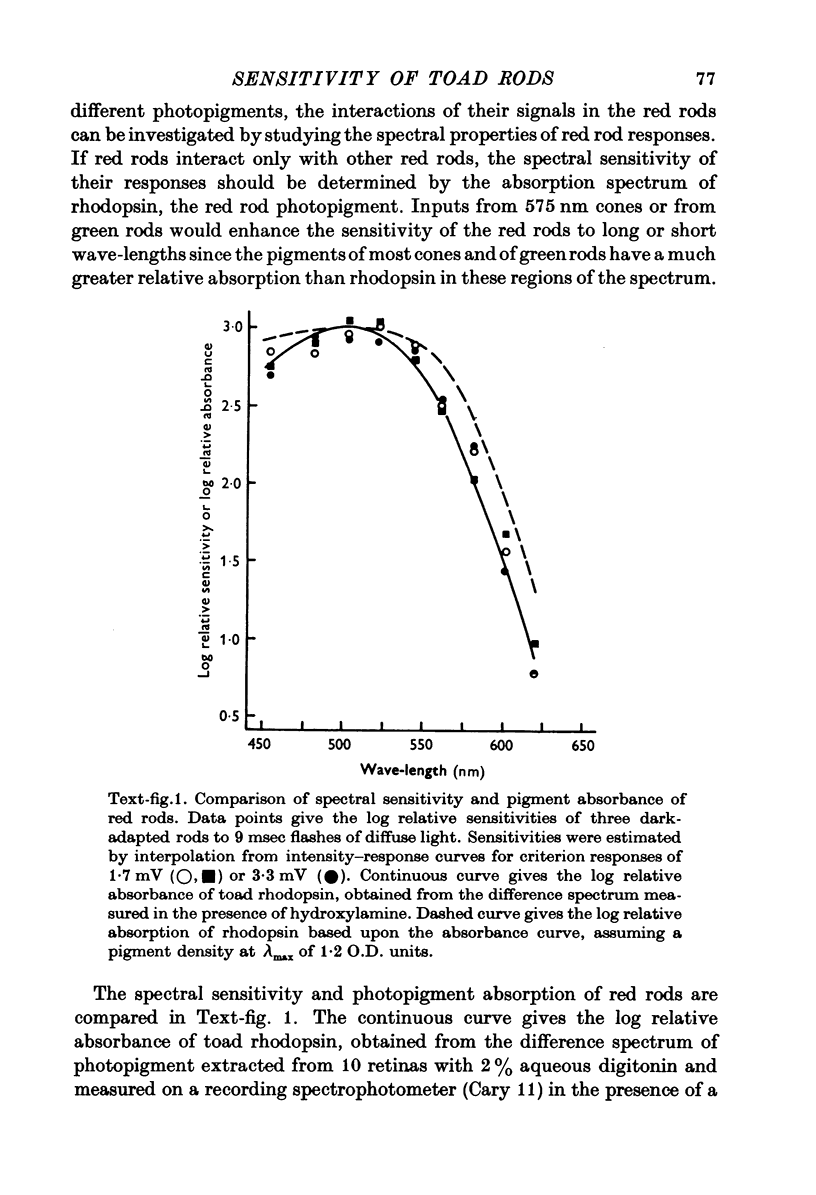
Abstract
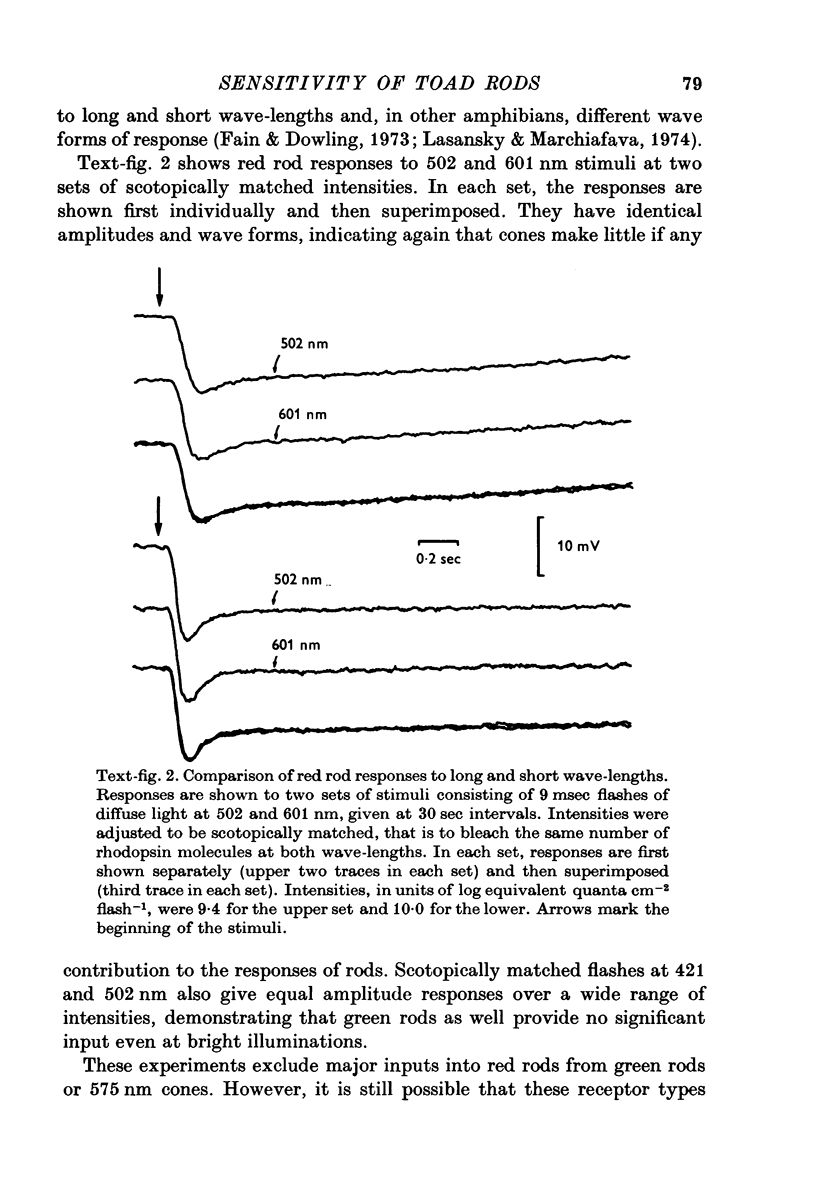
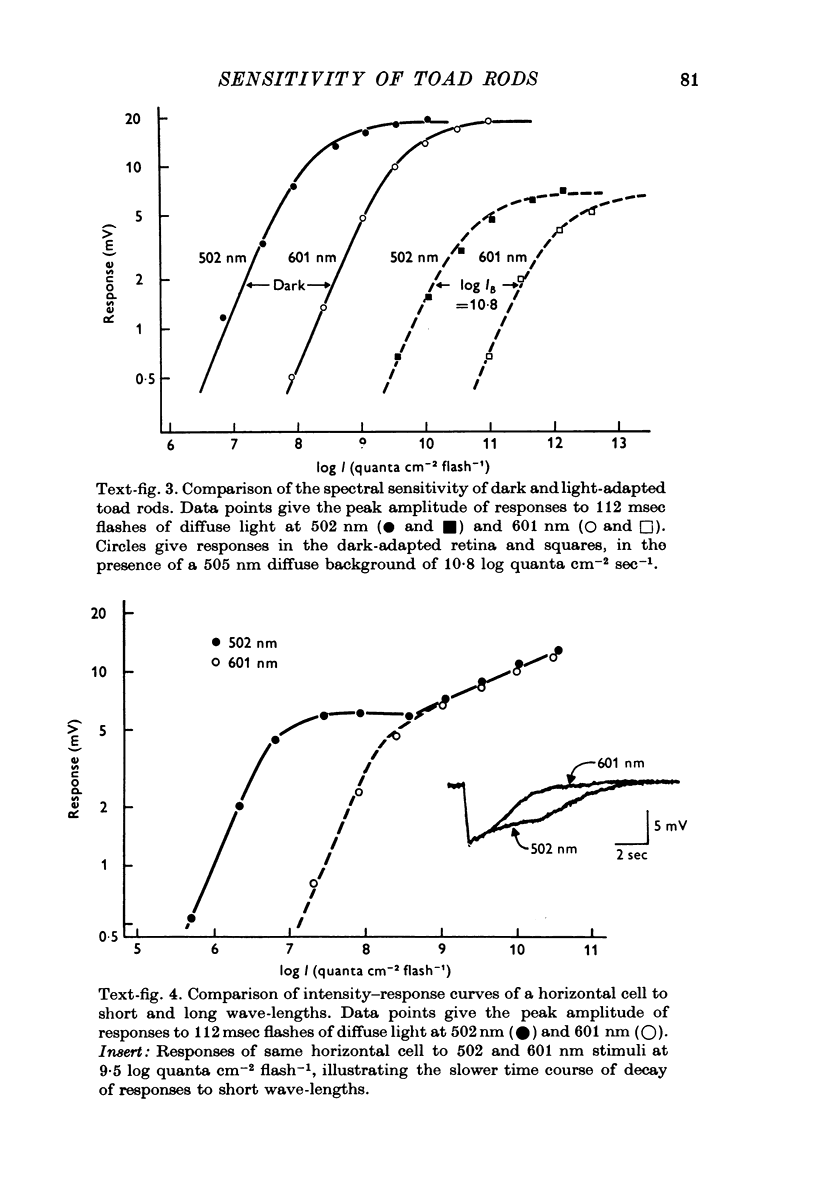
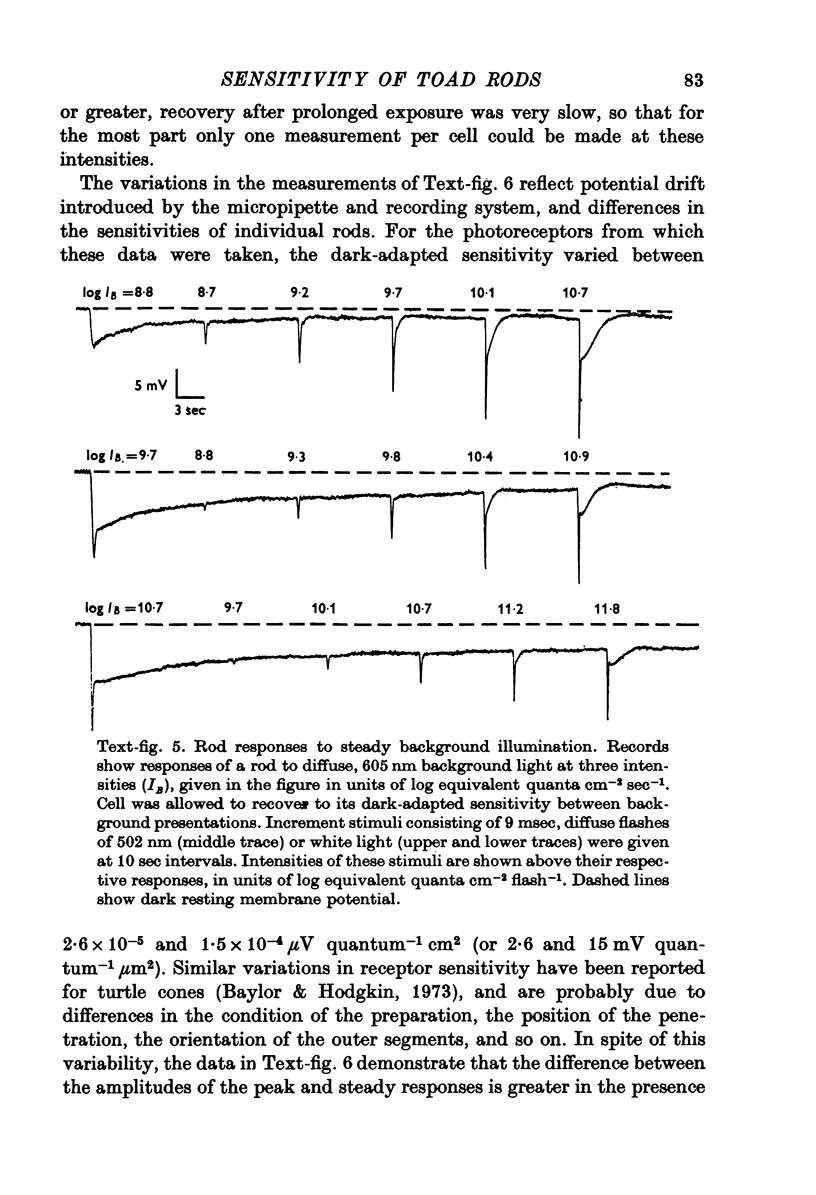
1. There are five morphological types of photoreceptors in the retina of the toad, Bufo marinus: red and green rods, single cones, and the principal and accessory members of double cones. The largest and most abundant of these is the red rod. 2. Intracellular recordings were used to investigate the dependence of the sensitivity of red rod responses on wave-length and background light. 3. The spectral sensitivity of dark-adapted and moderately light-adapted red rods can be satisfactorily fitted with the absorbance spectrum of the red rod photopigment. There are no significant contributions to red rod responses from cones or green rods. 4. In contrast, L-type horizontal cells, whose responses are dominated by input from the red rods near threshold, can be shown also to receive input from cones. 5. Steady background light produces a response in the red rods consisting of an initial hyperpolarization, followed by a decay of potential to a steady-state plateau level. The slow decay of response amplitude is accompanied by an increase in sensitivity to increment test flashes. 6. The increment sensitivity at steady-state decreases with increasing background intensity according to a modified Weber-Fechner relation. The dependence of increment sensitivity on the wave-length of the background light can be predicted by the red rod spectral sensitivity, showing that cones do not influence the light adaptation of rods. 7. At a backgound intensity of 11-5 log equivalent quanta cm-2sec-1, sensitivity begins to deviate from the Weber-Fechner relation. In background light one log unit brighter, the rods are completely saturated. 8. Small responses having the spectral sensitivity of cones can be recorded from saturated rods. These potentials have a prominent off response whose wave form resembles the d-wave of the e.r.g. 9. A comparison of the increment--sensitivity curves of single receptors shows that rods are light-adapted by backgrounds one thousand times dimmer than those which affect cones. The increment--sensitivity curves of rods and cones cross, so that single cones become more sensitive than single rods even before the rods begin to saturate.
Full text
PDF










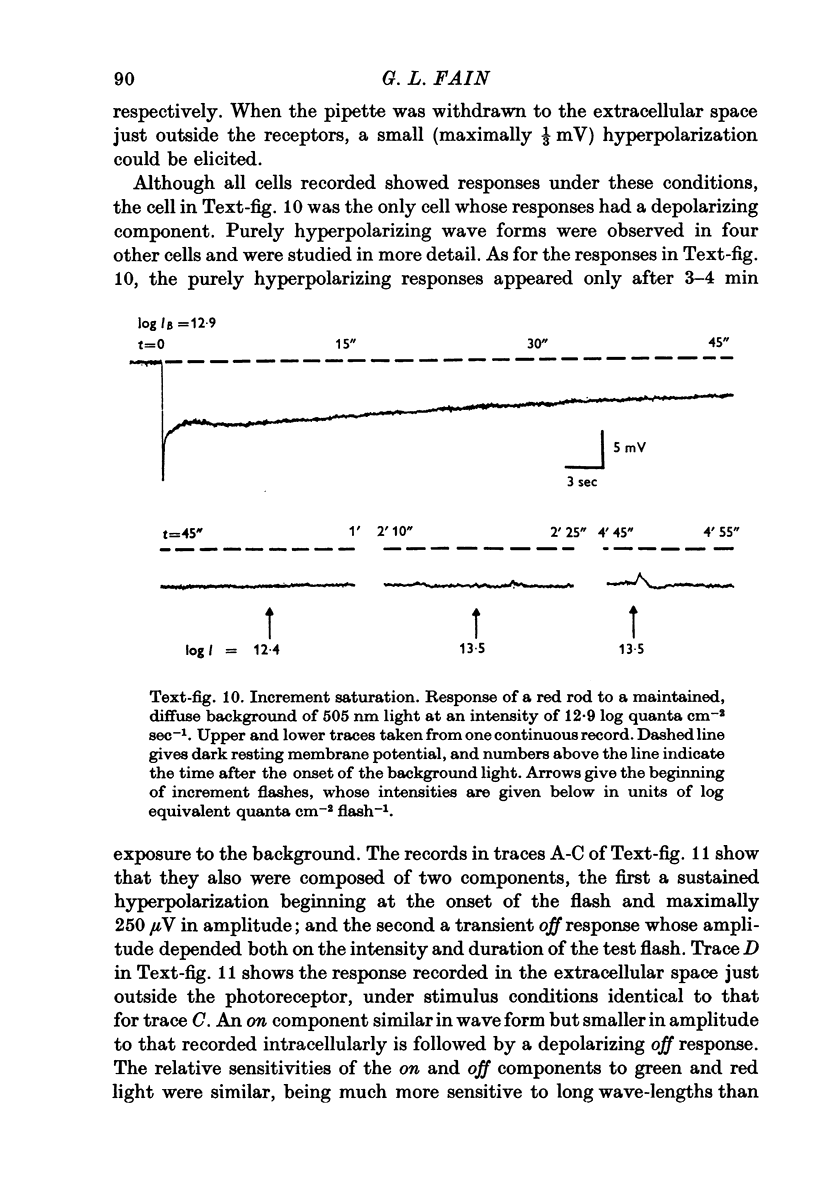
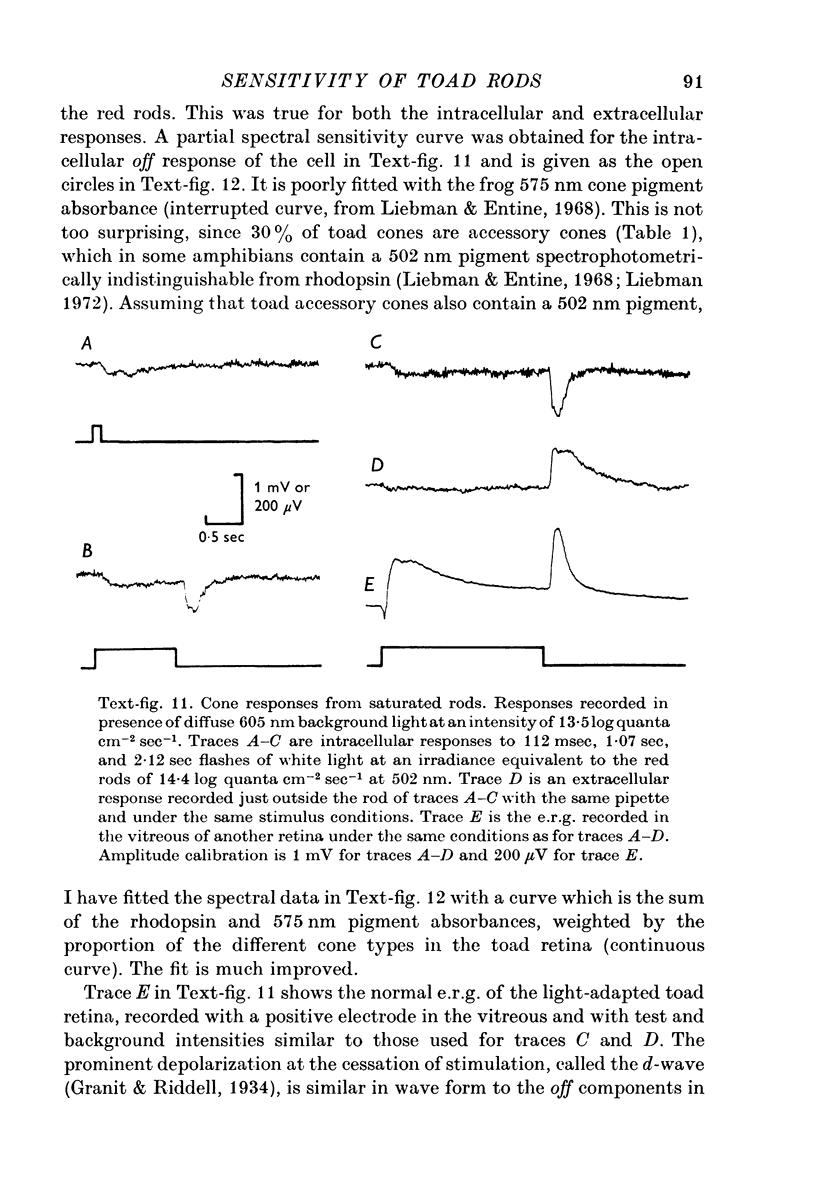
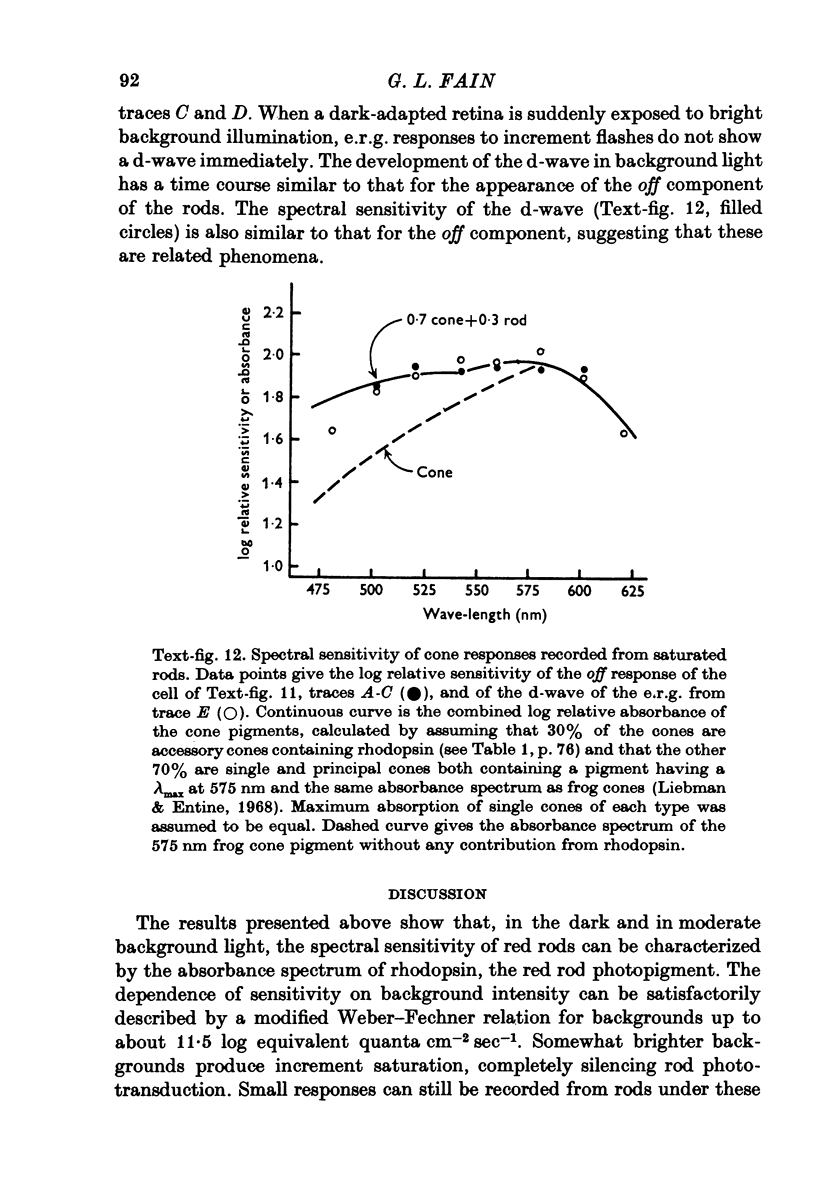



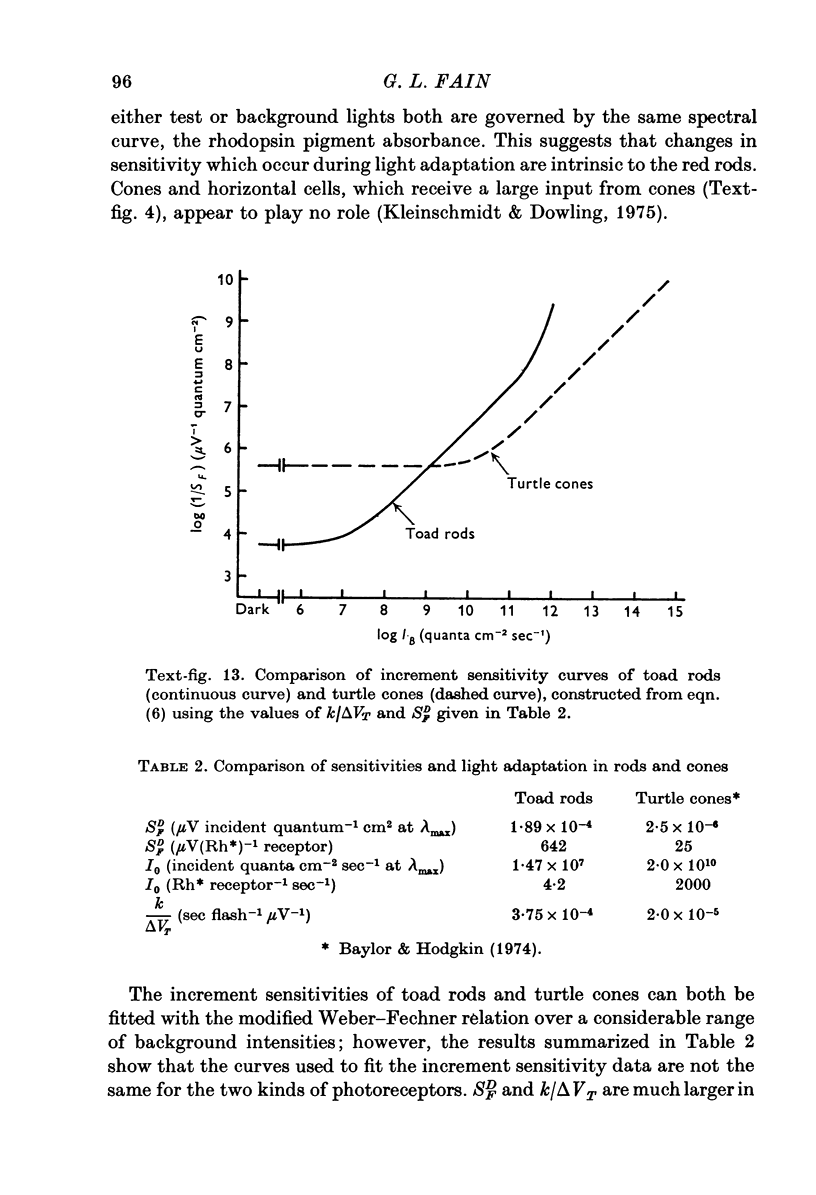





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Light path and photon capture in turtle photoreceptors. J Physiol. 1975 Jun;248(2):433–464. doi: 10.1113/jphysiol.1975.sp010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownds D., Brodie A. E. Light-sensitive swelling of isolated frog rod outer segments as an in vitro assay for visual transduction and dark adaptation. J Gen Physiol. 1975 Oct;66(4):407–425. doi: 10.1085/jgp.66.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENTON E. J., WYLLIE J. H. Study of the photosensitive pigments in the pink and green rods of the frog. J Physiol. 1955 Jan 28;127(1):81–89. doi: 10.1113/jphysiol.1955.sp005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J. The visual pigment of the green rods. Vision Res. 1967 Jan;7(1):1–16. doi: 10.1016/0042-6989(67)90022-3. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. Visual adaptation of the rhodopsin rods in the frogs retina. J Physiol. 1968 Nov;199(1):59–87. doi: 10.1113/jphysiol.1968.sp008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E. The site of visual adaptation. Science. 1967 Jan 20;155(3760):273–279. doi: 10.1126/science.155.3760.273. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., GUNKEL R. D., RUSHTON W. A. Increment thresholds in a subject deficient in cone vision. J Physiol. 1961 Apr;156:179–192. doi: 10.1113/jphysiol.1961.sp006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Dowling J. E. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973 Jun 15;180(4091):1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Gold G. H., Dowling J. E. Receptor coupling in the toad retina. Cold Spring Harb Symp Quant Biol. 1976;40:547–561. doi: 10.1101/sqb.1976.040.01.051. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Interactions of rod and cone signals in the mudpuppy retina. J Physiol. 1975 Nov;252(3):735–769. doi: 10.1113/jphysiol.1975.sp011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski S. R., Pak W. L. Intracellular recordings of rod responses during dark-adaptation. J Physiol. 1975 May;247(2):363–391. doi: 10.1113/jphysiol.1975.sp010936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R., Riddell L. A. The electrical responses of light- and dark-adapted frogs' eyes to rhythmic and continuous stimuli. J Physiol. 1934 Mar 29;81(1):1–28. doi: 10.1113/jphysiol.1934.sp003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Naka K. I. Identification of intracellular responses in the frog retina. Brain Res. 1972 Jul 13;42(1):59–71. doi: 10.1016/0006-8993(72)90042-x. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON S. E. AN ELECTRON MICROSCOPIC CLASSIFICATION OF THE RETINAL RECEPTORS OF THE LEOPARD FROG (RANA PIPIENS). J Ultrastruct Res. 1964 Jun;10:390–416. doi: 10.1016/s0022-5320(64)80018-6. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C. THE FINE STRUCTURE OF THE ALBINO RABBIT IRIS WITH SPECIAL REFERENCE TO THE IDENTIFICATION OF ADRENERGIC AND CHOLINERGIC NERVES AND NERVE ENDINGS IN ITS INTRINSIC MUSCLES. Am J Anat. 1964 Mar;114:173–205. doi: 10.1002/aja.1001140202. [DOI] [PubMed] [Google Scholar]
- Raviola E., Gilula N. B. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter T. The synthesis of photosensitive pigments in the rods of the frog's retina. Vision Res. 1966 Feb;6(1):15–38. doi: 10.1016/0042-6989(66)90011-3. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Cones excite rods in the retina of the turtle. J Physiol. 1975 Apr;246(3):639–651. doi: 10.1113/jphysiol.1975.sp010908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974 Jan;236(1):211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Incremental responses to light recorded from pigment epithelial cells and horizontal cells of the cat retina. J Physiol. 1971 Aug;217(1):93–110. doi: 10.1113/jphysiol.1971.sp009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Rod and cone contributions to S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1319–1329. doi: 10.1016/0042-6989(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K., SMITH P. H. Iodopsin. J Gen Physiol. 1955 May 20;38(5):623–681. doi: 10.1085/jgp.38.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dudek F. E., Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol. 1975 Feb;65(2):119–134. doi: 10.1085/jgp.65.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]




