Abstract
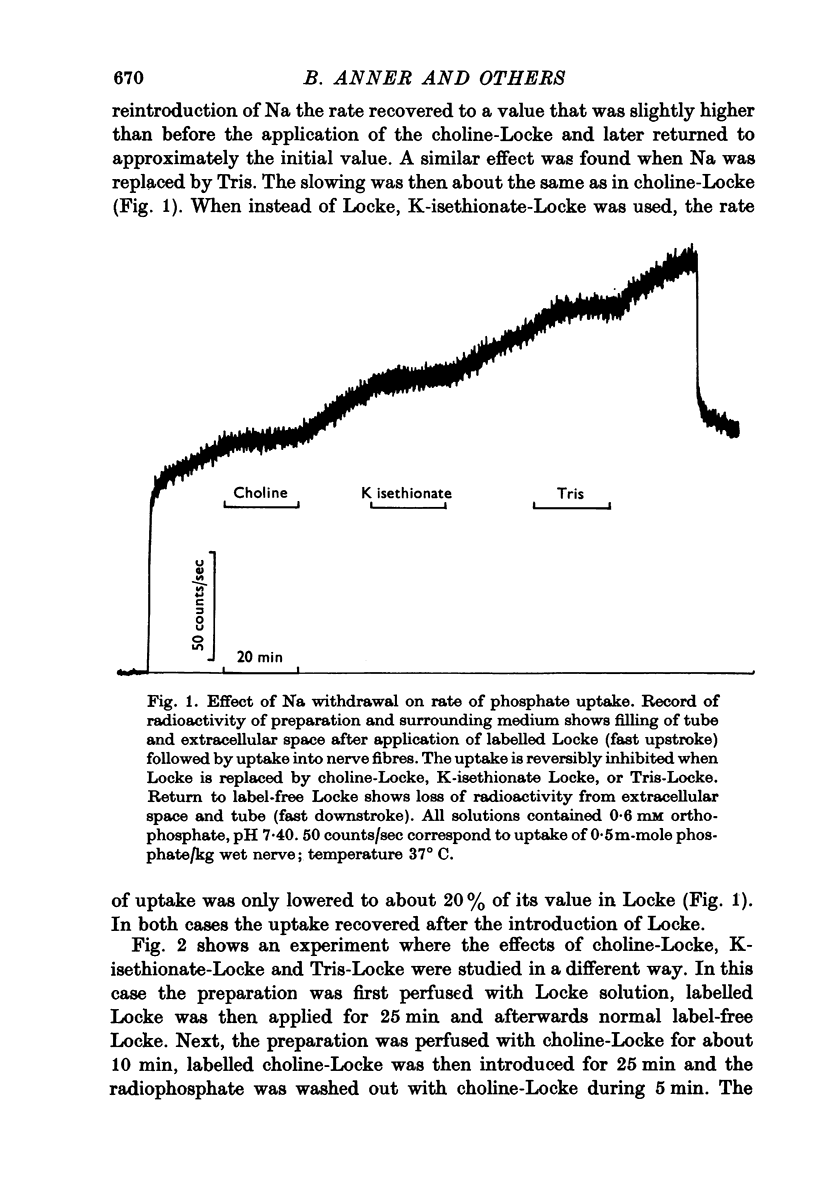
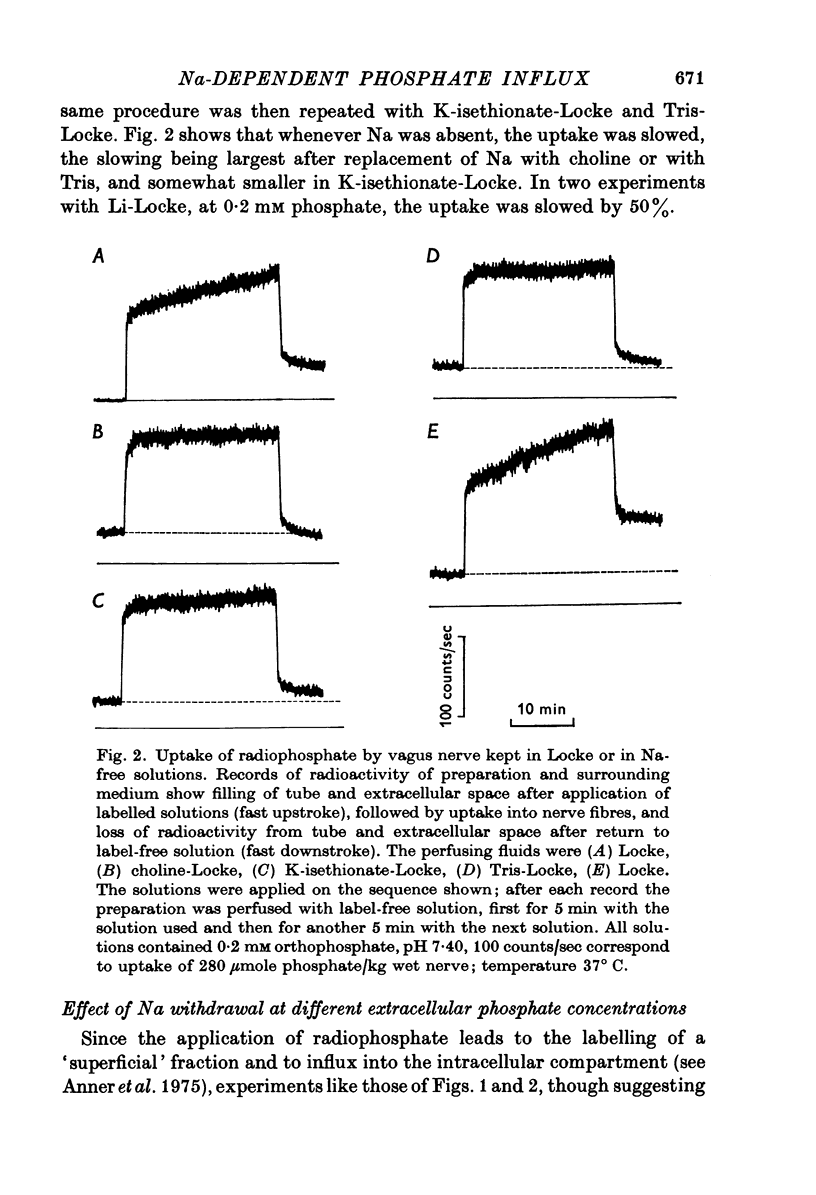
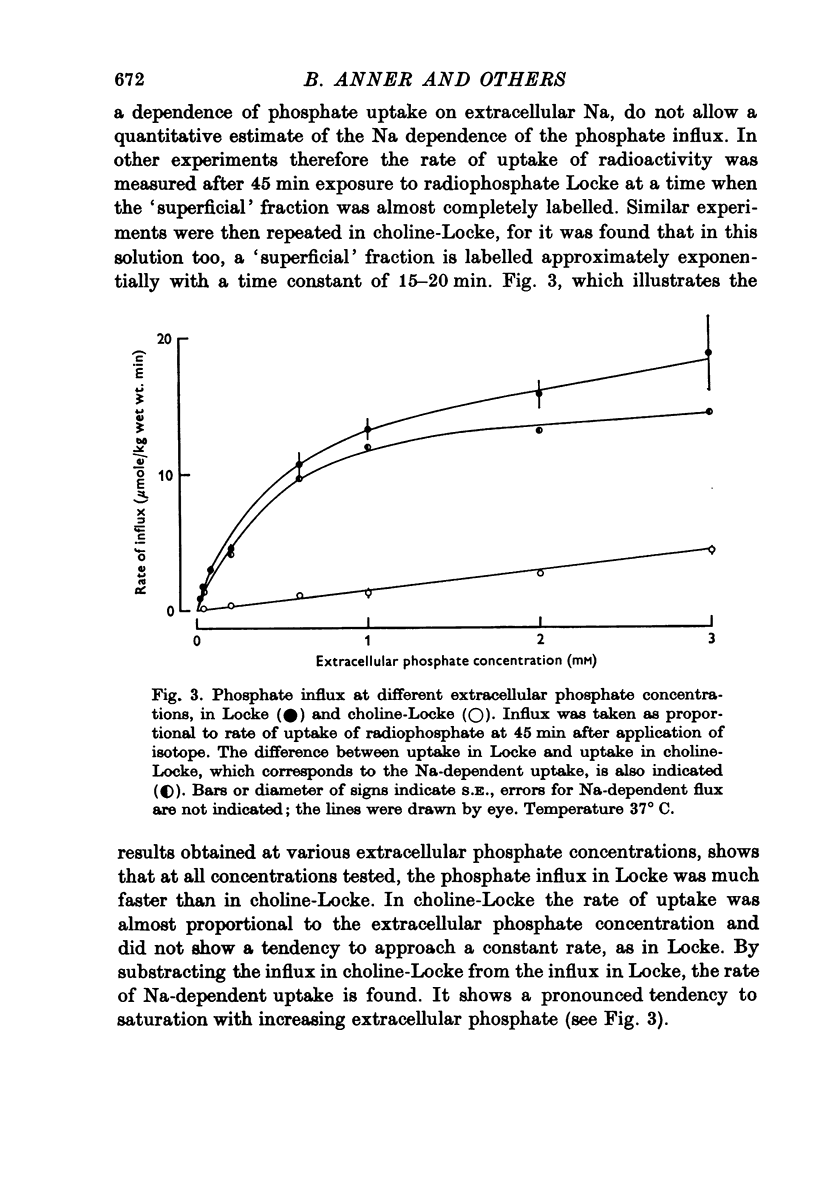
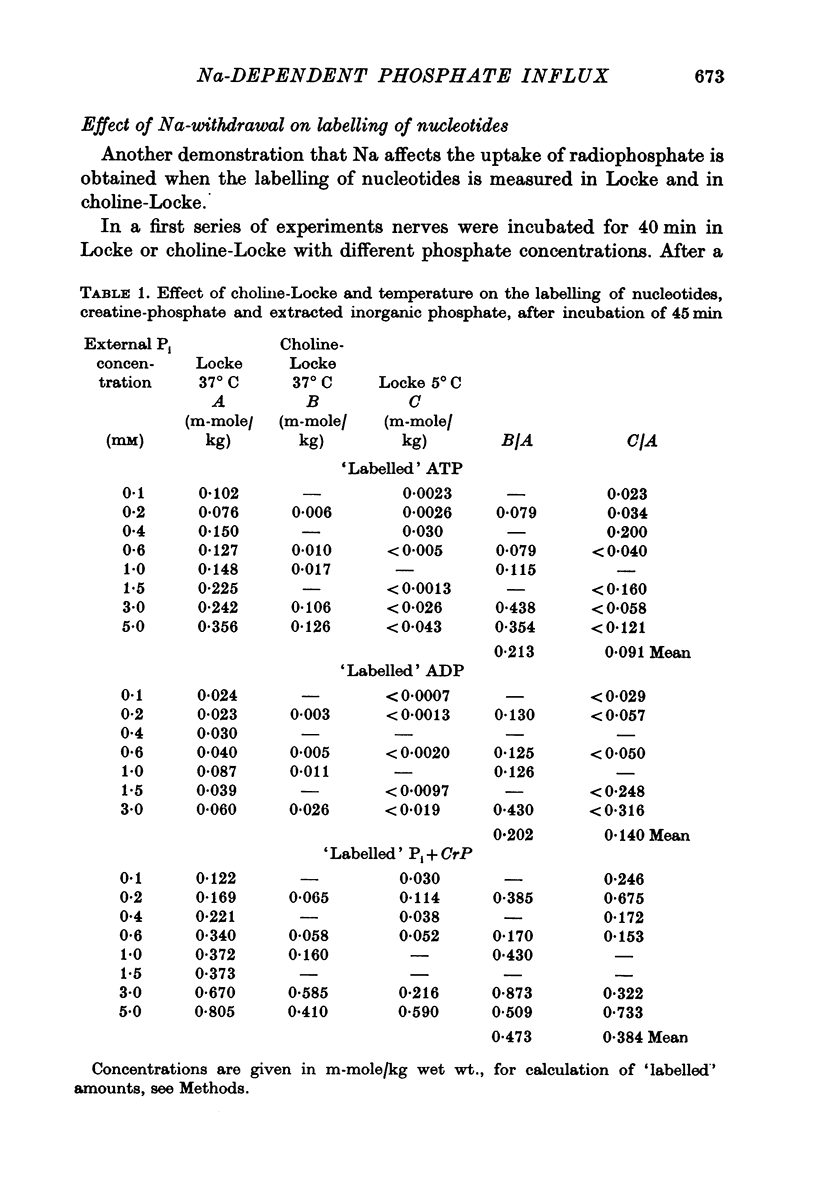
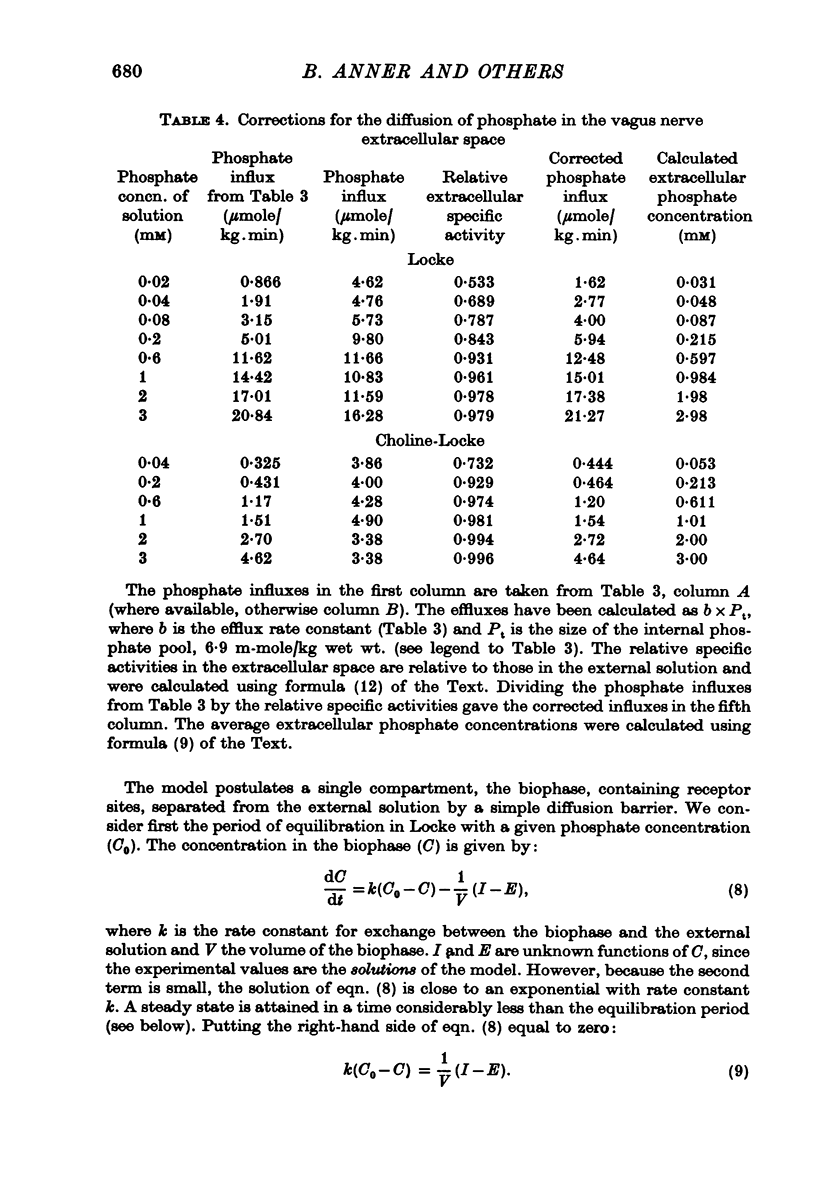
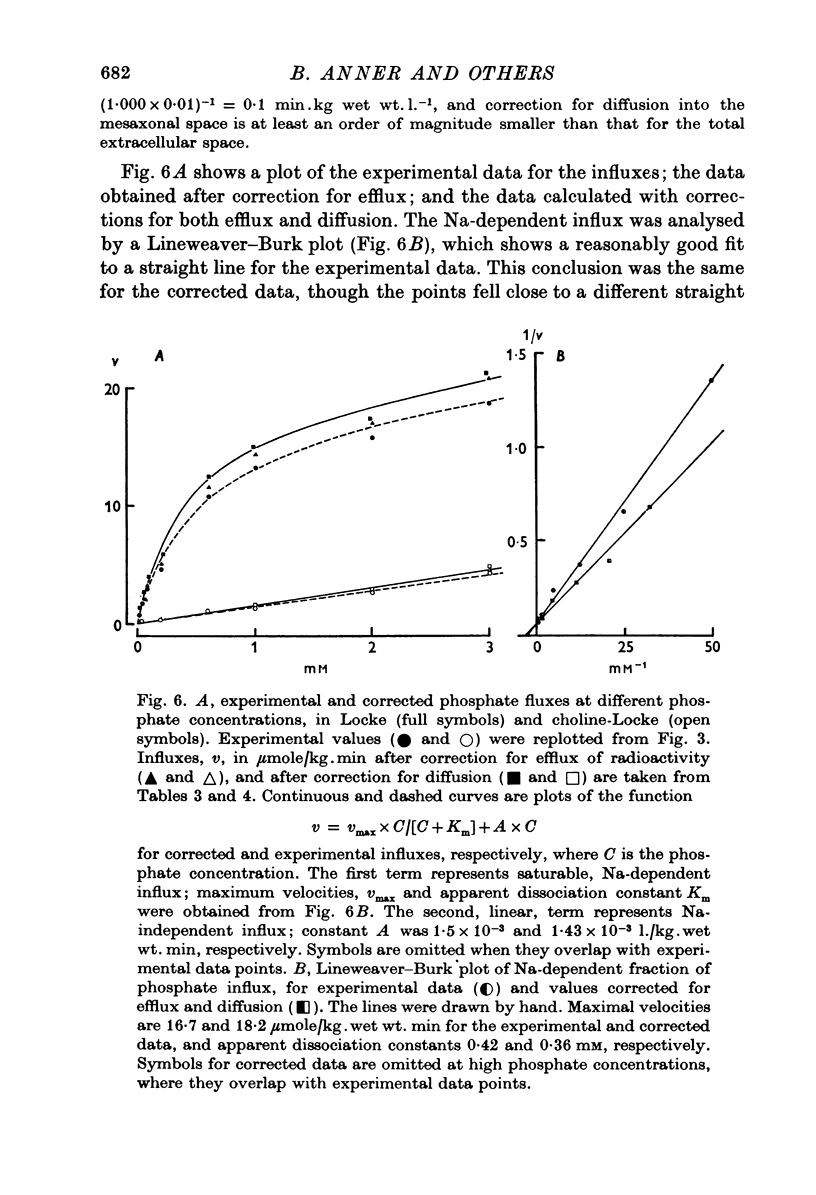
1. The rate of uptake of radiophosphate was measured in desheathed vagus nerves of rabbits mounted in an apparatus where the incubating solution flowed along the preparation. 2. Replacement of the Na of the Locke by either choline or Tris slowed the rate of uptake to about 10% of its value in Na; with K it was slowed to 20%; and with Li to 50%. 3. Measurements of the rate of uptake at different extracellular phosphate concentrations showed that in choline-Locke the influx of phosphate was proportional to the extracellular phosphate concentration, while in Locke the rate of inward flow showed a tendency to saturation with increasing phosphate concentrations. 4. Extracts of nerves after 45 min exposure to labelled solutions showed for different phosphate concentrations a slowing of the labelling of ATP, ADP and creatine-phosphate (CrP) and inorganic phosphate (Pi) when Locke was replaced by choline-Locke. 5. A similar slowing was found when, at 0-2 mM phosphate, the labelling of these compounds was measured at different times: application of choline-Locke reduced the rate of incorporation of 32P to about 15% of the rate in Locke. 6. In preparations that were loaded with radiophosphate and then washed with inactive solution, and the effluent fractionated, over 90% of the radioactivity was found in the inorganic phosphate fraction. 7. The efflux of 32P showed an initial exponential phase with a time constant of 20-30 min and a much slower one. The slow efflux had a rate constant of 0-0014 min-1 in 0-2 mM external phosphate. 8. Increasing the external phosphate concentration increased the efflux. 9. Prolonged incubation in choline-Locke reduced the efflux. 10. The influx of phosphate was calculated for different external phosphate concentrations using the rate of uptake of radiophosphate measured 45 min after the application of isotope and the corresponding efflux rate coefficient and the intracellular specific activities of the labelled compounds. 11. A correction was also introduced for diffusion, using the "limited biophase model". 12. The Na-sensitive influx, i.e. the difference between influx in Locke and influx in choline-Locke, showed saturation kinetics. 13. A Lineweaver-Burk plot for Na-sensitive uncorrected influxes gave a vmax of 16-7 mumole/kg wet wt. min and an apparent Km of 0-42 mM; for the corrected fluxes these values were 18-2 and 0-36. 14. It is concluded that a large part of phosphate influx and some of the phosphate efflux is mediated by a specific Na-dependent phosphate transport system. 15. This system seems to be present also in other types of nervous tissues and probably in many types of animal cells.
Full text
PDF


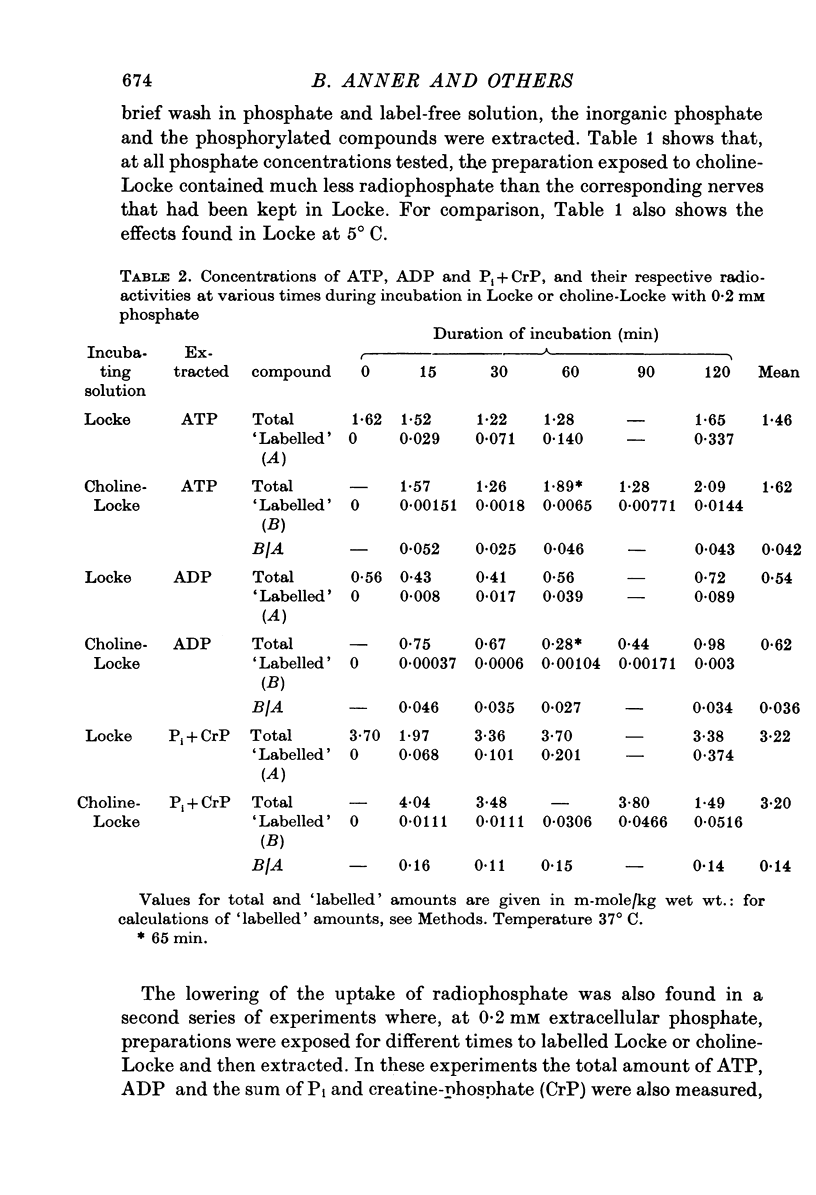
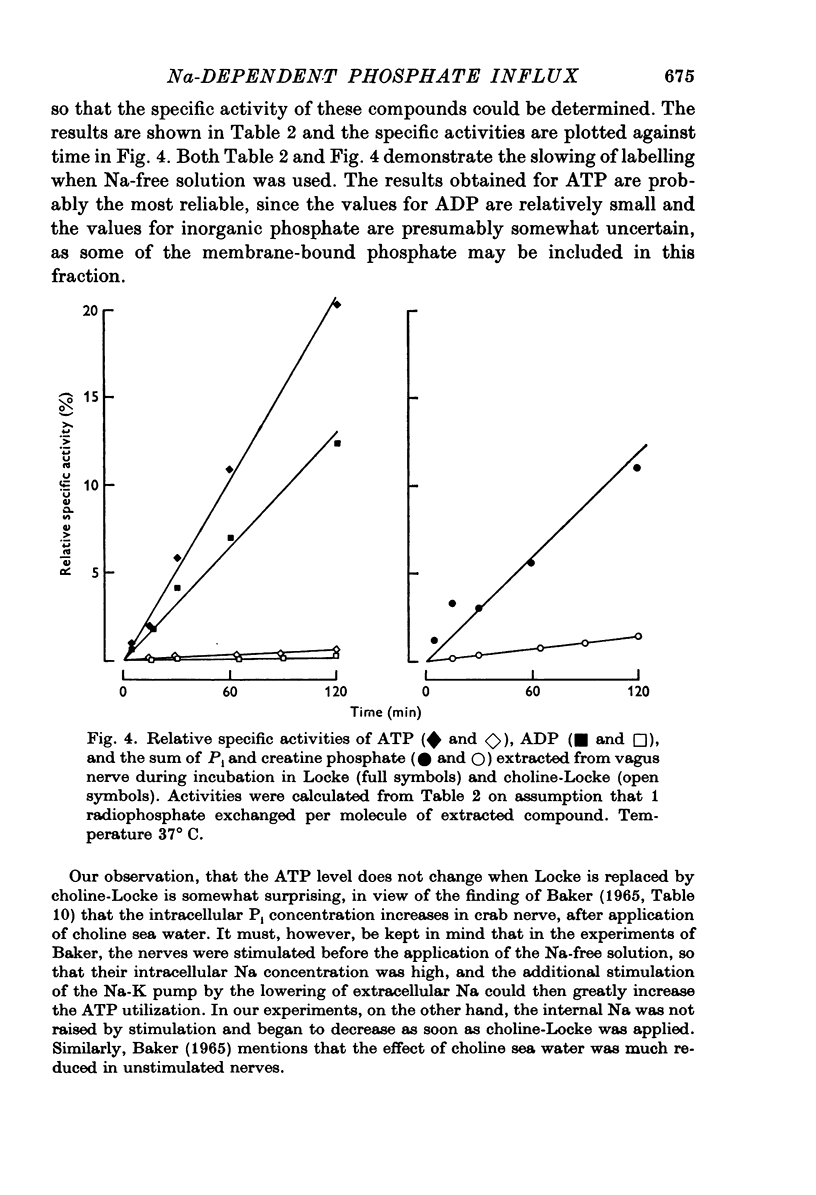
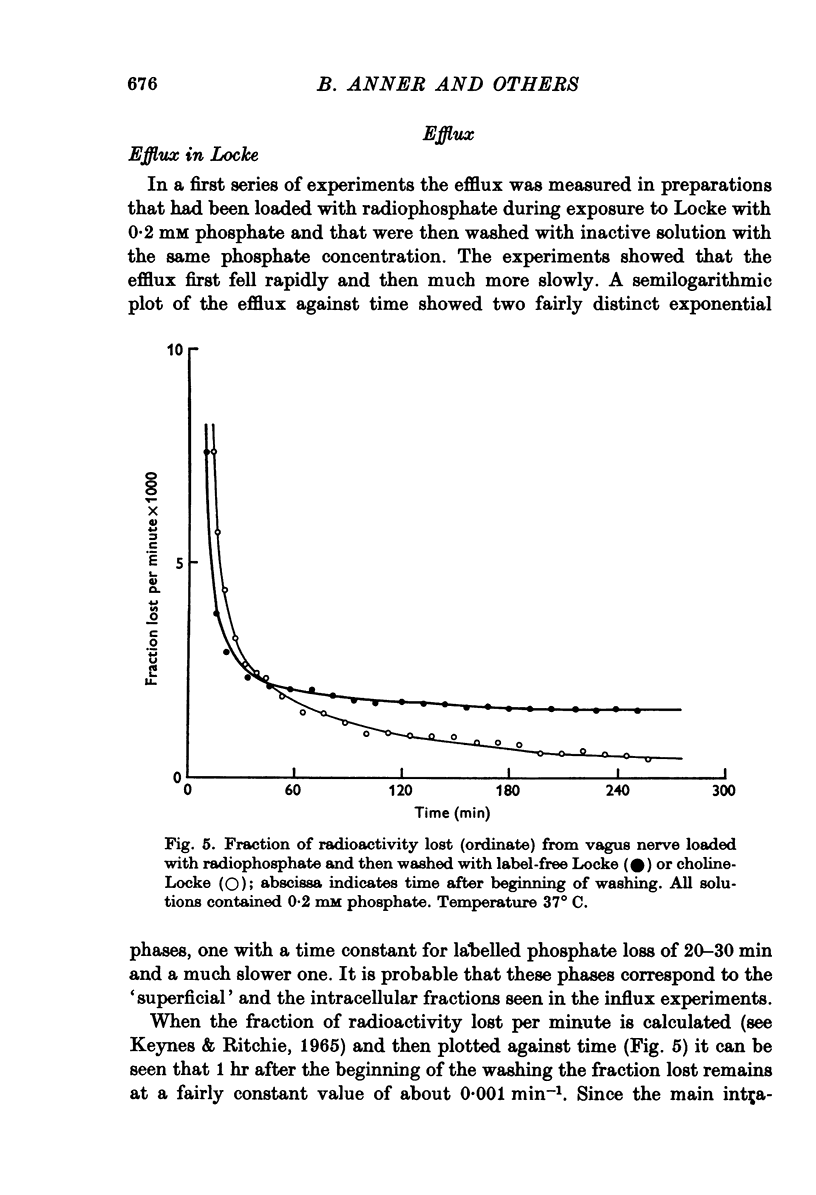

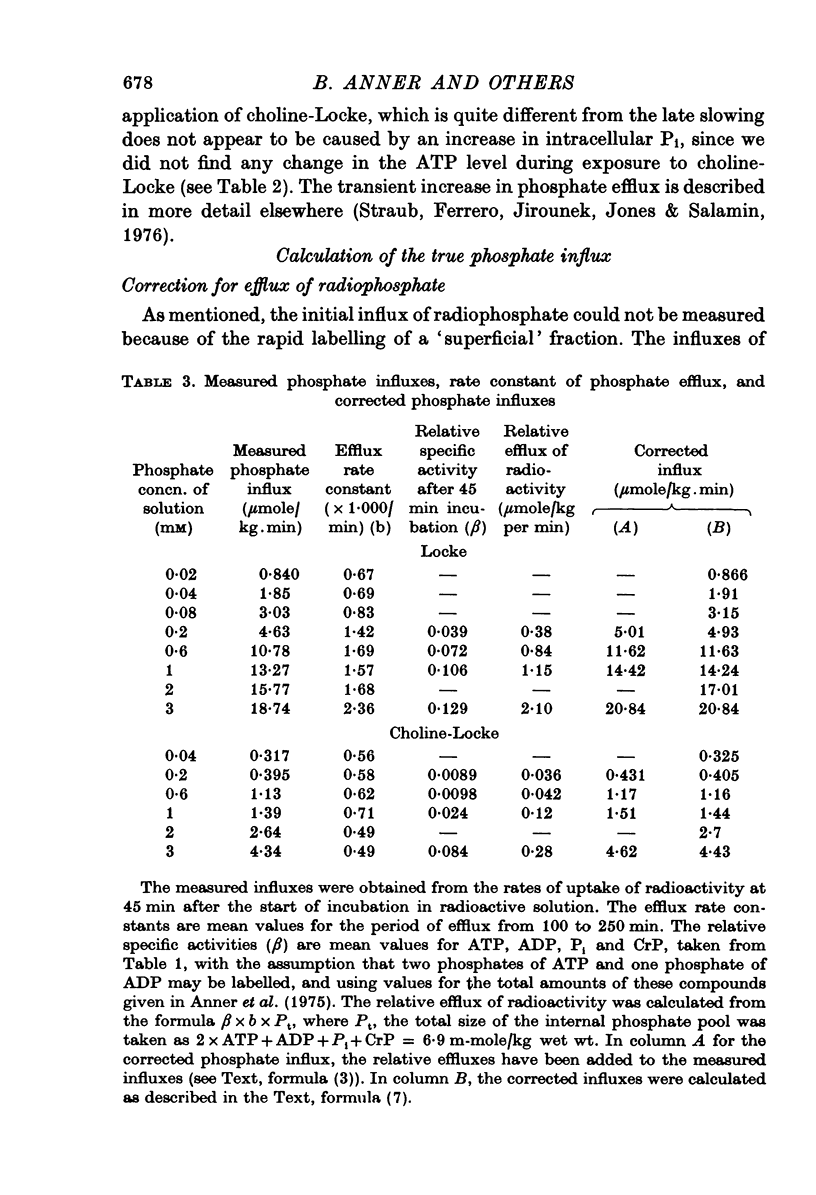






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABOOD L. G., KOYAMA I. PHOSPHATE INCORPORATION IN DESHEATHED NERVES: EFFECTS OF POTASSIUM AND CALCIUM IONS. Science. 1963 Sep 27;141(3587):1277–1278. doi: 10.1126/science.141.3587.1277. [DOI] [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Straub R. W. Inhibition of intracellular orthophosphate uptake in rabbit vagus nerve by Na withdrawal and low temperature. J Physiol. 1973 Jul;232(1):47P–48P. [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Straub R. W. Uptake of orthophosphate by rabbit vagus nerve fibres. J Physiol. 1975 Jun;247(3):759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K., de Rouffignac C., Roinel N., Rumrich G., Ullrich K. J. Renal phosphate transport: inhomogeneity of local proximal transport rates and sodium dependence. Pflugers Arch. 1975;356(4):287–298. doi: 10.1007/BF00580003. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Lamb J. F. Proceedings: Na-dependent phosphate transport in cultured cells. J Physiol. 1975 Sep;251(1):58P–59P. [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. THE RATE OF FORMATION AND TURNOVER OF PHOSPHORUS COMPOUNDS IN SQUID GIANT AXONS. J Physiol. 1964 May;171:119–131. doi: 10.1113/jphysiol.1964.sp007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ritchie J. M. The kinetics of the interaction between tetrodotoxin and mammalian nonmyelinated nerve fibers. Mol Pharmacol. 1972 May;8(3):285–292. [PubMed] [Google Scholar]
- Ferrero J., Jirounek P., Jones G. J., Salamin A., Straub R. W. Proceedings: Effects of ions and temperature on the efflux of orthophosphate from rabbit vagus nerve fibres. J Physiol. 1976 Jan;254(1):64P–65P. [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958 Dec 30;144(3):442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Effect of frequency of electrical stimulation on the concentration of intermediary metabolites in mammalian non-myelinated fibres. J Physiol. 1959 Oct;148:353–361. doi: 10.1113/jphysiol.1959.sp006292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Sodium, potassium, and intestinal transport of glucose, 1-tyrosine, phosphate, and calcium. Am J Physiol. 1963 Jul;205:107–111. doi: 10.1152/ajplegacy.1963.205.1.107. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. The kinetics of action of acetylcholine antagonists in smooth muscle. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):488–510. doi: 10.1098/rspb.1966.0045. [DOI] [PubMed] [Google Scholar]
- Siegenthaler P. A., Belsky M. M., Goldstein S. Phosphate uptake in an obligately marine fungus: a specific requirement for sodium. Science. 1967 Jan 6;155(3758):93–94. doi: 10.1126/science.155.3758.93. [DOI] [PubMed] [Google Scholar]


