Abstract
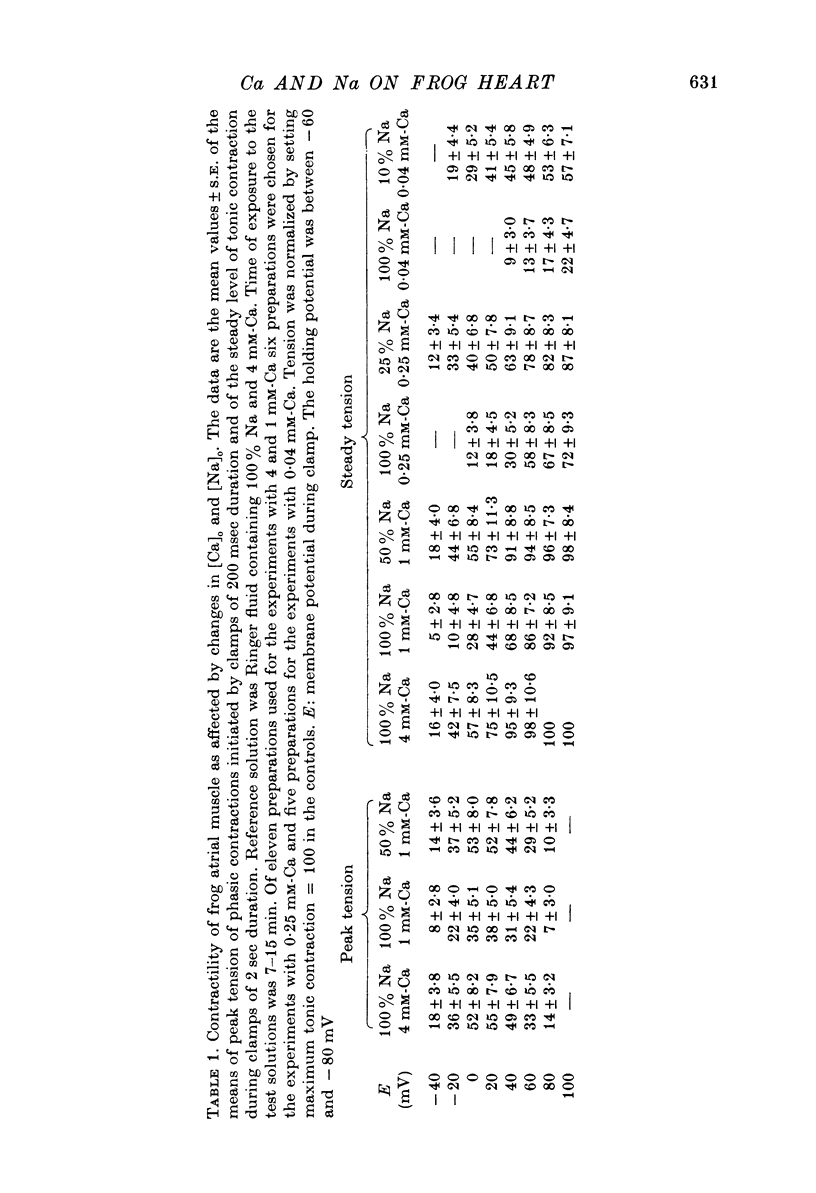
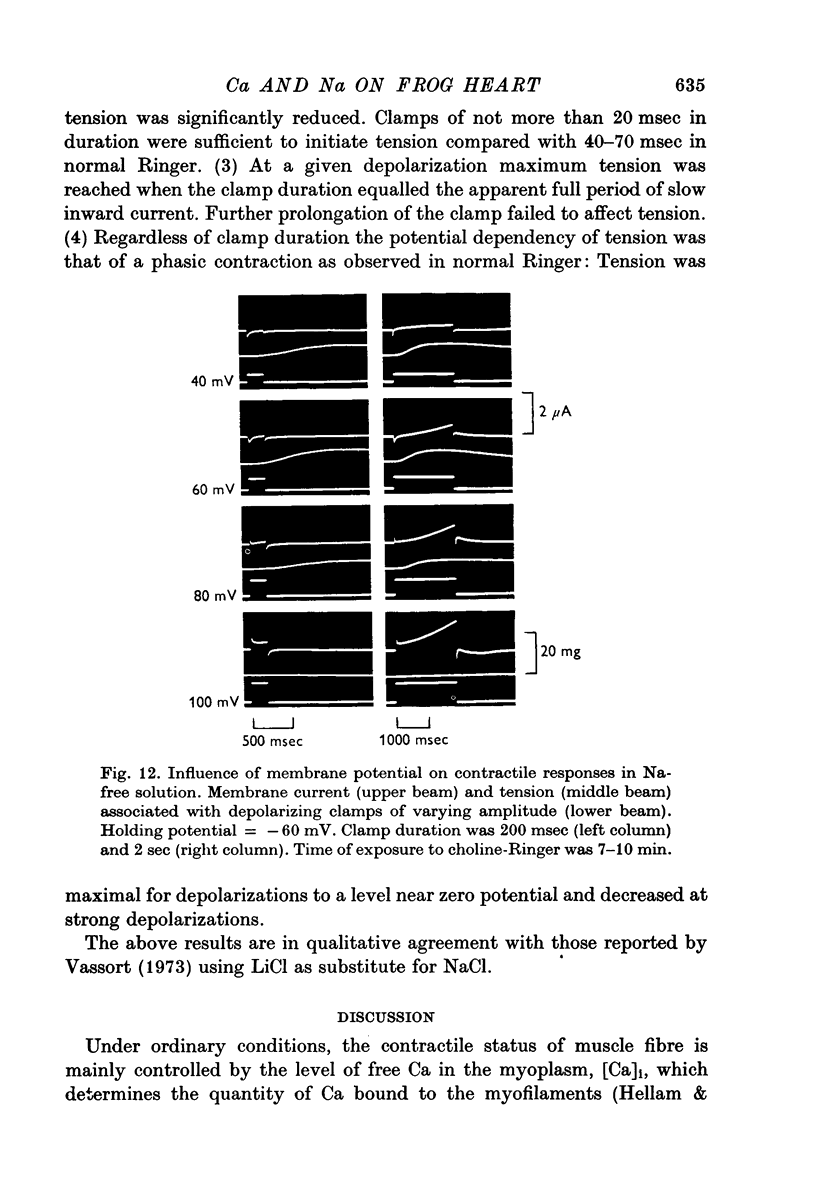
1. In double sucrose-gap voltage-clamped frog atrial fibres the influence of [Ca]o and [Na]o on membrane current and contraction was investigated. 2. The slow (secondary) inward current varied with [Ca]o but was almost insensitive to changes in [Na]o. In contrast, the phasic (transient) contraction initiated by the slow inward current was affected by both [Ca]o and [Na]o. 3. With moderate changes of [Ca]o and [Na]o from normal, the strength of phasic contraction at a given depolarization followed the [Ca]o/[Na]2o ratio approximately. This was best seen at membrane potentials near zero level. 4. Under the same conditions, tonic (sustained) contractions associated with prolonged depolarizations were strictly correlated to the [Ca]o/[Na]2o ratio at any potential. No interrelation between tonic tension and steady-state current was found. 5. With extensive changes in [Ca]o and [Na]o, the sensitivity of both phasic and tonic tension to the [Ca]o/[Na]2o ratio declined, the negative effect of [Na]o becoming smaller than was expected from this ratio. 6. In Na-free choline-Ringer, a strong contracture developed followed by a spontaneous relaxation. Starting from the relaxed state, application of depolarizing clamps gave rise to phasic contractions with a very slow relaxation while tonic contractions were apparently lacking. 7. The results are interpreted in terms of an energy-dependent carrier mechanism exchanging one Ca for two Na ions across the cell membrane. The model implies a strong asymmetry in the rate constants governing the chemical reactions on both sides of the membrane. The system is thought to operate close to equilibrium at any potential, thereby determining the steady level of myoplasmic Ca. The equilibrium itself is considered to shift upon depolarization. Assuming that [Na]i is constant, the steady level of [Ca]i is expected to be proportional to the [Ca]o/[Na]2o ratio, the scale factor being a function of membrane potential. 8. The carrier model suggests the occurrence of a depolarization-induced inward transfer of Ca which might be involved in the generation of tonic contractions. 9. The apparent lack of tonic contractions in the absence of external Na ions may be explained by a suppression of carrier-mediated Ca influx normally occurring upon depolarization. 10. The antagonistic effects of [Ca]o and [Na]o on phasic contraction are understood as being due to alterations of the Ca pumping system rather than changes in slow inward current.
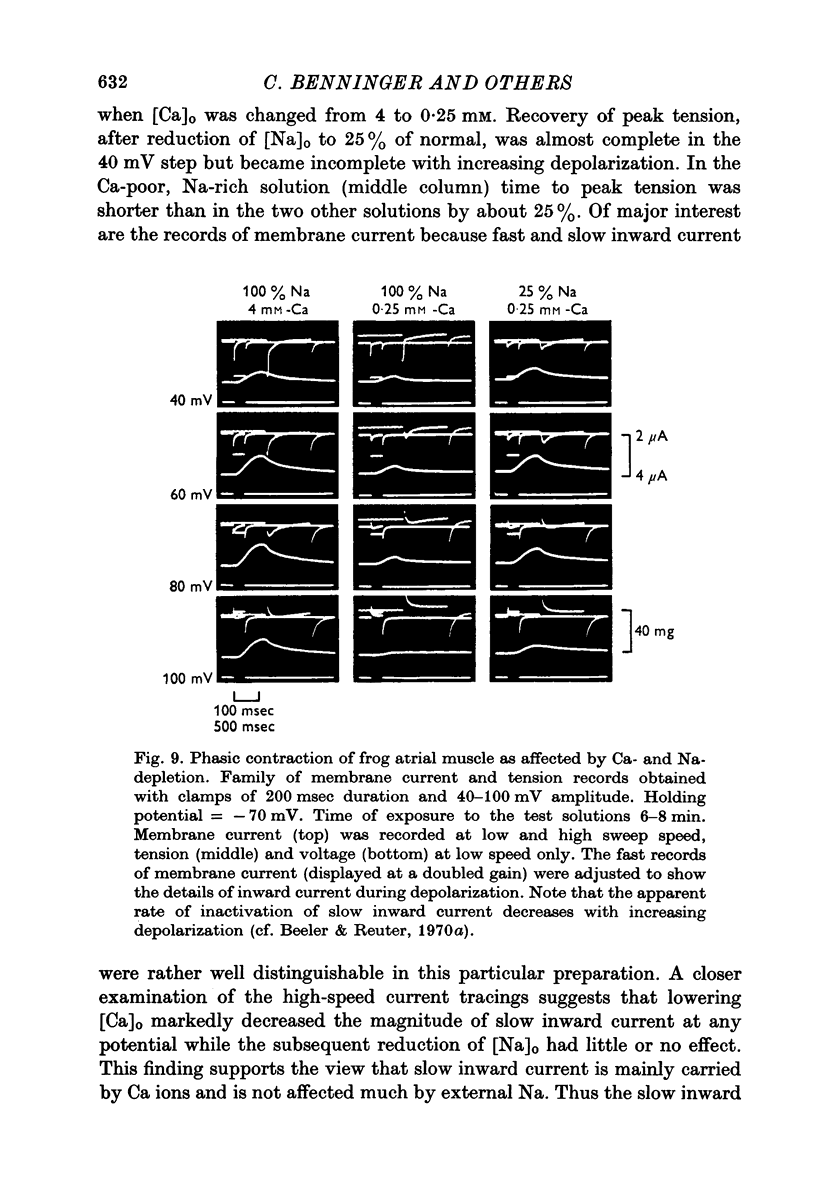
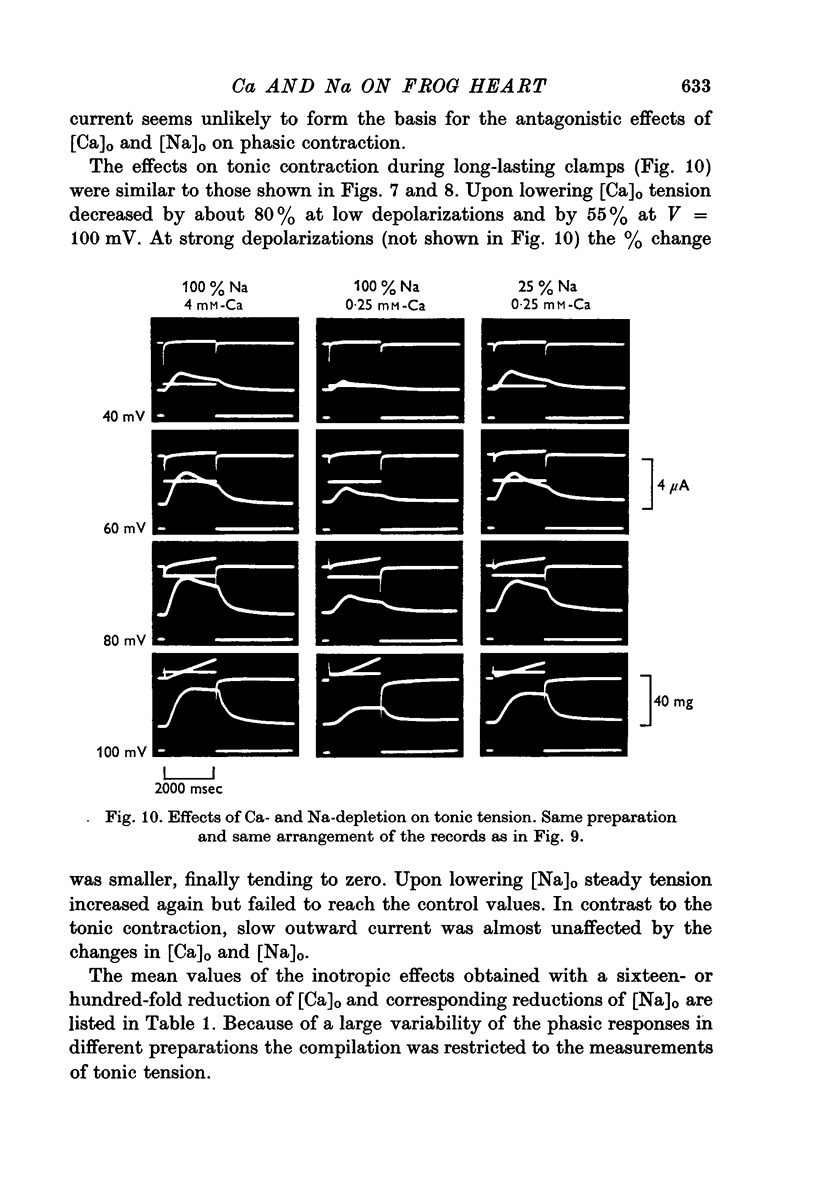
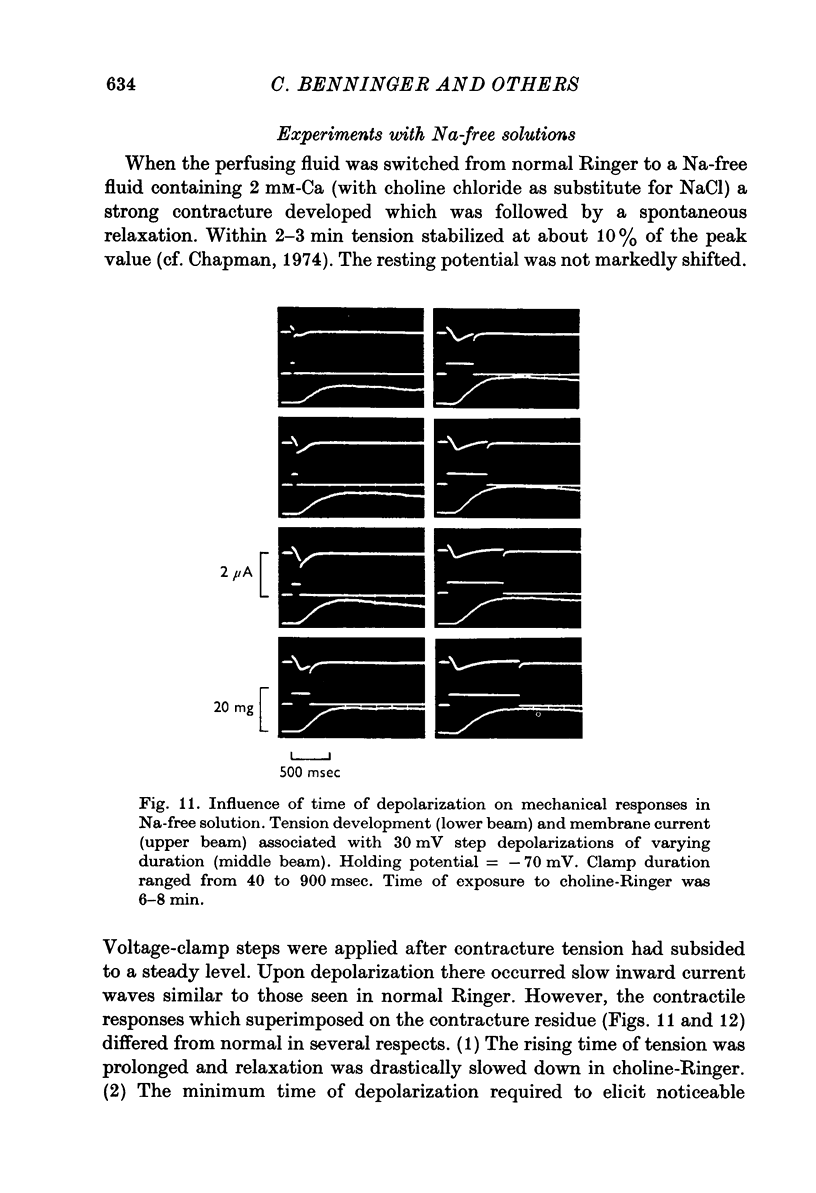
Full text
PDF




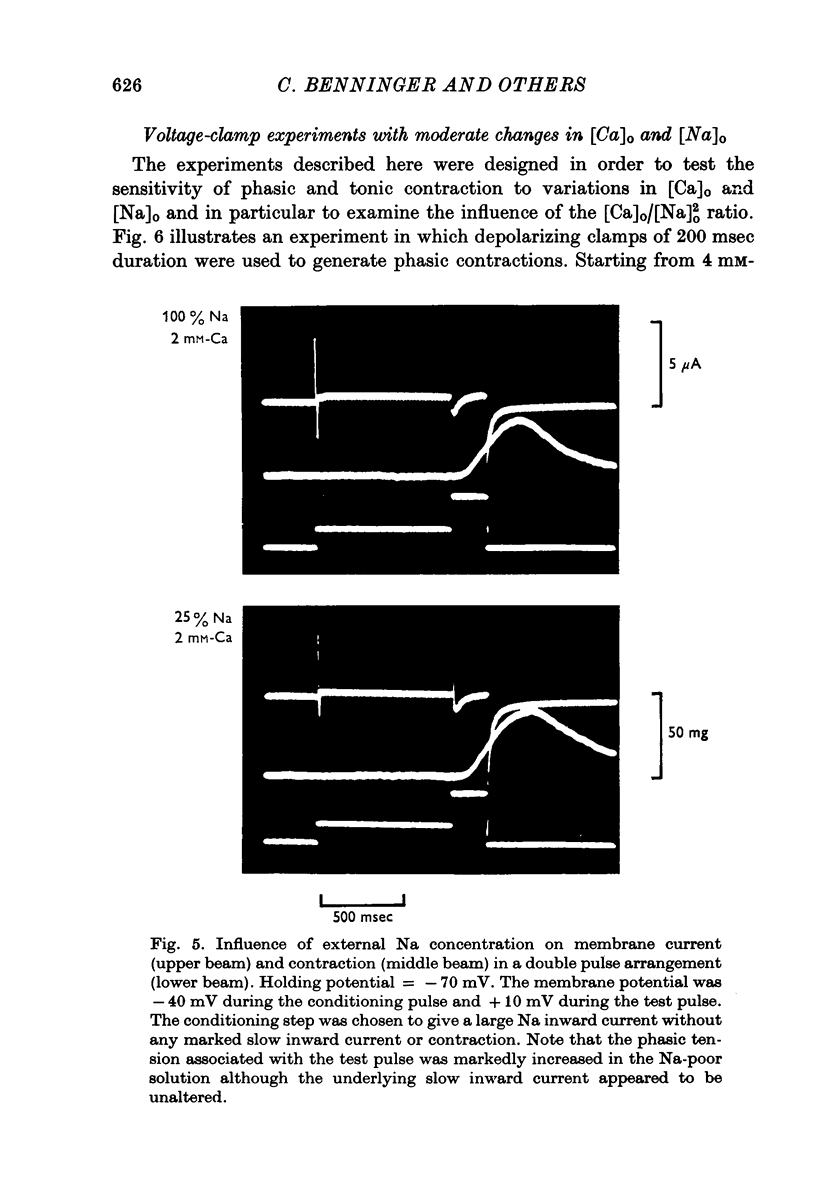
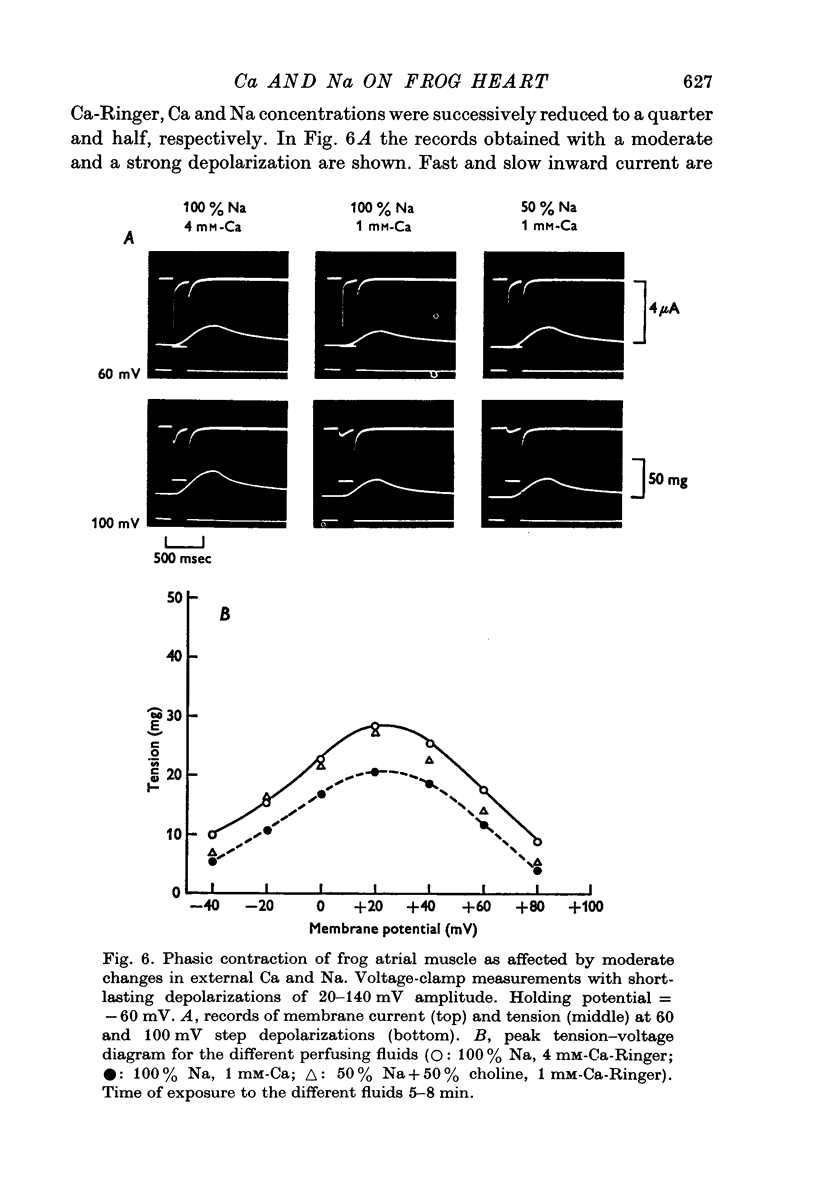
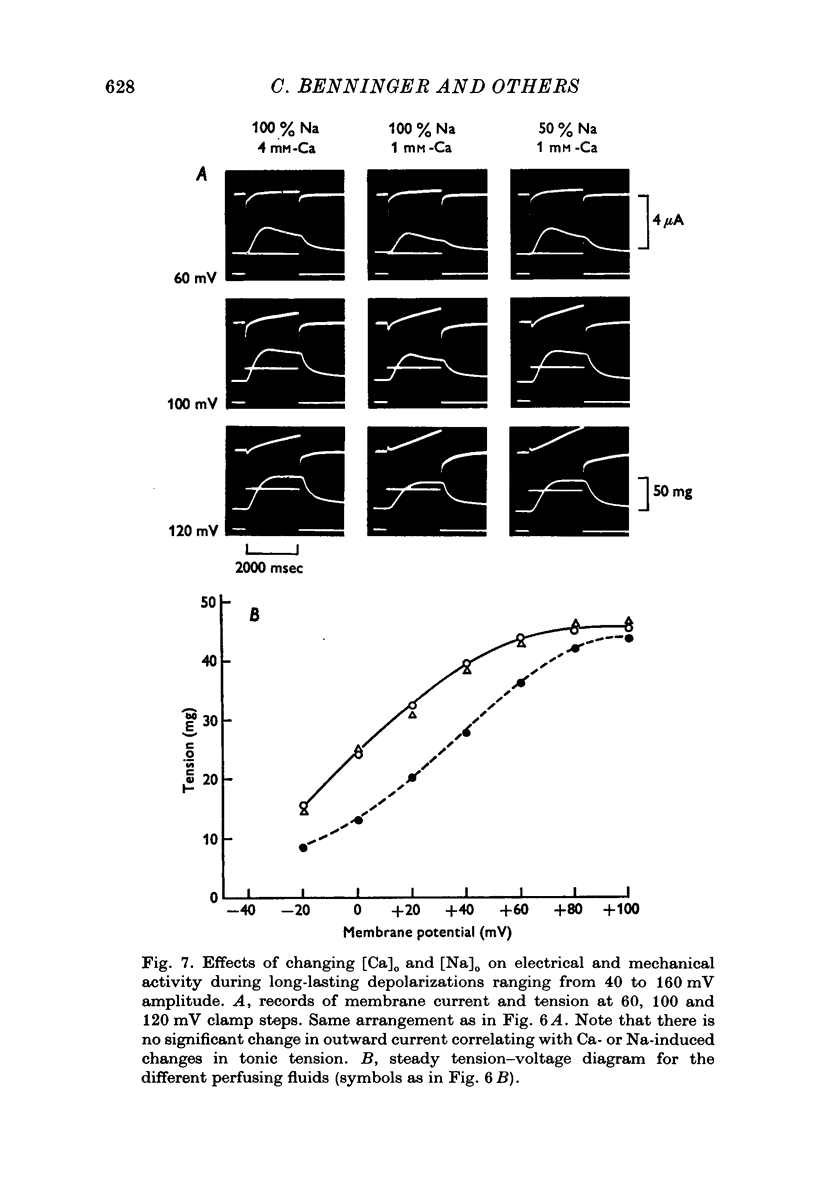

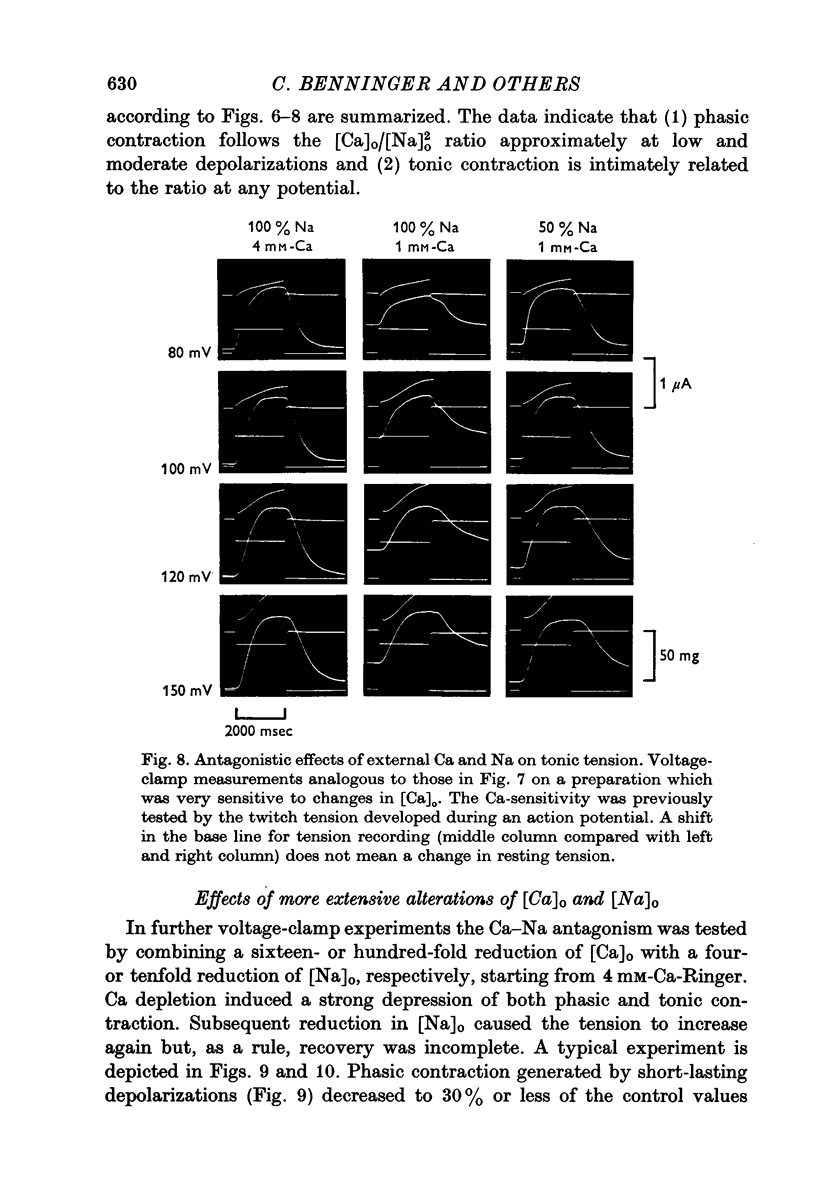









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
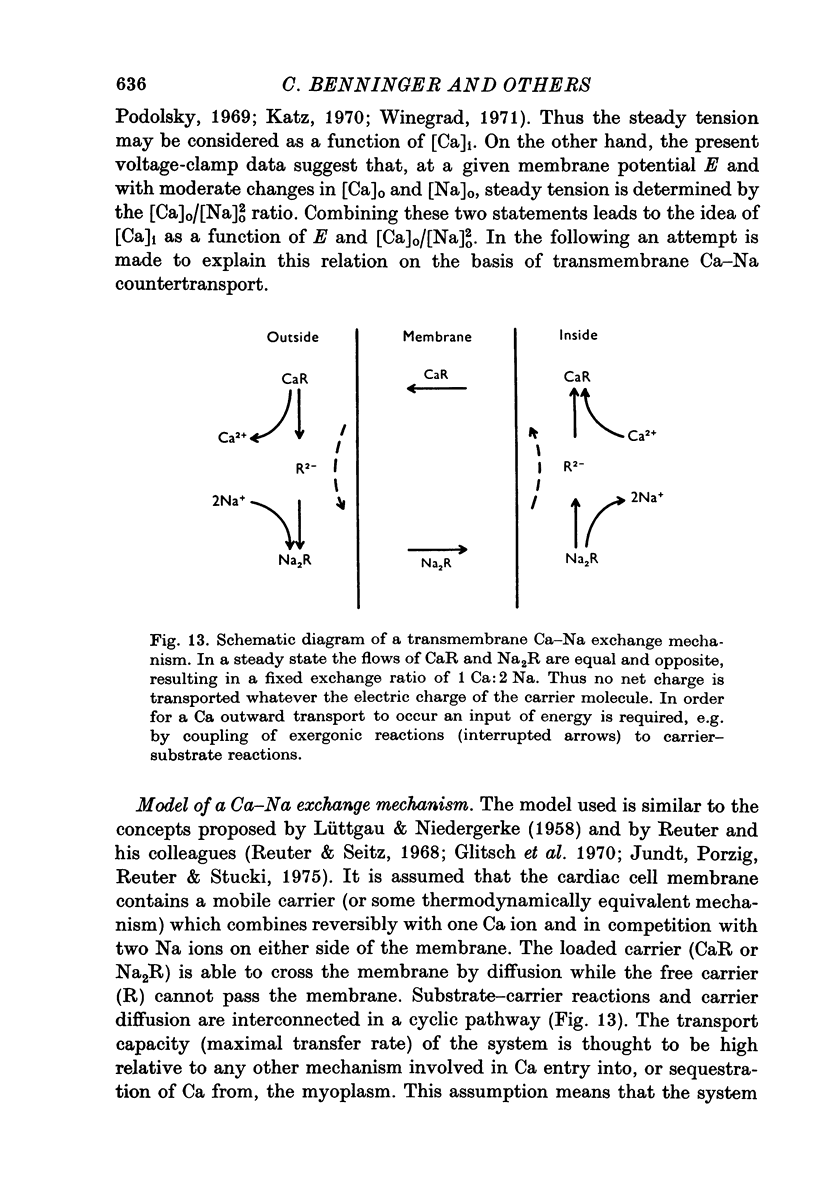
- Baker P. F., Glitsch H. G. Does metabolic energy participate directly in the Na+-dependent extrusion of Ca2+ -Ca2+ ions from squid giant axons? J Physiol. 1973 Aug;233(1):44P–46P. [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busselen P., Carmeliet E. Protagonistic effects of Na and Ca on tension development in cardiac muscle at low extracellular Na concentrations. Nat New Biol. 1973 May 9;243(123):57–59. [PubMed] [Google Scholar]
- Chapman R. A. A study of the contractures induced in frog atrial trabeculae by a reduction of the bathing sodium concentration. J Physiol. 1974 Mar;237(2):295–313. doi: 10.1113/jphysiol.1974.sp010483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The dependence of the contractile force generated by frog auricular trabeculae upon the external calcium concentration. J Physiol. 1971 May;215(1):139–162. doi: 10.1113/jphysiol.1971.sp009462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. The action of ions and lipoids upon the frog's heart. J Physiol. 1913 Oct 17;47(1-2):66–107. doi: 10.1113/jphysiol.1913.sp001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly I. de B., Clark A. J. The action of ions upon the frog's heart. J Physiol. 1921 Mar 15;54(5-6):367–383. doi: 10.1113/jphysiol.1921.sp001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einwächter H. M., Haas H. G., Kern R. Membrane current and contraction in frog atrial fibres. J Physiol. 1972 Dec;227(1):141–171. doi: 10.1113/jphysiol.1972.sp010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. High potassium and low sodium contractures in sheep cardiac muscle. J Gen Physiol. 1971 Nov;58(5):483–510. doi: 10.1085/jgp.58.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Kimoto Y., Kato Y. A study on the excitation-contraction coupling of the bullfrog ventricle with voltage clamp technique. Jpn J Physiol. 1971 Apr;21(2):159–173. doi: 10.2170/jjphysiol.21.159. [DOI] [PubMed] [Google Scholar]
- Haas H. G., Glitsch H. G., Kern R. Kalium-Fluxe und Membranpotential am Froschvorhof in Abhängigkeit von der Kalium-Aussenkonzentration. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;288(1):43–64. [PubMed] [Google Scholar]
- Haas H. G., Kern R., Benninger C., Einwächter H. M. Effects of prenylamine on cardiac membrane currents and contractility. J Pharmacol Exp Ther. 1975 Mar;192(3):688–701. [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., McGuigan J. A. Contractures in a superfused frog's ventricle. J Physiol. 1966 Oct;186(2):261–283. doi: 10.1113/jphysiol.1966.sp008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L. Ca2+ transport by mitochondria and its possible role in the cardiac contraction-relaxation cycle. Circ Res. 1974 Sep;35 (Suppl 3):83–90. [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Sensitivity of calcium efflux from squid axons to changes in membrane potential. J Gen Physiol. 1975 Feb;65(2):135–152. doi: 10.1085/jgp.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. The ionic nature of slow inward current and its relation to contraction. Pflugers Arch. 1972;334(1):24–38. doi: 10.1007/BF00585998. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dual effect of calcium on the action potential of the frog's heart. J Physiol. 1966 May;184(2):291–311. doi: 10.1113/jphysiol.1966.sp007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R., Trautwein W. The dependence of cardiac contraction on depolarization and slow inward current. Pflugers Arch. 1971;323(3):187–203. doi: 10.1007/BF00586383. [DOI] [PubMed] [Google Scholar]
- Ramón F., Anderson N., Joyner R. W., Moore J. W. Axon voltage-clamp simulations. A multicellular preparation. Biophys J. 1975 Jan;15(1):55–69. doi: 10.1016/S0006-3495(75)85791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter M. Der Einfluss der Natriumionen auf die Beziehung zwischen Frequenz und Kraft der Kontraktion des isolierten Meerschweinchenmyokards. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(3):261–286. [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S. A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. J Physiol. 1883 Jan;4(1):29–42.3. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- Scholz H. Uber Unterschiede im Kontrakturverhalten bei Ventrikel- und Vorhofspräparaten aus Warmblüterherzen. Pflugers Arch. 1969;312(3):63–81. doi: 10.1007/BF00588532. [DOI] [PubMed] [Google Scholar]
- Scholz H. Uber die Wirkung von Calcium and Natrium auf die Kaliumkontraktur isolierter Meerschweinchenvorhöfe. Pflugers Arch. 1969;308(4):315–332. doi: 10.1007/BF00587183. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Z Zellforsch Mikrosk Anat. 1969;98(3):437–468. [PubMed] [Google Scholar]
- Tarr M., Trank J. W. An assessment of the double sucrose-gap voltage clamp technique as applied to frog atrial muscle. Biophys J. 1974 Sep;14(9):627–643. doi: 10.1016/S0006-3495(74)85940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr M. Two inward currents in frog atrial muscle. J Gen Physiol. 1971 Nov;58(5):523–543. doi: 10.1085/jgp.58.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. Influence of sodium ions on the regulation of frog myocardial contractility. Pflugers Arch. 1973 Mar 30;339(3):224–240. doi: 10.1007/BF00587374. [DOI] [PubMed] [Google Scholar]
- Vassort G., Rougier O. Membrane potential and slow inward current dependence of frog cardiac mechanical activity. Pflugers Arch. 1972;331(3):191–203. doi: 10.1007/BF00589126. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W., KOLLER H. Die Calciumwirkung am Froschherzen als Funktion des Ionengleichgewichts zwischen Zellmembran und Umgebung. Helv Physiol Pharmacol Acta. 1948;6(2):208–221. [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R., Tritthart H., Walter B. Correlation of Na-withdrawal effects on Ca-mediated action potentials and contractile activity in cat ventricular myocardium. Pflugers Arch. 1974;350(4):299–307. doi: 10.1007/BF00592638. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium binding and release in frog heart. J Gen Physiol. 1973 Dec;62(6):693–706. doi: 10.1085/jgp.62.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. Studies of cardiac muscle with a high permeability to calcium produced by treatment with ethylenediaminetetraacetic acid. J Gen Physiol. 1971 Jul;58(1):71–93. doi: 10.1085/jgp.58.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]


