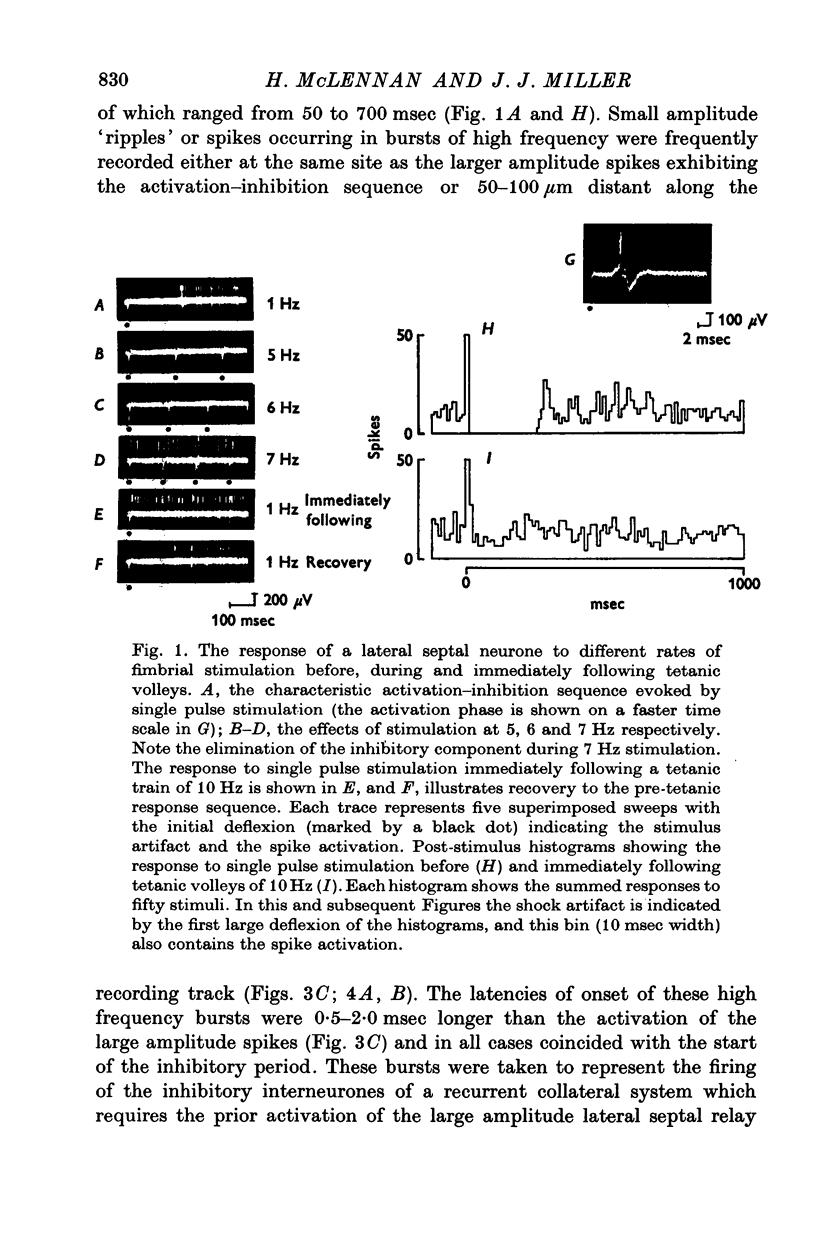
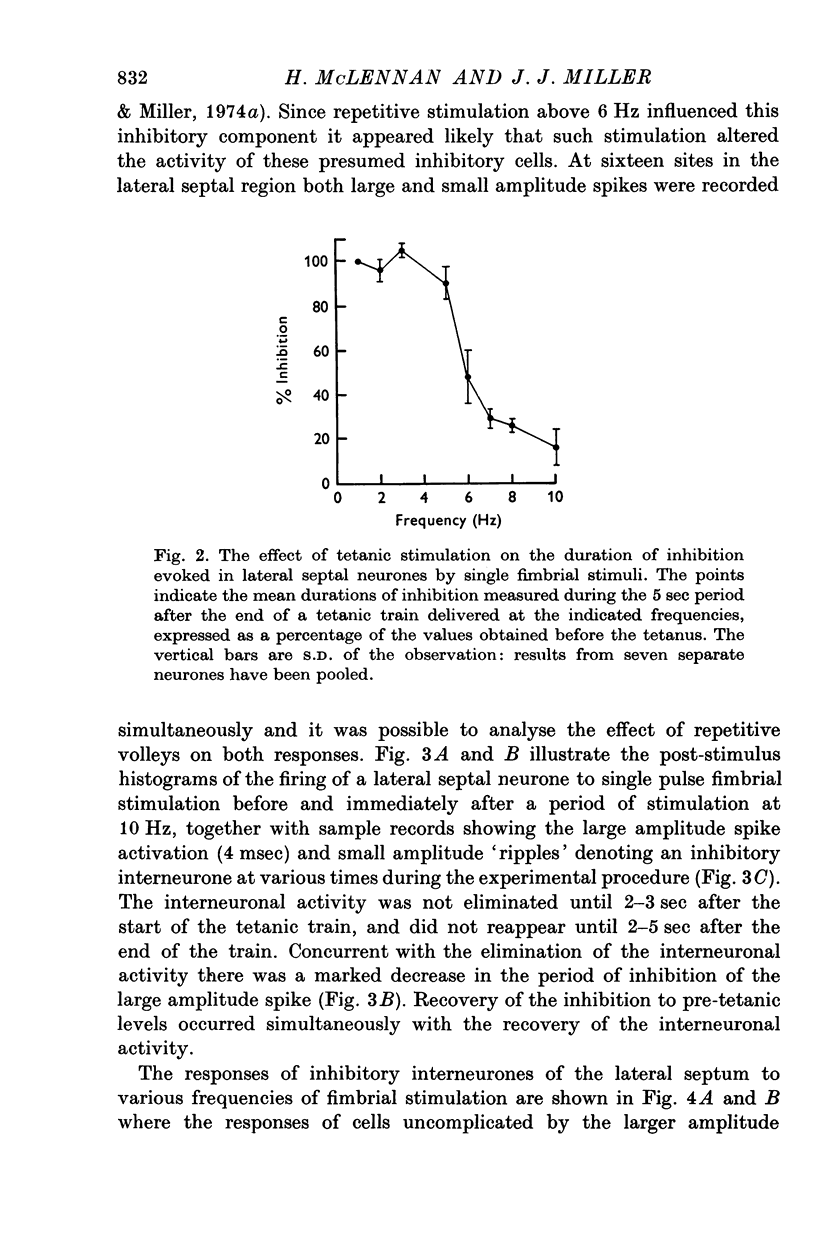
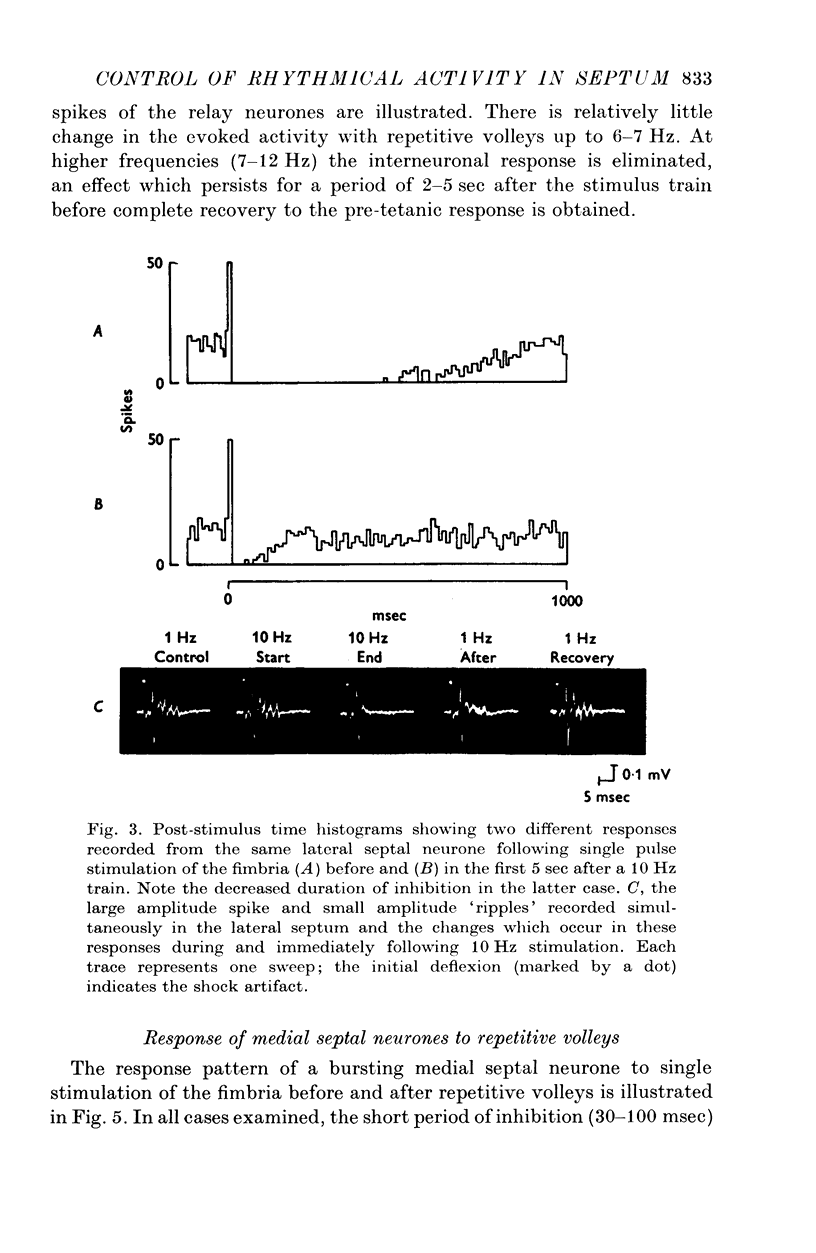
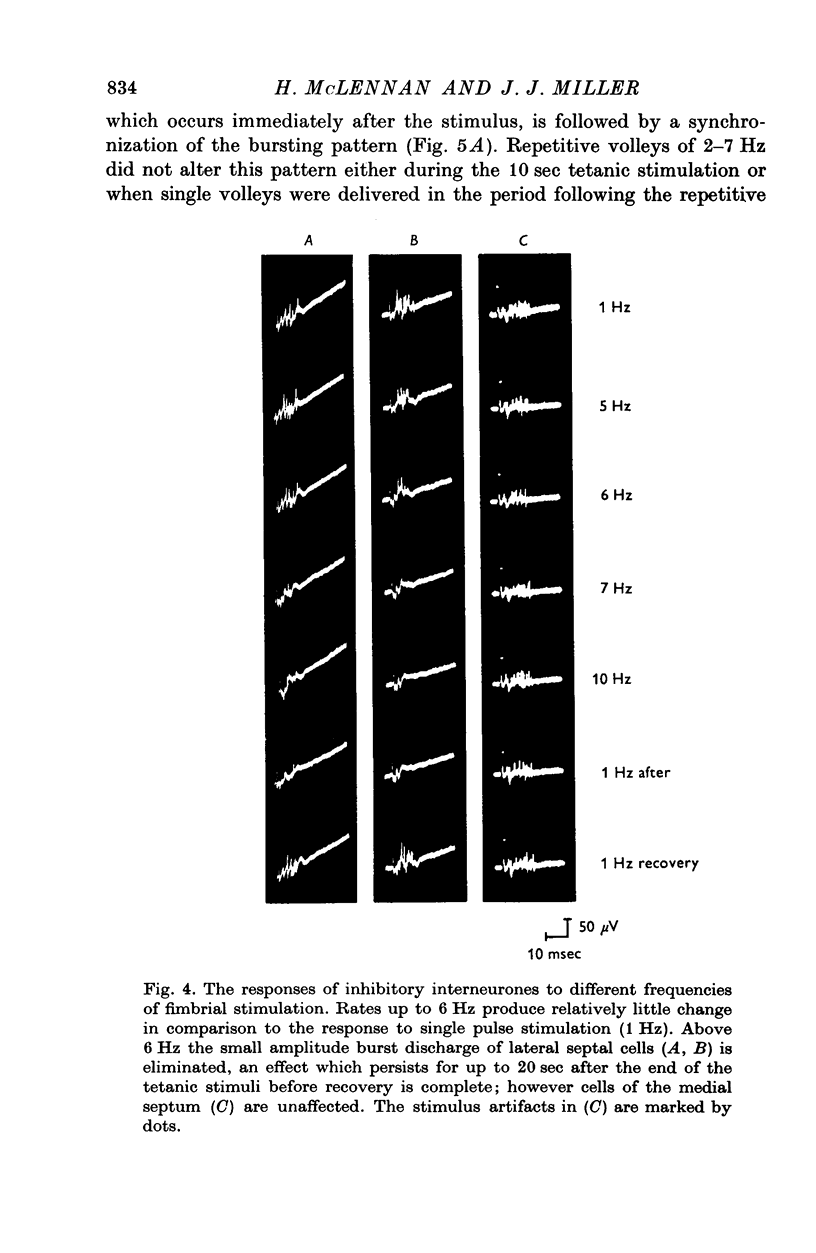
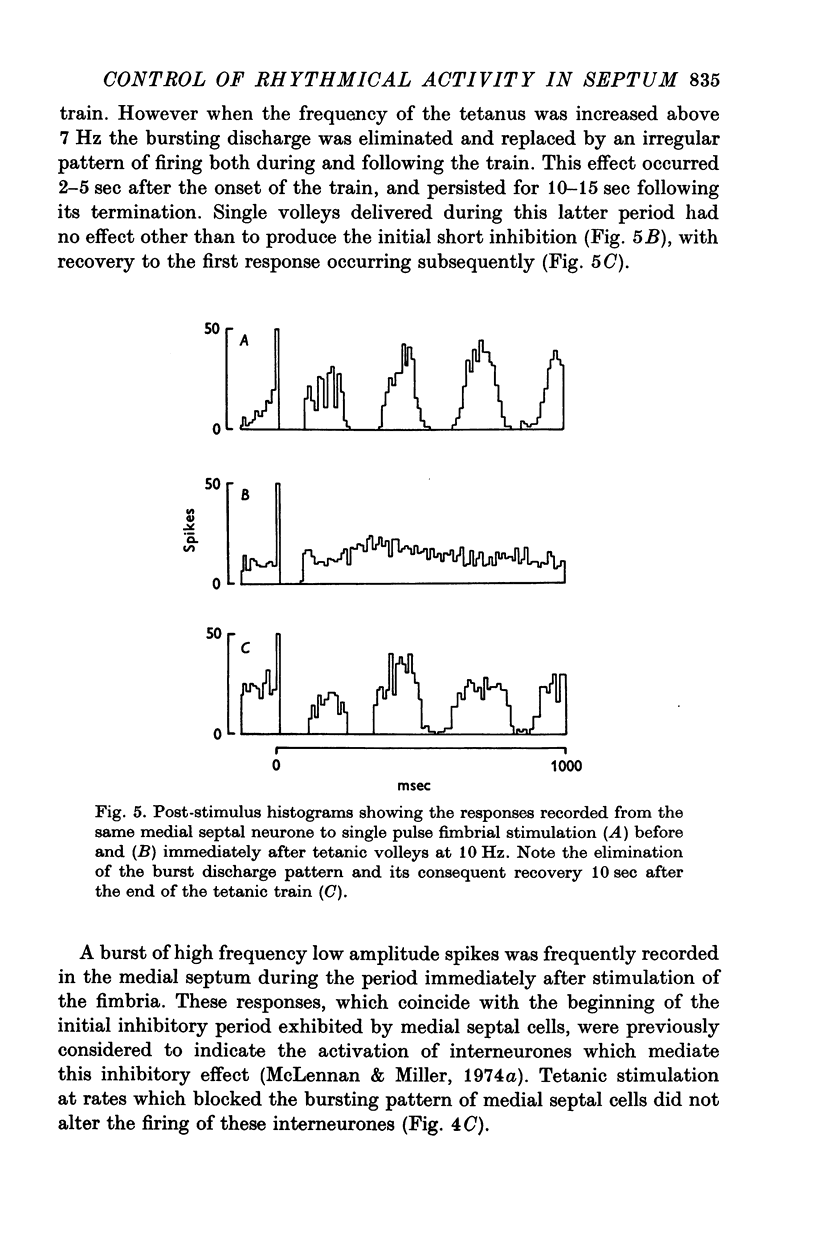
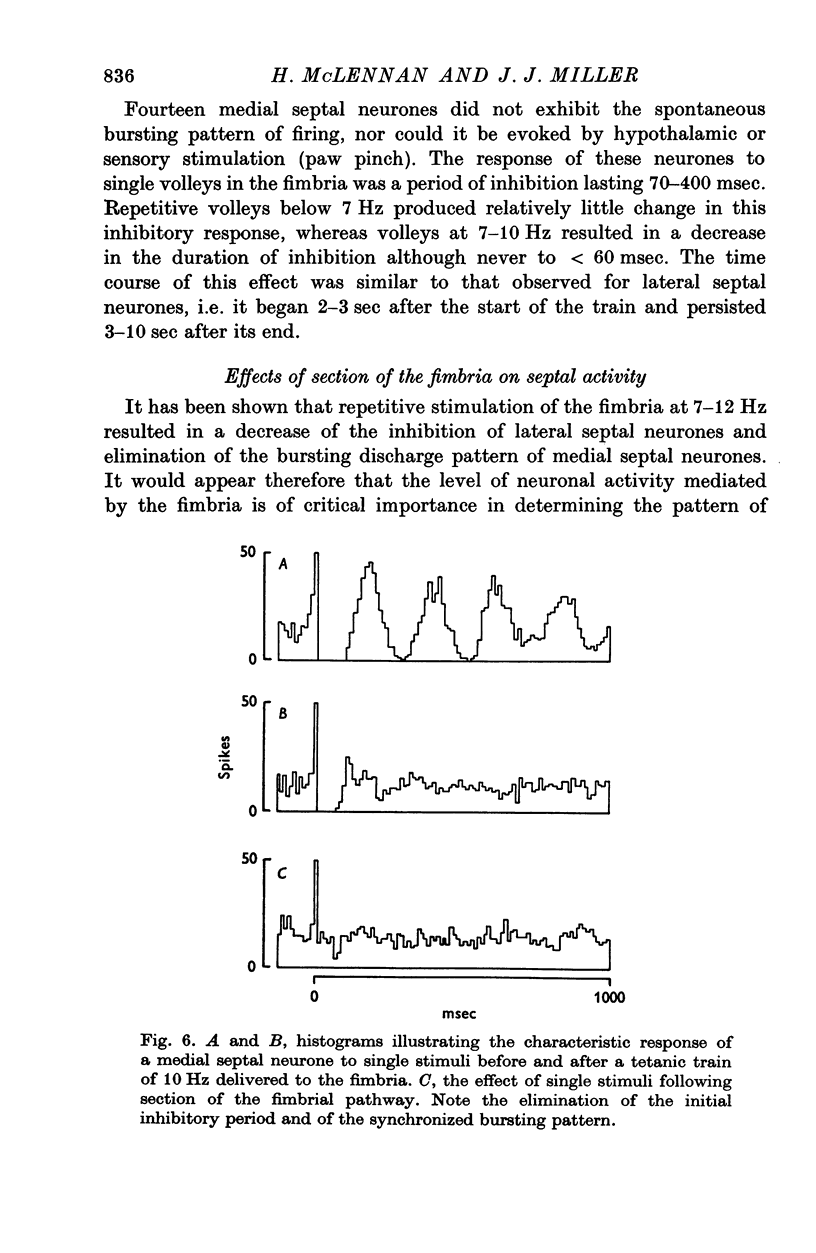
Abstract
1. The response patterns of identified neurones in the medical and lateral septal regions to varying rates of repetitive stimulation of the fimbria were investigated in rats anaesthetized with urethane. 2. Neurones in the lateral septum which characteristically respond to single pulse stimulation of the fimbria with an activation-inhibition sequence, exhibited a reduction or complete elimination of the inhibitory component both during and following tetanic volleys delivered at 7-12 HZ. Stimulation at lower frequencies did not alter the response. 3. Concurrently with this effect on the inhibitory component of the response exhibited by lateral septal cells, repetitive volleys eliminate the small amplitude burst discharges which are correlated with the onset of the inhibitory period and are considered to indicate the firing of inhibitory interneurones. 4. Tetanic stimulation of the fimbria at rates which eliminate this interneuronal response in the lateral septum, produce an irregular pattern of firing in medial septal neurones which previously exhibited a synchronized bursting discharge to single pulses. 5. Ipsilateral section of the fimbrial input to the septum resulted in the elimination of the burst discharge pattern exhibited by medial septal neurones. 6. The results suggest that a frequency gating mechanism in the lateral septum, activation of which is dependent upon the level of hippocampal output, is responsible for controlling the firing pattern of medical septal neurones.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anchel H., Lindsley D. B. Differentiation of two reticulo-hypothalamic systems regulating hippocampal activity. Electroencephalogr Clin Neurophysiol. 1972 Mar;32(3):209–226. doi: 10.1016/0013-4694(72)90171-x. [DOI] [PubMed] [Google Scholar]
- Anderson P., Lomo T. Mode of activation of hippocampal pyramidal cells by excitatory synapses on dendrites. Exp Brain Res. 1966;2(3):247–260. [PubMed] [Google Scholar]
- DeFrance J. F., Kitai S. T., Shimono T. Electrophysiological analysis of the hippocampal septal projections. II. Functional characteristics. Exp Brain Res. 1973 Jul 30;17(5):463–476. doi: 10.1007/BF00234862. [DOI] [PubMed] [Google Scholar]
- Gogolák G., Petsche H., Sterc J., Stumpf C. Septum cell activity in the rabbit under reticular stimulation. Brain Res. 1967 Aug;5(4):508–510. doi: 10.1016/0006-8993(67)90022-4. [DOI] [PubMed] [Google Scholar]
- Gogolák G., Stumpf C., Petsche H., Sterc J. The firing pattern of septal neurons and the form of the hippocampal theta wave. Brain Res. 1968 Feb;7(2):201–207. doi: 10.1016/0006-8993(68)90098-x. [DOI] [PubMed] [Google Scholar]
- Macadar O., Roig J. A., Monti J. M., Budelli R. The functional relationship between septal and hippocampal unit activity and hippocampal theta rhythm. Physiol Behav. 1970 Dec;5(12):1443–1449. doi: 10.1016/0031-9384(70)90134-4. [DOI] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. Gamma-aminobutyric acid and inhibition in the septal nuclei of the rat. J Physiol. 1974 Mar;237(3):625–633. doi: 10.1113/jphysiol.1974.sp010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol. 1974 Mar;237(3):607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETSCHE H., GOGOLAK G., VANZWIETEN P. A. RHYTHMICITY OF SEPTAL CELL DISCHARGES AT VARIOUS LEVELS OF RETICULAR EXCITATION. Electroencephalogr Clin Neurophysiol. 1965 Jul;19:25–33. doi: 10.1016/0013-4694(65)90004-0. [DOI] [PubMed] [Google Scholar]
- PETSCHE H., STUMPF C., GOGOLAK G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Raisman G. The connexions of the septum. Brain. 1966 Jun;89(2):317–348. doi: 10.1093/brain/89.2.317. [DOI] [PubMed] [Google Scholar]
- STUMPF C., PETSCHE H., GOGOLAK G. The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. II. The differential influence of drugs upon both the septal cell firing pattern and the hippocampus theta activity. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:212–219. doi: 10.1016/0013-4694(62)90031-7. [DOI] [PubMed] [Google Scholar]


