Abstract
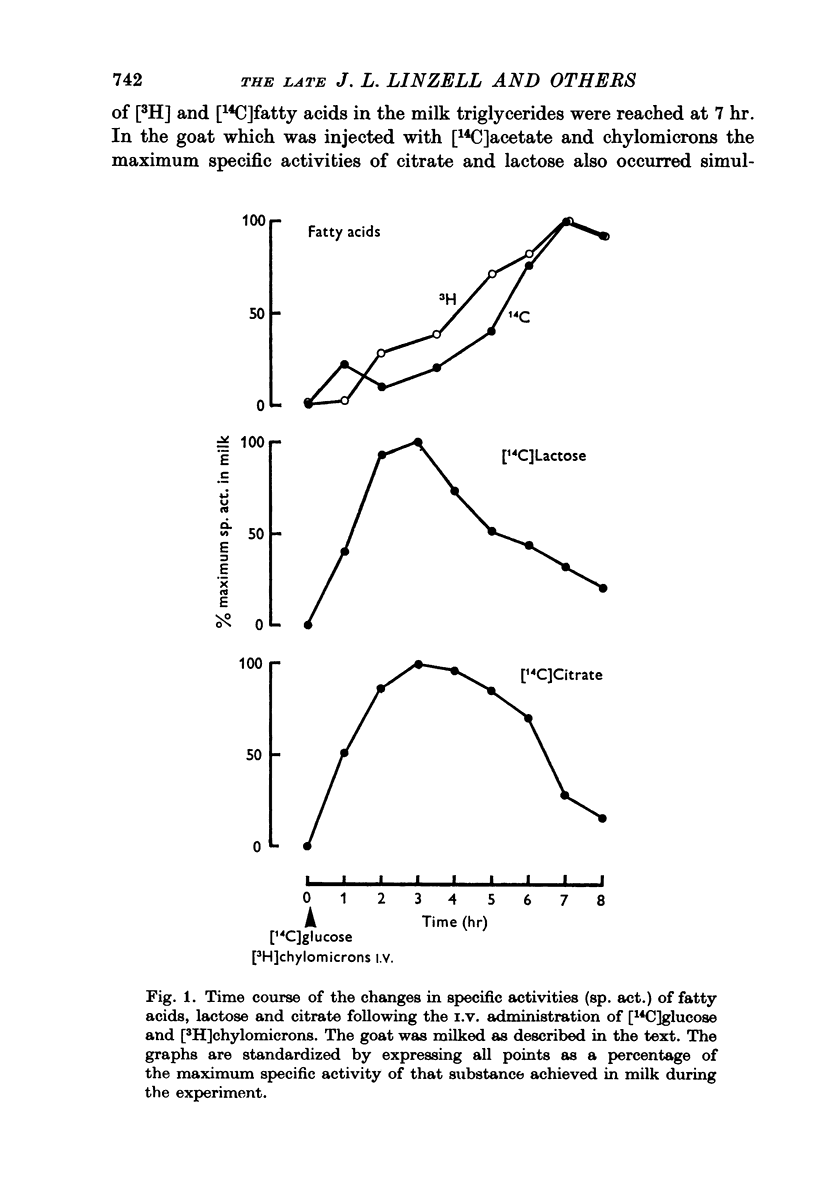
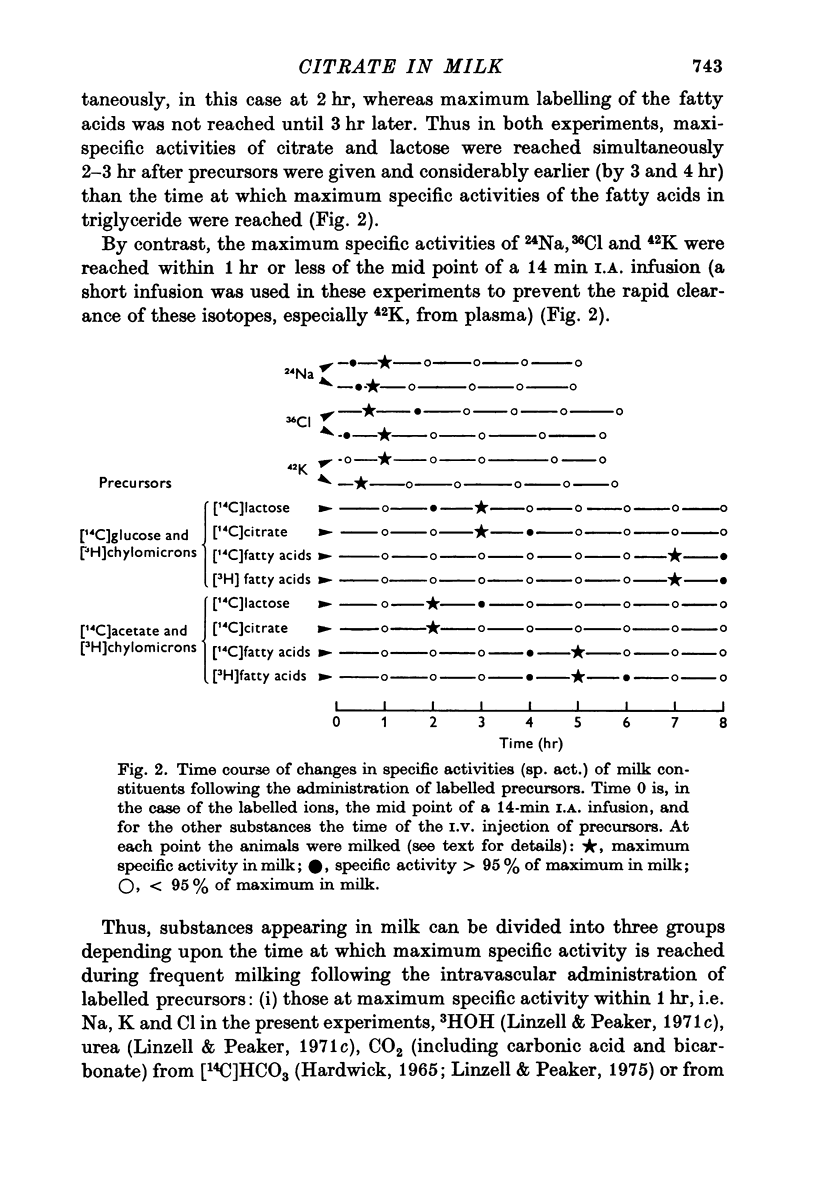
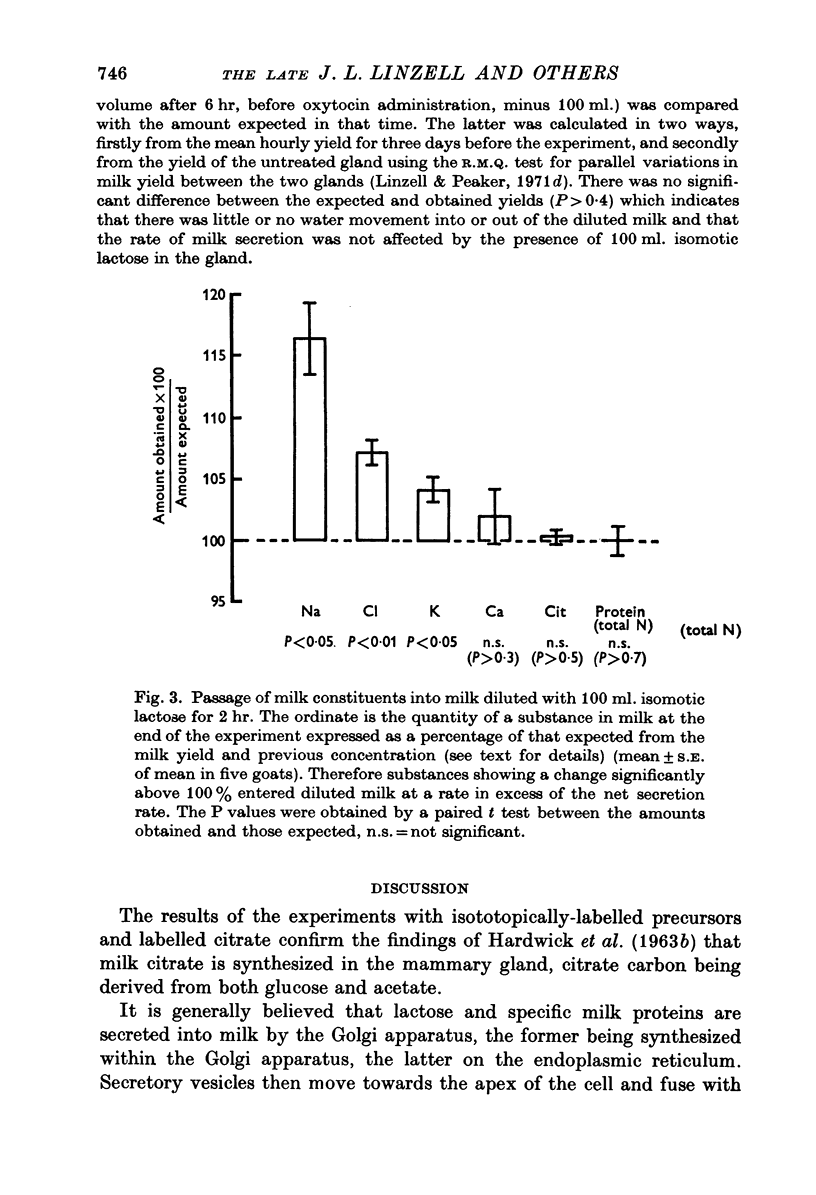
1. The time course of changes in specific activities of citrate, lactose and fatty acids in milk during frequent milking, following the I.V. administration of labelled glucose, acetate and chylomicrons in goats has been studied. Peak specific activities of lactose and citrate in milk were reached at 2-3 hr, while peak specific activites of fatty acids were reached at 5-7 hr. 2. Following short I.A. infusions of 24Na, 36Cl, and 42K, peak specific activities in milk were reached in 1 hr or less. 3. The mammary epithelium of lactating goats was found to be virtually impermeable to labelled citrate in both directions. 4. Labelled citrate had an apparent volume of distribution in lactating guinea-pigs mammary slices in vitro similar to that of extracellular space markers. 5. Treatment of goats with large doses of oxytocin markedly increased the permeability of the secretory epithelium to labelled citrate. 6. In the goat mammary gland, citrate, protein and calcium failed to enter milk which had been diluted with isosmotic lactose by intraductal injection, whereas Na, K and Cl did enter, thus tending to restore the concentrations of these ions to normal. 7. It is suggested that citrate, which is formed within the sucretory cell, enters milk not by passage across the apical cell membrane but, in common with lactose and milk protein, by exocytosis of Golgi vesicles. It appears that citrate is held at high concentrations in milk by virtue of the impermeability of the mammary epithelium to the forms in which it occurs in milk.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauman D. E., Mellenberger R. W., Derrig R. G. Fatty acid synthesis in sheep mammary tissue. J Dairy Sci. 1973 Oct;56(10):1312–1318. doi: 10.3168/jds.S0022-0302(73)85352-4. [DOI] [PubMed] [Google Scholar]
- Fleet I. R., Goode J. A., Hamon M. H., Laurie M. S., Linzell J. L., Peaker M. Secretory activity of goat mammary glands during pregnancy and the onset of lactation. J Physiol. 1975 Oct;251(3):763–773. doi: 10.1113/jphysiol.1975.sp011120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet I. R., Linzell J. L., Peaker M. The use of an autoanalyzer for the rapid analysis of milk constituents affected by subclinical mastitis. Br Vet J. 1972 Jun;128(6):297–300. doi: 10.1016/s0007-1935(17)36934-8. [DOI] [PubMed] [Google Scholar]
- Gumaa K. A., McLean P., Greenbaum A. L. Calculation of the intracellular distribution of acetyl CoA and CoA, based on the use of citrate synthase as an equilibrium enzyme. FEBS Lett. 1973 Jan 15;29(2):193–196. doi: 10.1016/0014-5793(73)80559-9. [DOI] [PubMed] [Google Scholar]
- HARDWICK D. C. THE INCORPORATION OF CARBON DIOXIDE INTO MILK CITRATE IN THE ISOLATED PERFUSED GOAT UDDER. Biochem J. 1965 Apr;95:233–237. doi: 10.1042/bj0950233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick D. C., Linzell J. L., Mepham T. B. The metabolism of acetate and glucose by the isolated perfused udder. 2. The contribution of acetate and glucose to carbon dioxide and milk constituents. Biochem J. 1963 Aug;88(2):213–220. doi: 10.1042/bj0880213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick D. C. The fate of acetyl groups derived from glucose in the isolated perfused goat udder. Biochem J. 1966 Apr;99(1):228–231. doi: 10.1042/bj0990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T. W., Huang C. M., Morre D. J. Membranes of mammary gland. V. Isolation of Golgi apparatus and rough endoplasmic reticulum from bovine mammary gland. J Dairy Sci. 1972 Nov;55(11):1577–1585. doi: 10.3168/jds.s0022-0302(72)85724-2. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J., Cheetham R. D. Lactose synthesis by a golgi apparatus fraction from rat mammary gland. Nature. 1970 Dec 12;228(5276):1105–1106. doi: 10.1038/2281105a0. [DOI] [PubMed] [Google Scholar]
- Lascelles A. K., Hardwick D. C., Linzell J. L., Mepham T. B. The transfer of [3H]stearic acid from chylomicra to milk fat in the goat. Biochem J. 1964 Jul;92(1):36–42. doi: 10.1042/bj0920036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Changes in colostrum composition and in the permeability of the mammary epithelium at about the time of parturition in the goat. J Physiol. 1974 Nov;243(1):129–151. doi: 10.1113/jphysiol.1974.sp010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Intracellular concentrations of sodium, potassium and chloride in the lactating mammary gland and their relation to the secretory mechanism. J Physiol. 1971 Aug;216(3):683–700. doi: 10.1113/jphysiol.1971.sp009547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Mechanism of milk secretion. Physiol Rev. 1971 Jul;51(3):564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M., Taylor J. C. The effects of prolactin and oxytocin on milk secretion and on the permeability of the mammary epithelium in the rabbit. J Physiol. 1975 Dec;253(2):547–563. doi: 10.1113/jphysiol.1975.sp011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The distribution and movements of carbon dioxide, carbonic acid and bicarbonate between blood and milk in the goat. J Physiol. 1975 Jan;244(3):771–782. doi: 10.1113/jphysiol.1975.sp010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The permeability of mammary ducts. J Physiol. 1971 Aug;216(3):701–716. doi: 10.1113/jphysiol.1971.sp009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L. The effect of very frequent milking and of oxytocin on the yield and composition of milk in fed and fasted goats. J Physiol. 1967 May;190(2):333–346. doi: 10.1113/jphysiol.1967.sp008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaker M., Linzell J. L. Citrate in milk: a harbinger of lactogenesis. Nature. 1975 Feb 6;253(5491):464–464. doi: 10.1038/253464a0. [DOI] [PubMed] [Google Scholar]
- Peaker M. Recent advances in the study of monovalent ions movements across the mammary epithelium: relation to onset of lactation. J Dairy Sci. 1975 Jul;58(7):1042–1047. doi: 10.3168/jds.s0022-0302(75)84677-7. [DOI] [PubMed] [Google Scholar]
- SELDINGER S. I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953 May;39(5):368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- WOOD H. G., JOFFE S., GILLESPIE R., HANSEN R. G., HARDENBROOK H. Lactose synthesis. IV. The synthesis of milk constituents after unilateral injection of glycerol-1,3-C14 into the pudic artery. J Biol Chem. 1958 Dec;233(6):1264–1270. [PubMed] [Google Scholar]


