Abstract
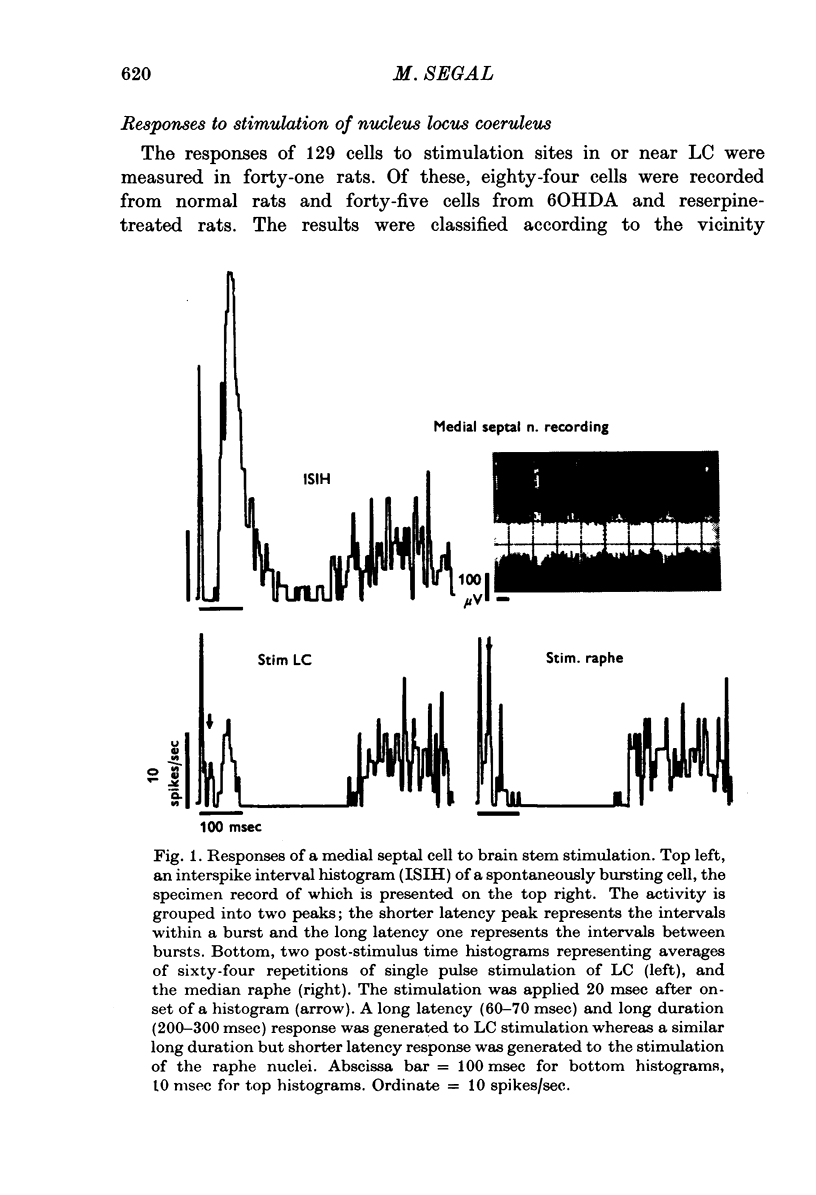
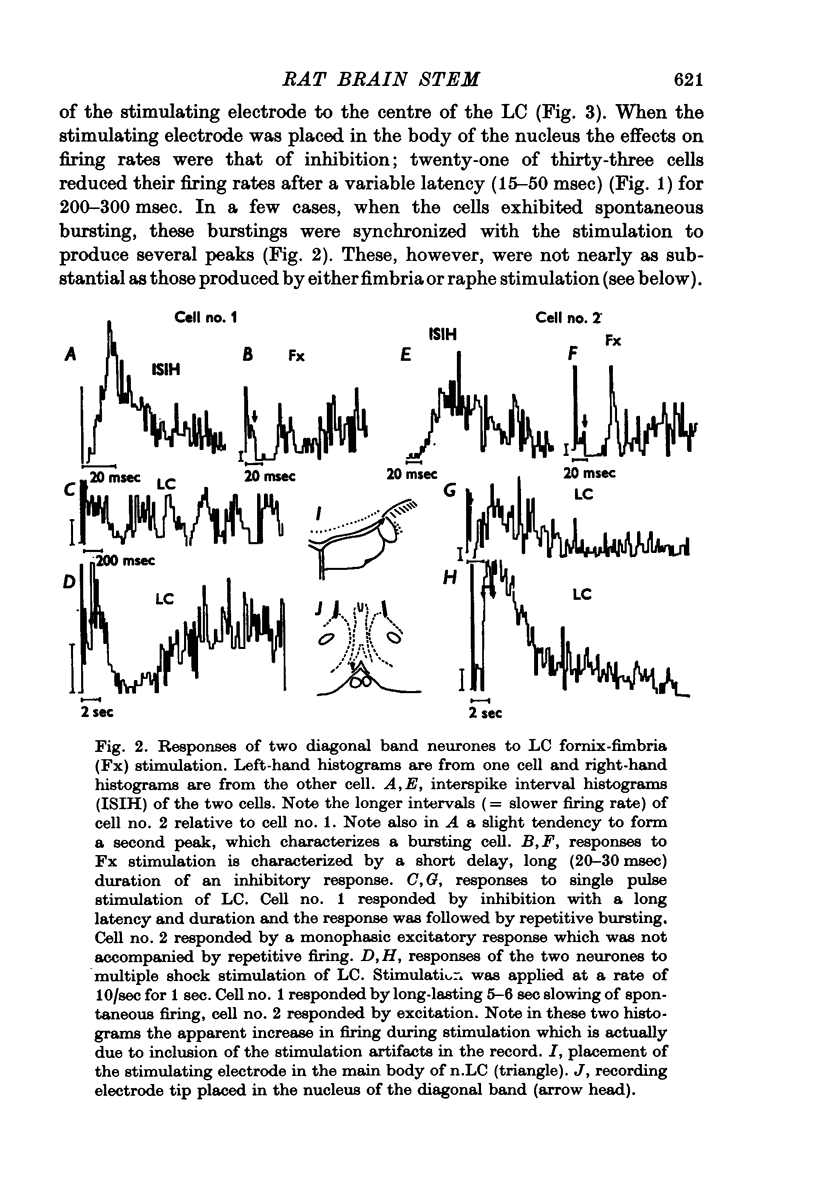
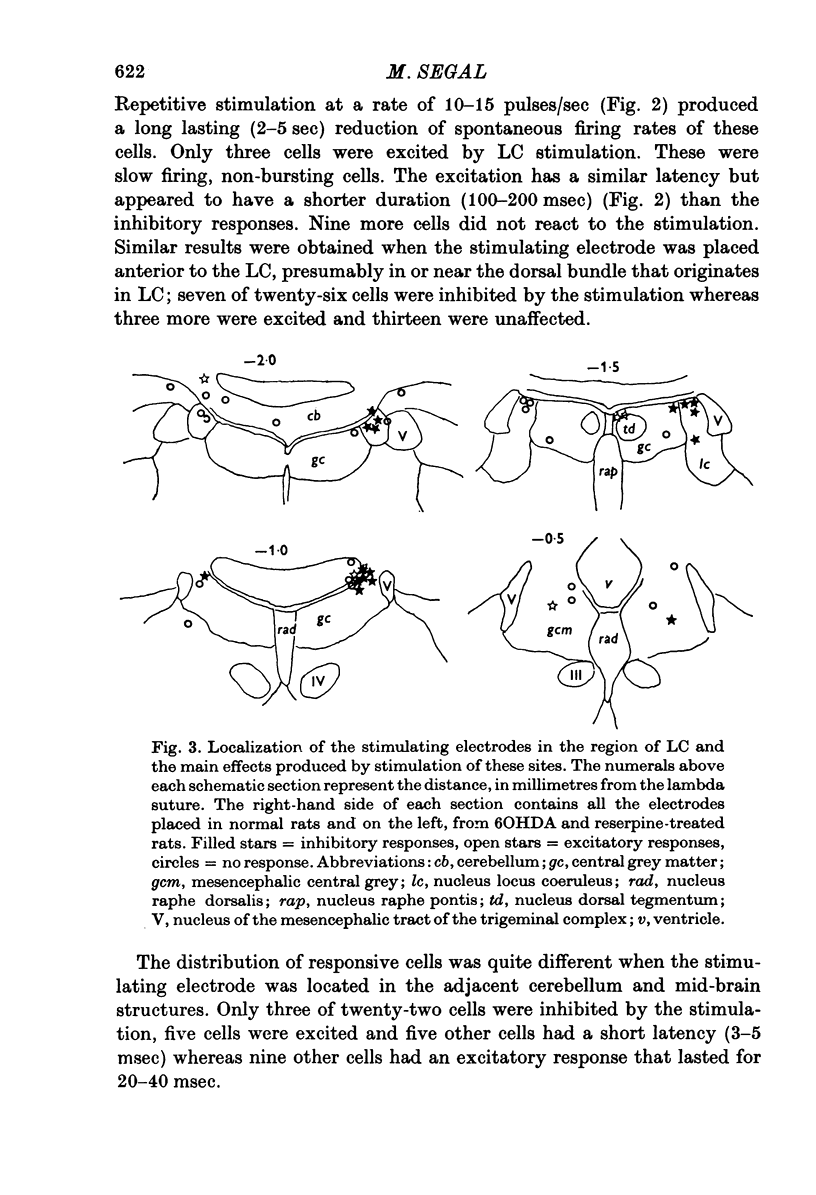
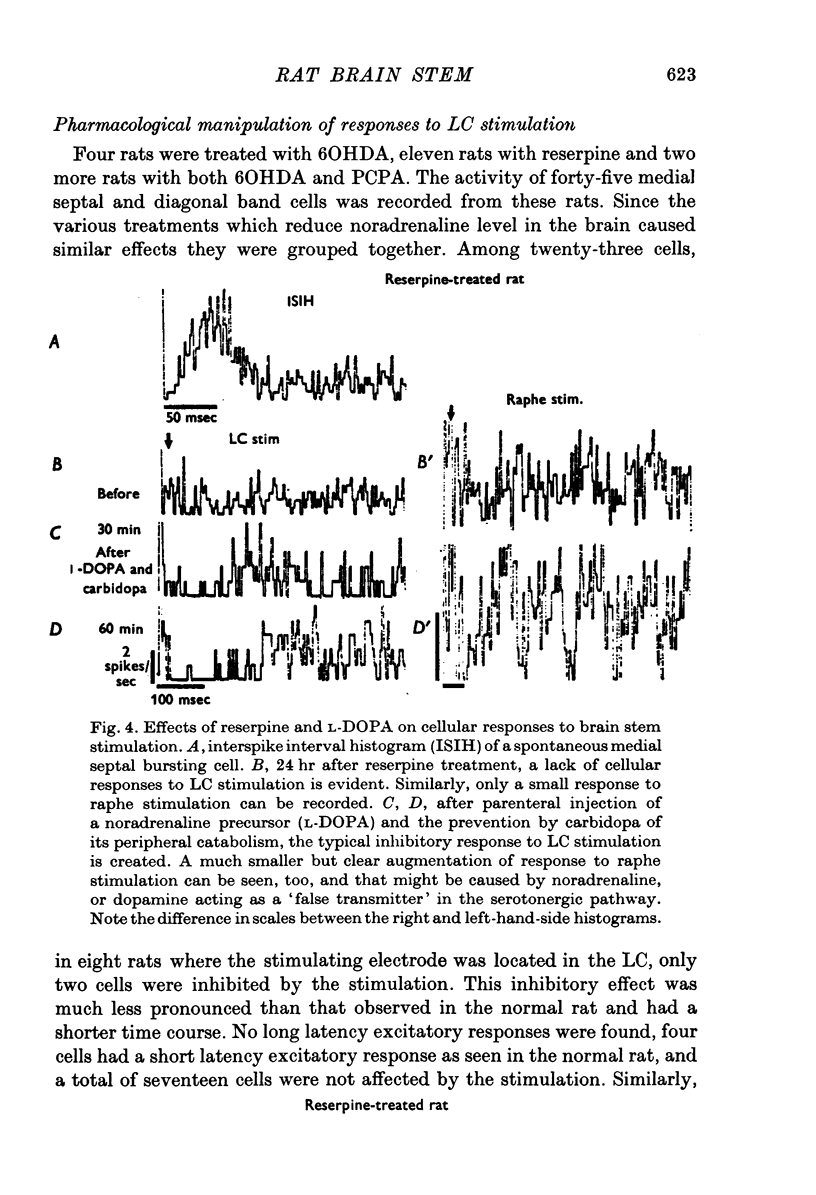
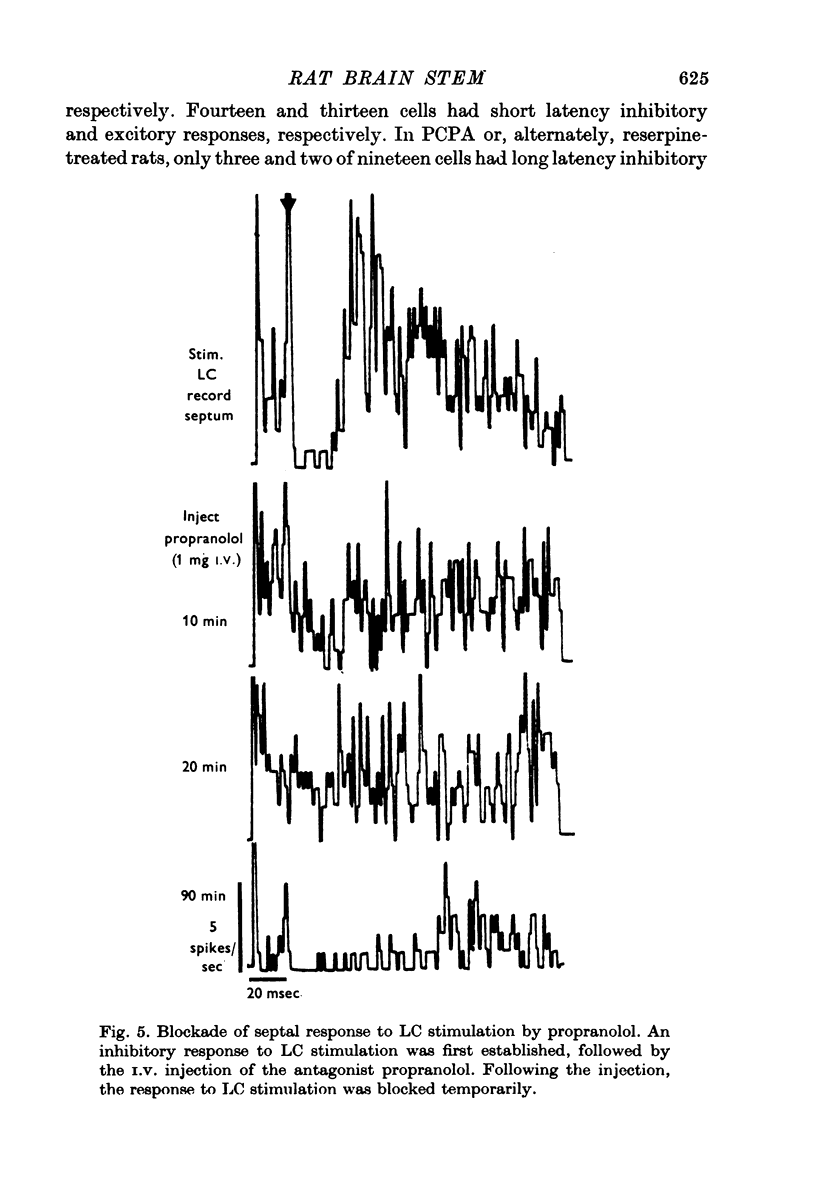
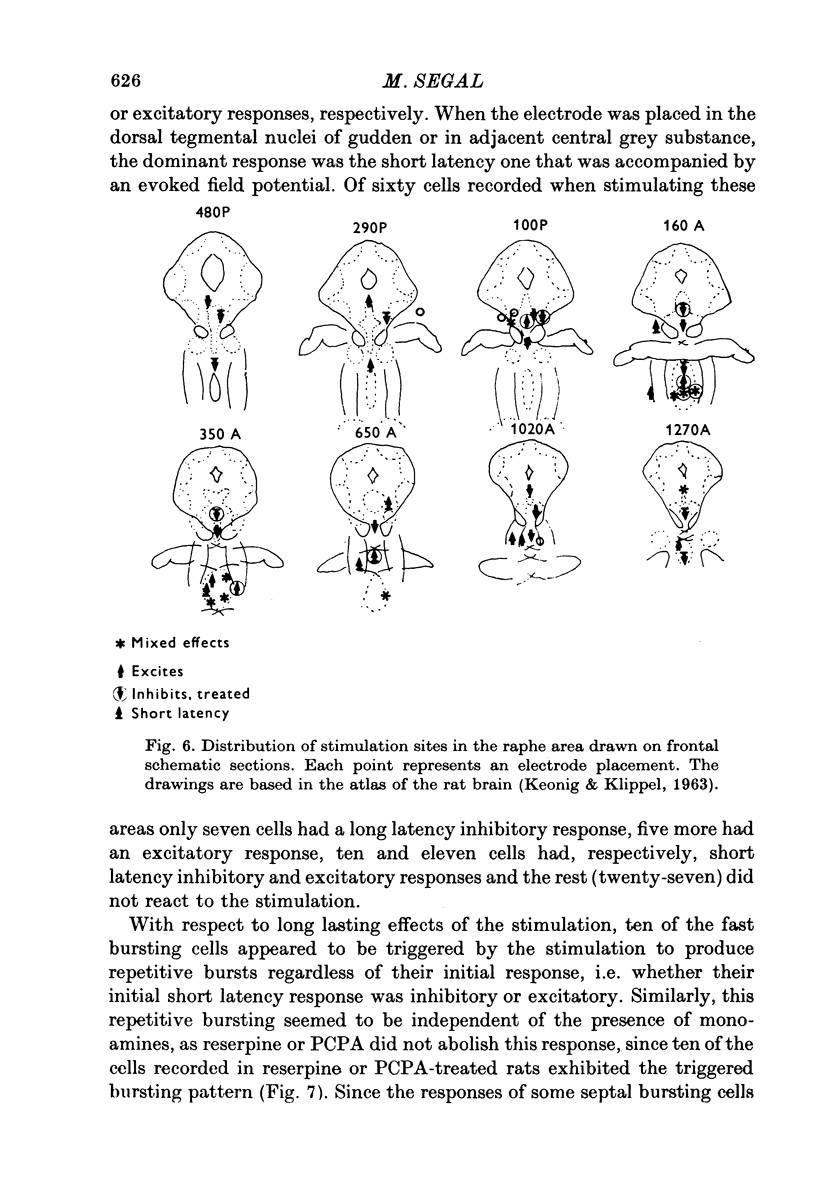
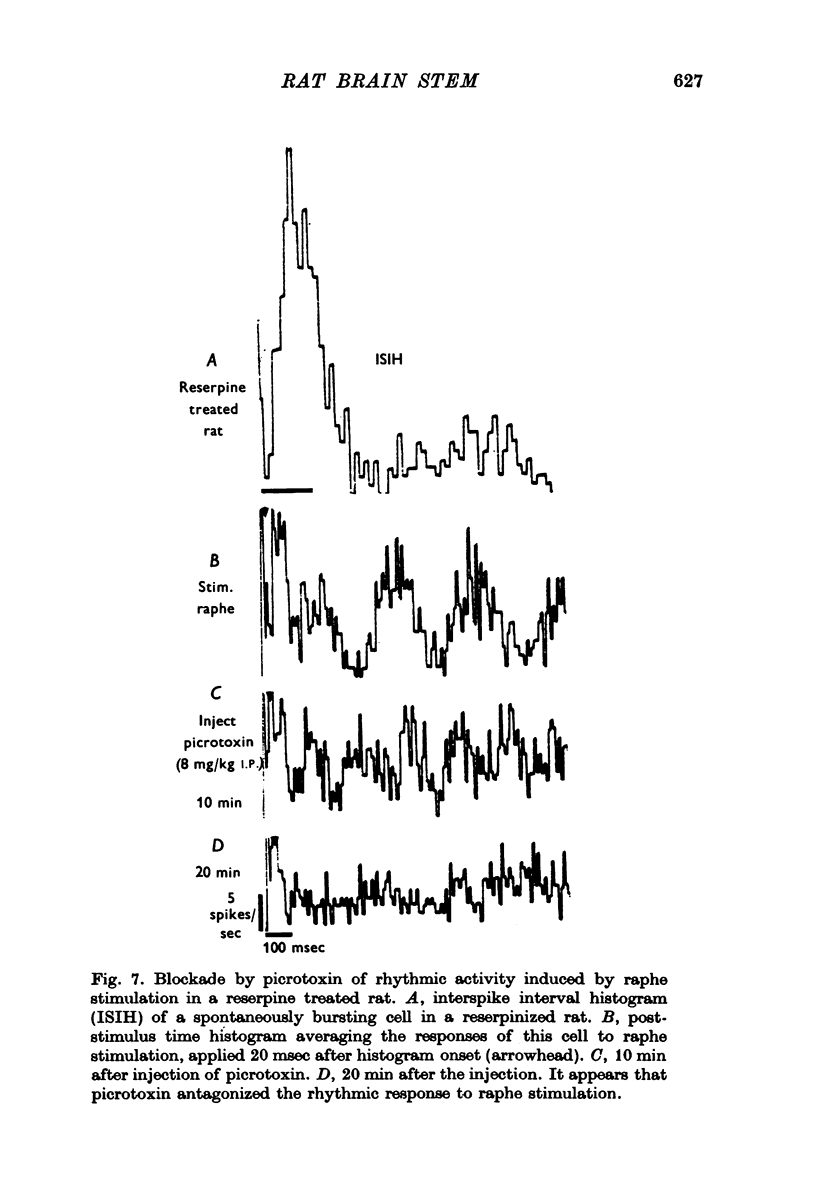
1. Activity of neurones in the medial septal nucleus and the diagonal band was recorded from urethane anaesthesized rats. Responses of the cells to electrical stimulation of the raphe nuclei and nucleus locus coeruleus (LC) were measured. 2. LC stimulation caused a long latency, 30-100 msec, and long duration, 100-300 msec cessation of spontaneous activity of most recorded neurones. When bursting-type neurones were recorded, the stimulation occasionally caused a synchronized repetitive bursting firing pattern. 3. Pre-treatment with drugs which interfere with catecholamine neurotransmission, i.e. reserpine and 6OHDA, prevented the appearance of cellular responses to LC stimulation. 4. Stimulation of the dorsal or the median raphe nuclei generated more complex and less clear-cut responses. These included several types of long (20-50 msec) and short (2-5 msec) latency responses. These responses were also accompanied in some cells by synchronized repetitive bursting. 5. Interference with serotonin neurotransmission with PPCA or reserpine reduced the detection of long latency responses. 6. Short latency responses accompanied by evoked field potentials were recorded also after stimulation of dorsal tegmental nucleus. 7. Rates of spontaneous firing cells were augmented after monoamine neurotransmission interruption whereas after fornix lesion, when there is supposedly an increased monoamine innervation of the septum, cells fire at lower rates than normal. 8. It is suggested that noradrenaline and serotonin may serve as neurotransmitters in the medial-septum-diagonal band areas.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownstein M., Saavedra J. M., Palkovits M. Norepinephrine and dopamine in the limbic system of the rat. Brain Res. 1974 Oct 25;79(3):431–436. doi: 10.1016/0006-8993(74)90440-5. [DOI] [PubMed] [Google Scholar]
- Conrad L. C., Leonard C. M., Pfaff D. W. Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1974 Jul;156(2):179–205. doi: 10.1002/cne.901560205. [DOI] [PubMed] [Google Scholar]
- DeFrance J. F., Kitai S. T., Shimono T. Electrophysiological analysis of the hippocampal-septal projections. I. Response and topographical characteristics. Exp Brain Res. 1973 Jul 30;17(5):447–462. doi: 10.1007/BF00234861. [DOI] [PubMed] [Google Scholar]
- Divac I. Magnocellular nuclei of the basal forebrain project to neocortex, brain stem, and olfactory bulb. Review of some functional correlates. Brain Res. 1975 Aug 15;93(3):385–398. doi: 10.1016/0006-8993(75)90178-x. [DOI] [PubMed] [Google Scholar]
- Herz A., Gogolák G. Mikroelektrophoretische Untersuchungen am Septum des Kaninchens. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Sep 15;285(4):317–330. [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain. 1967 Sep;90(3):521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- MOREST D. K. Connexions of the dorsal tegmental nucleus in rat and rabbit. J Anat. 1961 Apr;95:229–246. [PMC free article] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol. 1974 Mar;237(3):607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. Y., Björklund A., Stenevi U. Plastic changes in the adrenergic innervation of the rat septal area in response to denervation. Brain Res. 1971 Oct 8;33(1):13–35. doi: 10.1016/0006-8993(71)90303-9. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Iwama K. Antidromic activation of the rat locus coeruleus neurons from hippocampus, cerebral and cerebellar cortices. Brain Res. 1975 Dec 5;99(2):372–376. doi: 10.1016/0006-8993(75)90039-6. [DOI] [PubMed] [Google Scholar]
- PETSCHE H., GOGOLAK G., VANZWIETEN P. A. RHYTHMICITY OF SEPTAL CELL DISCHARGES AT VARIOUS LEVELS OF RETICULAR EXCITATION. Electroencephalogr Clin Neurophysiol. 1965 Jul;19:25–33. doi: 10.1016/0013-4694(65)90004-0. [DOI] [PubMed] [Google Scholar]
- PETSCHE H., STUMPF C., GOGOLAK G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Paul S. M., Heath R. G., Ellison J. P. Histochemical demonstration of a direct pathway from the fastigial nucleus to the septal region. Exp Neurol. 1973 Sep;40(3):798–805. doi: 10.1016/0014-4886(73)90113-1. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Segal M., Bloom F. E. A radioautographic study of the efferent pathways of the nucleus locus coeruleus. J Comp Neurol. 1974 May 1;155(1):15–42. doi: 10.1002/cne.901550103. [DOI] [PubMed] [Google Scholar]
- Raisman G. Neuronal plasticity in the septal nuclei of the adult rat. Brain Res. 1969 Jun;14(1):25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- Raisman G. The connexions of the septum. Brain. 1966 Jun;89(2):317–348. doi: 10.1093/brain/89.2.317. [DOI] [PubMed] [Google Scholar]
- Sasa M., Munekiyo K., Ikeda H., Takaori S. Noradrenaline-mediated inhibition by locus coeruleus of spinal trigeminal neurons. Brain Res. 1974 Nov 22;80(3):443–460. doi: 10.1016/0006-8993(74)91029-4. [DOI] [PubMed] [Google Scholar]
- Sasa M., Munekiyo K., Takaori S. Dorsal raphe stimulation produces inhibitory effect on trigeminal nucleus neurons. Brain Res. 1976 Jan 16;101(2):199–207. doi: 10.1016/0006-8993(76)90263-8. [DOI] [PubMed] [Google Scholar]
- Segal M., Bloom F. E. The action of norepinephrine in the rat hippocampus. II. Activation of the input pathway. Brain Res. 1974 May 31;72(1):99–114. doi: 10.1016/0006-8993(74)90653-2. [DOI] [PubMed] [Google Scholar]
- Segal M., Landis S. C. Afferents to the septal area of the rat studied with the method of retrograde axonal transport of horseradish peroxidase. Brain Res. 1974 Dec 27;82(2):263–268. doi: 10.1016/0006-8993(74)90603-9. [DOI] [PubMed] [Google Scholar]
- Segal M. Physiological and pharmacological evidence for a serotonergic projection to the hippocampus. Brain Res. 1975 Aug 22;94(1):115–131. doi: 10.1016/0006-8993(75)90881-1. [DOI] [PubMed] [Google Scholar]
- Segal M. Responses of septal nuclei neurons to microiontophoretically administered putative neurotransmitters. Life Sci. 1974 Apr 1;14(7):1345–1351. doi: 10.1016/0024-3205(74)90443-3. [DOI] [PubMed] [Google Scholar]
- Seiger A., Olson L. Late prenatal ontogeny of central monoamine neurons in the rat: Fluorescence histochemical observations. Z Anat Entwicklungsgesch. 1973 Aug 30;140(3):281–318. doi: 10.1007/BF00525058. [DOI] [PubMed] [Google Scholar]


