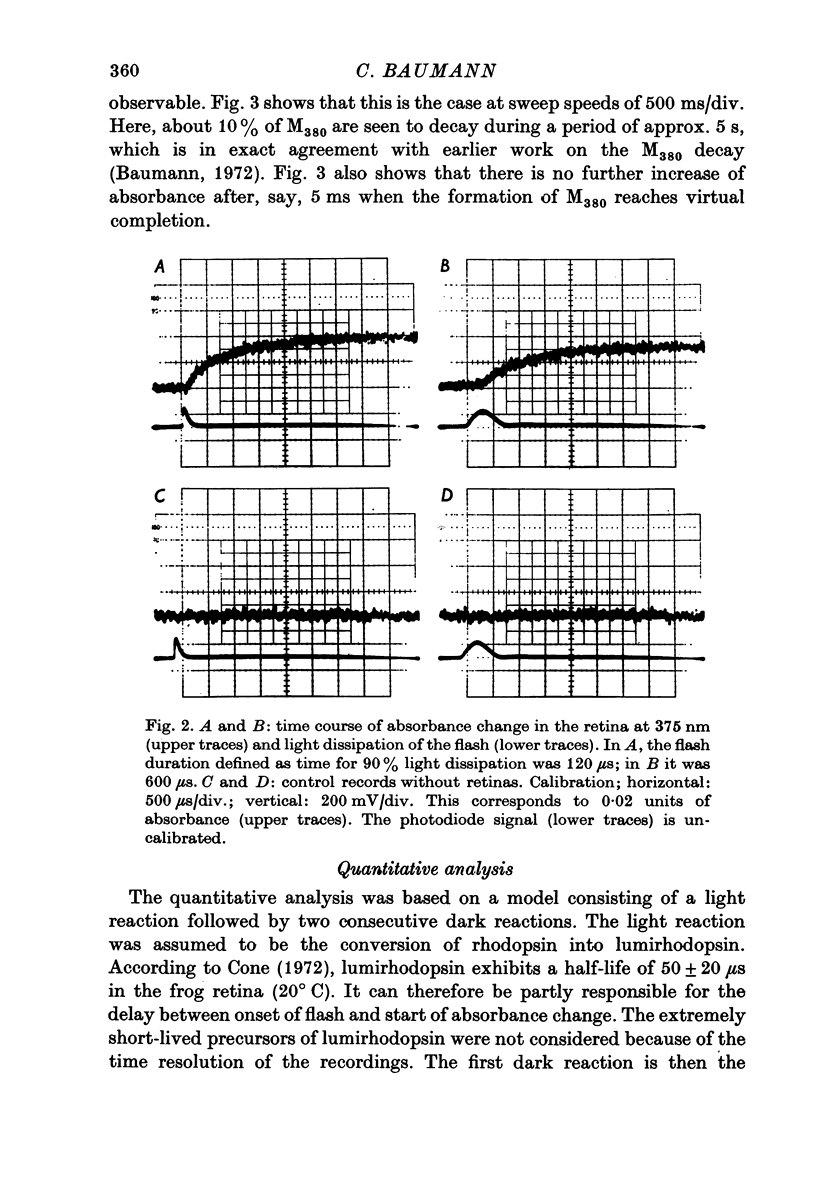
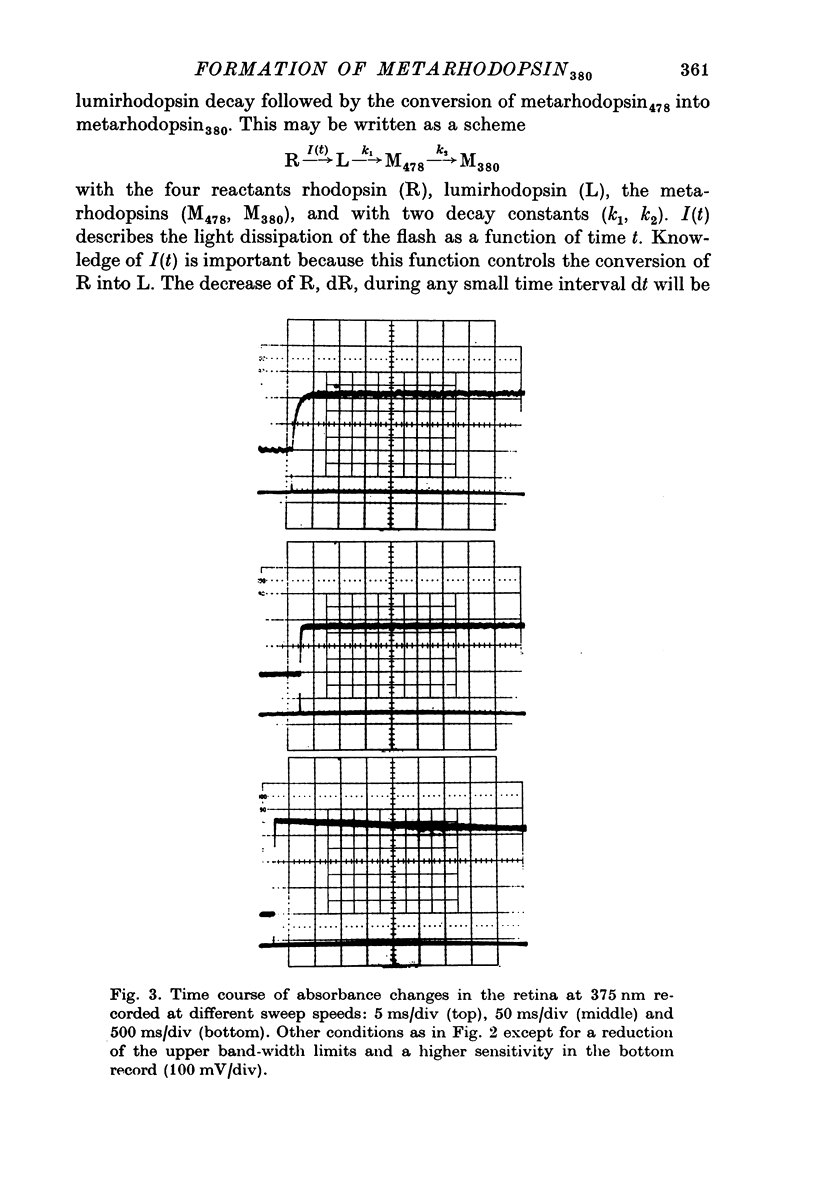
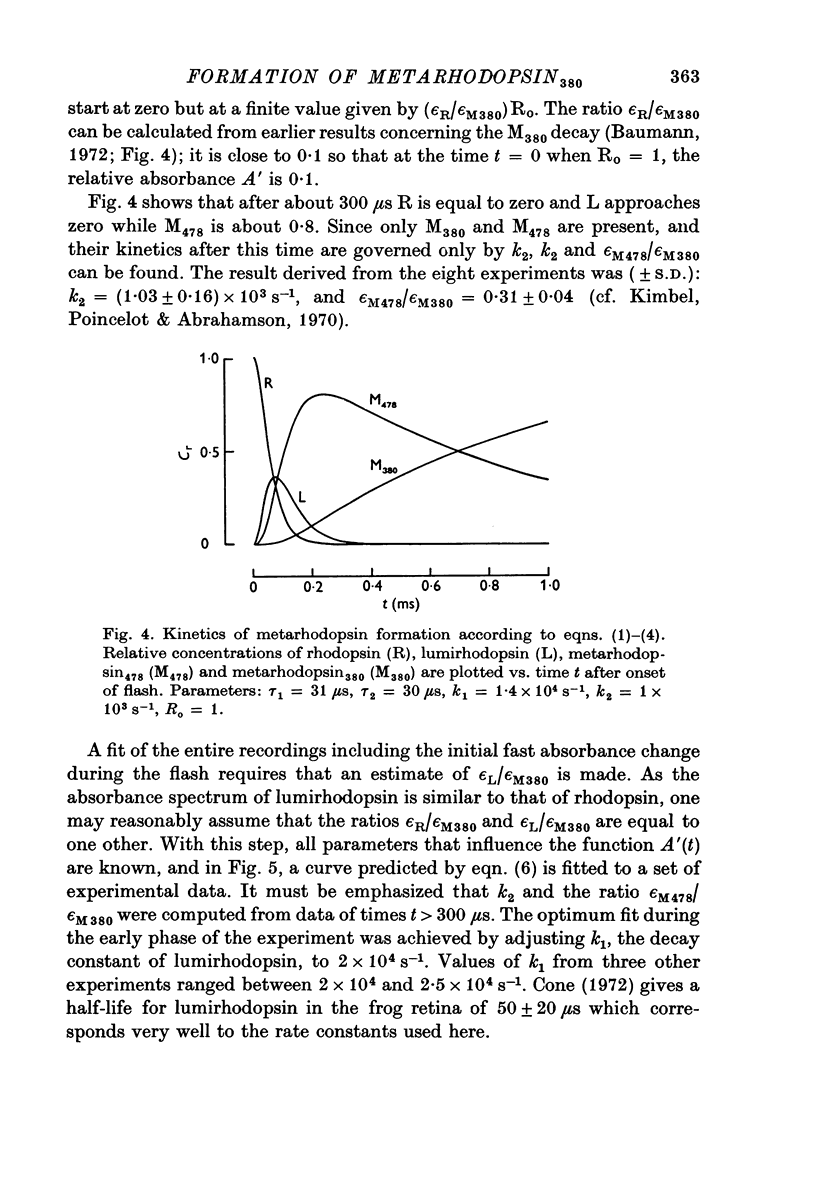
1. The formation of metarhodopsin380 (metarhodopsin II) was studied in isolated frog retinas exposed to intense flashes of 120 mus duration. 2. A rapid increase in absorbance at 375 nm during the flash was followed by a slower absorbance increase in the subsequent dark period. The slower increase showed virtual completion after 5 ms. 3. The fast absorbance increase during the flash was due to the formation of metarhodopsin478. The rate of this reaction was dependent on the time course of the flash and on the decay rate of lumirhodopsin. 4. Kinetic analysis indicates that three consecutive reactions occur: the light-controlled formation of lumirhodopsin, its first-order decay to metarhodopsin478 and the conversion of metarhodopsin478 into metarhodopsin380. At 21 degrees C, the decay constants were 2 X 10(4) S-1 (lumirhodopsin) and 1 X 10(3) S-1 (metarhodopsin478), respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann C., Bender S. Kinetics of rhodopsin bleaching in the isolated human retina. J Physiol. 1973 Dec;235(3):761–773. [PMC free article] [PubMed] [Google Scholar]
- Baumann C. Flash photolysis of rhodopsin in the isolated frog retina. Vision Res. 1970 Sep;10(9):789–798. doi: 10.1016/0042-6989(70)90158-6. [DOI] [PubMed] [Google Scholar]
- Baumann C. Kinetics of slow thermal reactions during the bleaching of rhodopsin in the perfused frog retina. J Physiol. 1972 May;222(3):643–663. doi: 10.1113/jphysiol.1972.sp009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Emrich H. M., Reich R. Uber Primärreaktionen beim Schvorgang. Thermodynamischer und kinetischer Einfluss des pH-Wertes auf die Metarhodopsin-I-II-Umwandlung. Protonenverbrauch als Auswirkung einer Konformationsänderung. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):577–591. [PubMed] [Google Scholar]
- Falk G., Fatt P. Rapid hydrogen ion uptake of rod outer segments and rhodopsin solutions on illumination. J Physiol. 1966 Mar;183(1):211–224. doi: 10.1113/jphysiol.1966.sp007861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. N. Photoproducts of rhodopsin bleaching in the isolated, perfused frog retina. Vision Res. 1969 Dec;9(12):1415–1433. doi: 10.1016/0042-6989(69)90058-3. [DOI] [PubMed] [Google Scholar]
- Gyllenberg G., Reuter T., Sippel H. Long-lived photoproducts of rhodopsin in the retina of the frog. Vision Res. 1974 Dec;14(12):1349–1357. doi: 10.1016/0042-6989(74)90009-1. [DOI] [PubMed] [Google Scholar]
- Kimbel R. L., Jr, Poincelot R. P., Abramhamson E. W. Chromophore transfer from lipid to protein in bovine rhodopsin. Biochemistry. 1970 Apr 14;9(8):1817–1820. doi: 10.1021/bi00810a022. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Jagger W. S., Kaplan M. W., Bargoot F. G. Membrane structure changes in rod outer segments associated with rhodopsin bleaching. Nature. 1974 Sep 6;251(5470):31–36. doi: 10.1038/251031a0. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroy S. E., Erhardt F., Abrahamson E. W. Protein configuration changes in the photolysis of rhodopsin. II. The sequence of intermediates in thermal decay of cattle metarhodopsin in vitro. Biochim Biophys Acta. 1966 Feb 7;112(2):265–277. doi: 10.1016/0926-6585(66)90326-8. [DOI] [PubMed] [Google Scholar]
- Rushton W. A., Powell D. S. The early phase of dark adaptation. Vision Res. 1972 Jun;12(6):1083–1093. doi: 10.1016/0042-6989(72)90099-5. [DOI] [PubMed] [Google Scholar]
- WULFF V. J., ADAMS R. G., LINSCHITZ H., ABRAHAMSON E. W. Effect of flash illumination on rhodopsin in solution. Ann N Y Acad Sci. 1959 Nov 12;74(2):281–290. doi: 10.1111/j.1749-6632.1958.tb39551.x. [DOI] [PubMed] [Google Scholar]
- Williams T. P., Baker B. N., McDowell J. H. The influence of lipids on dynamic properties of rhodopsin. Exp Eye Res. 1974 Jan;18(1):69–75. doi: 10.1016/0014-4835(74)90044-x. [DOI] [PubMed] [Google Scholar]


