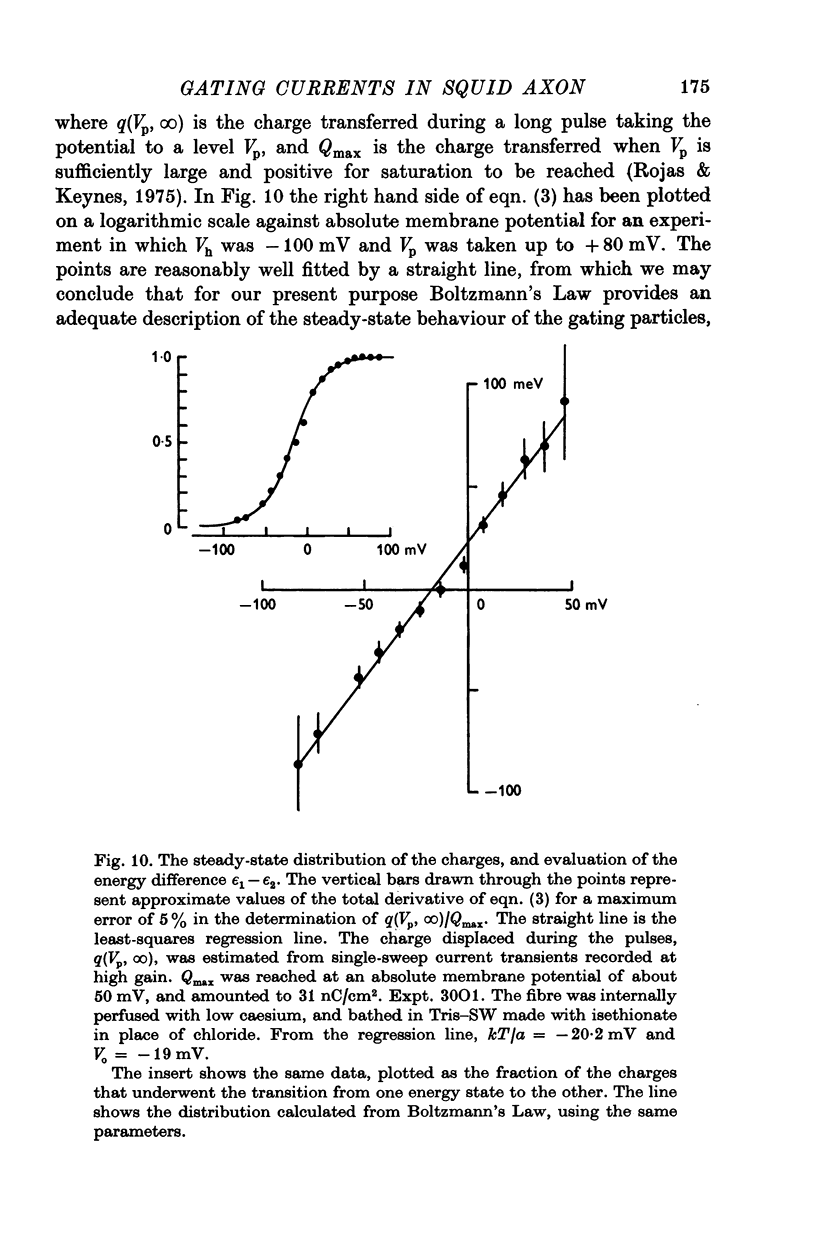
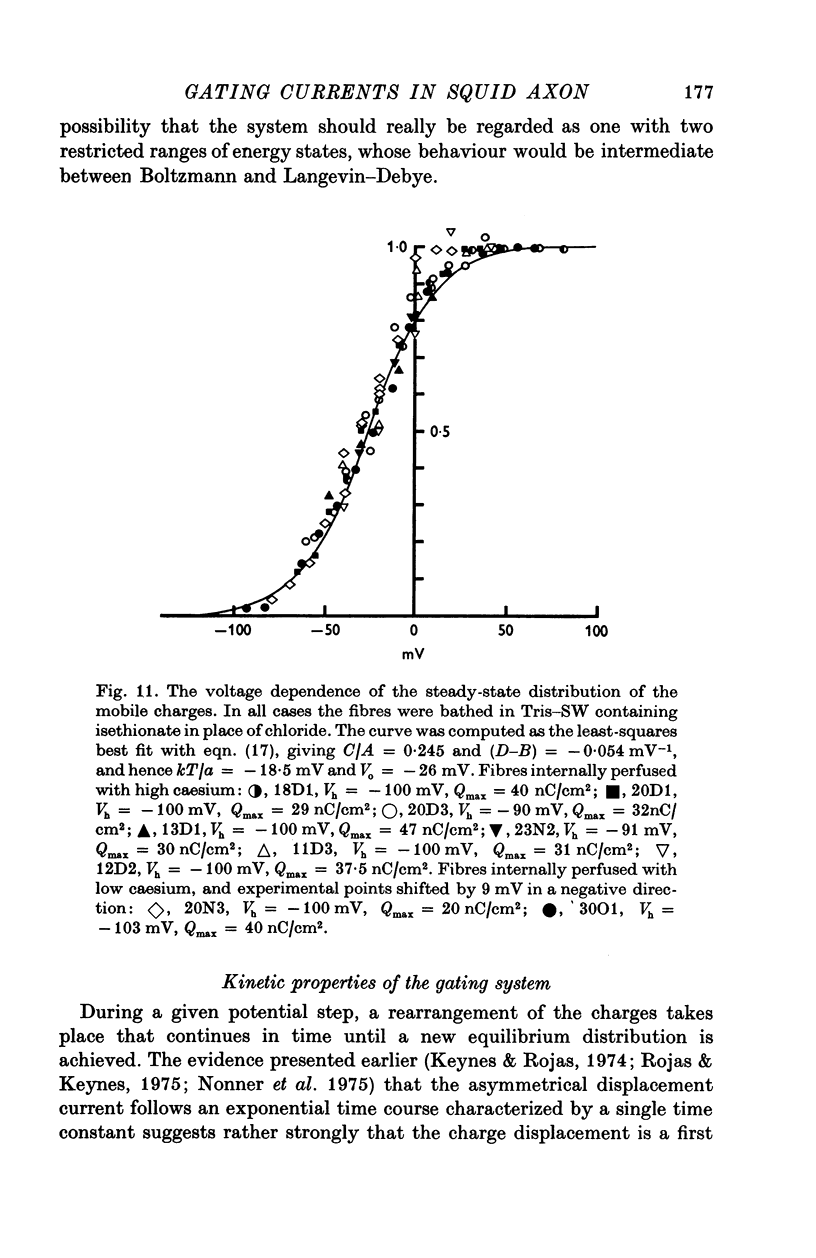
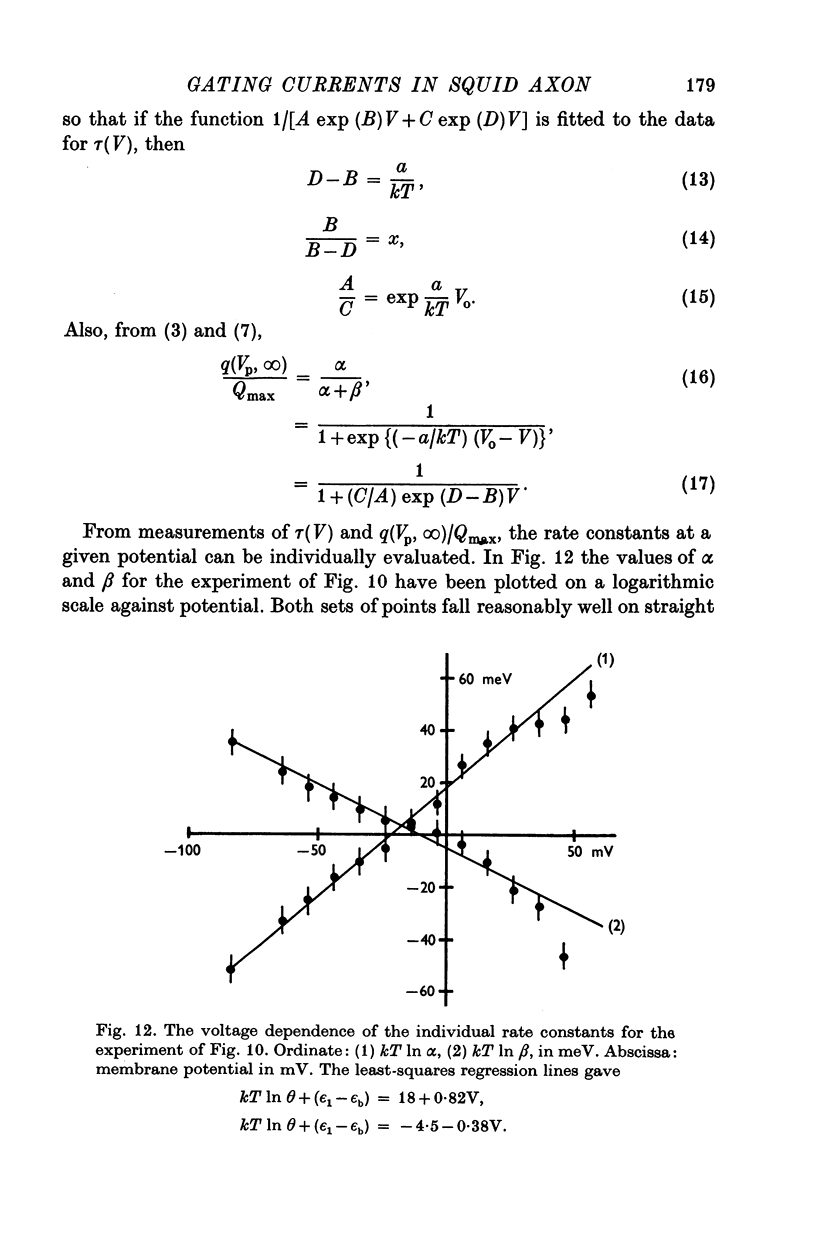
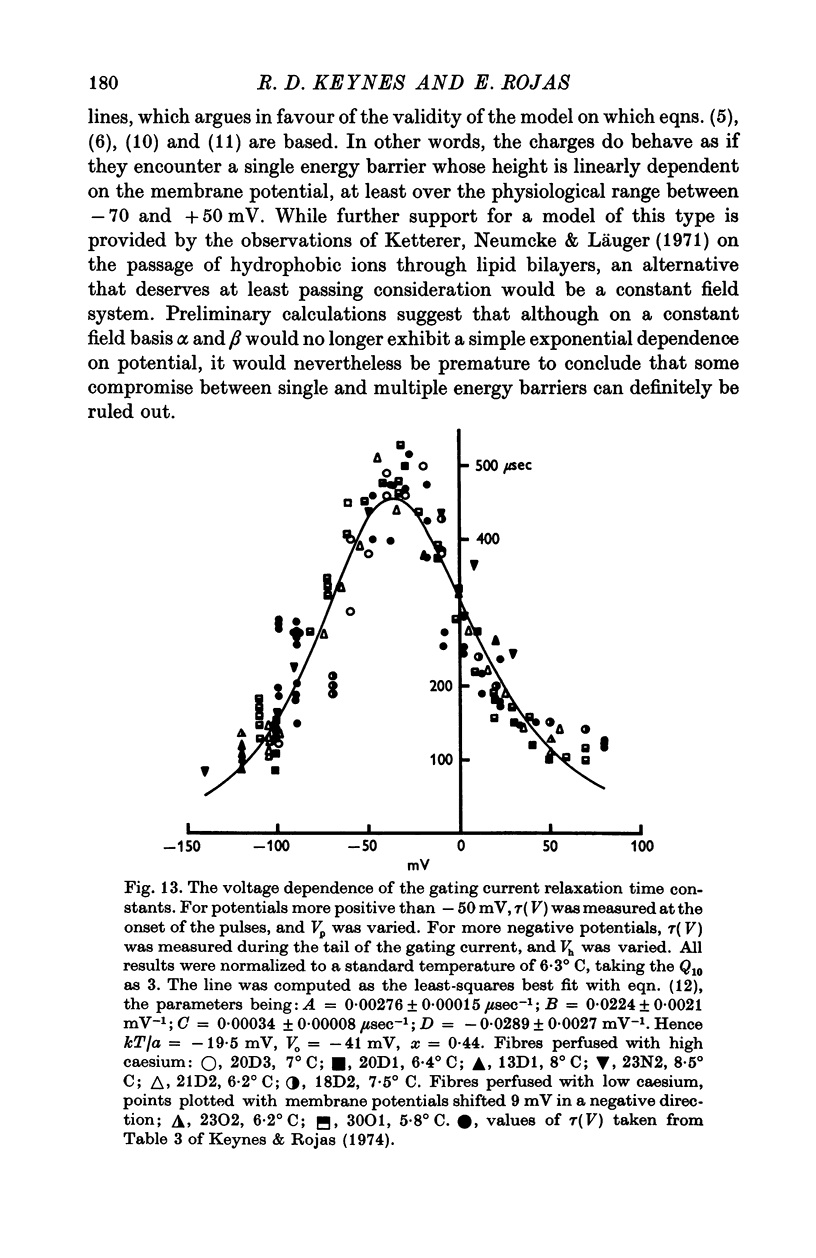
Abstract
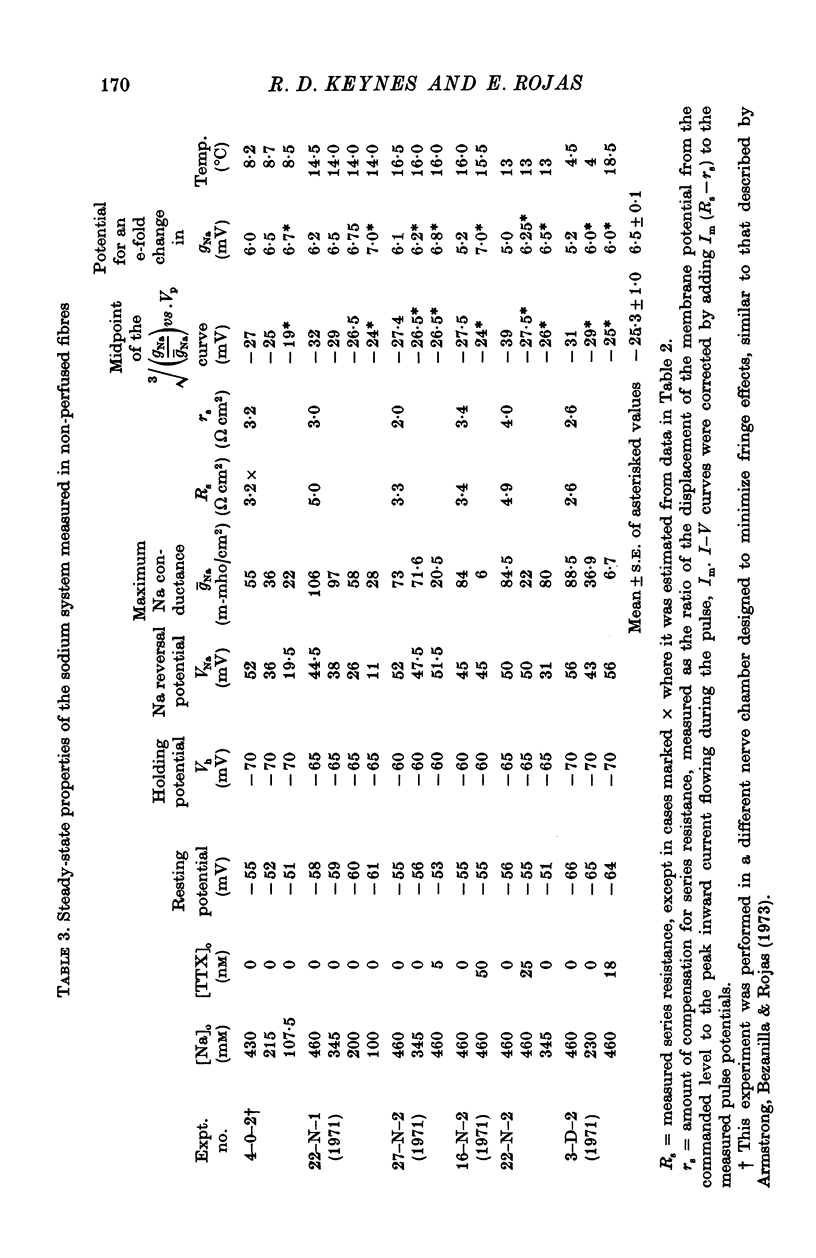
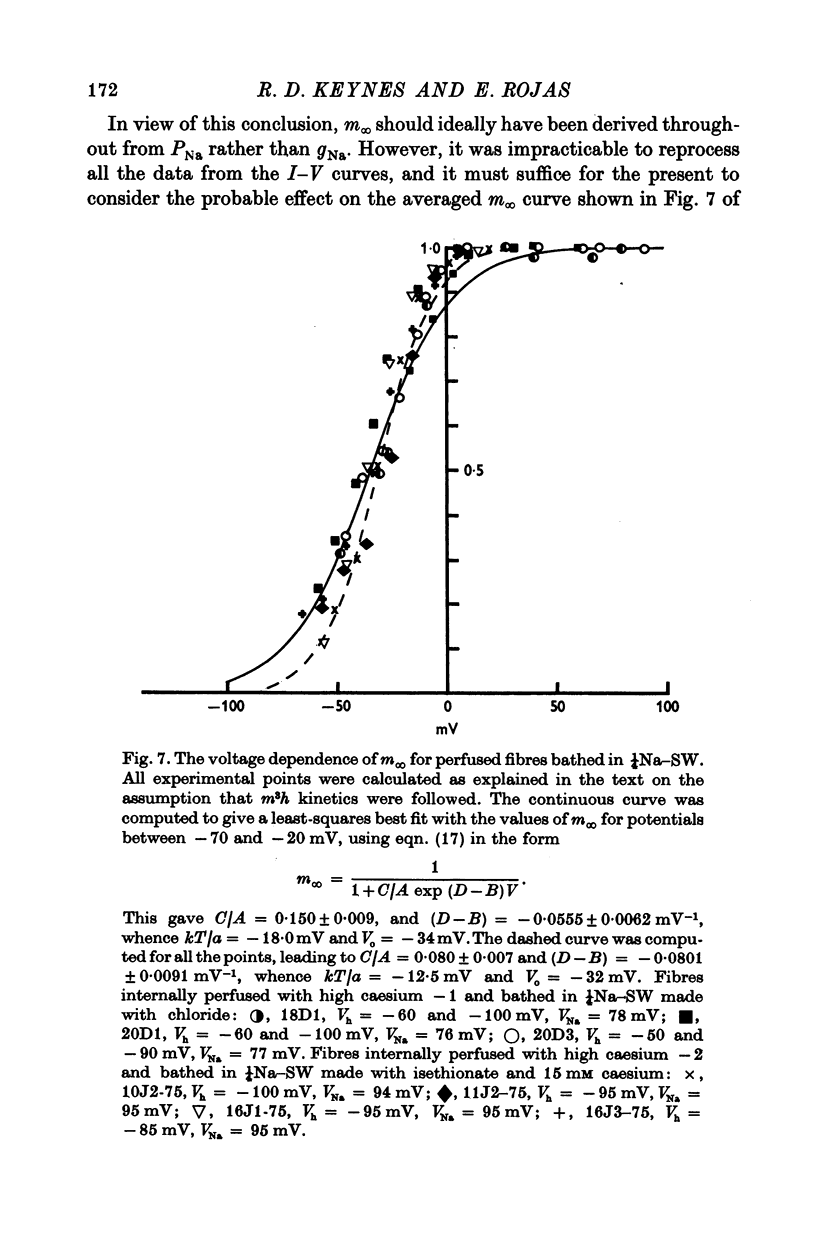
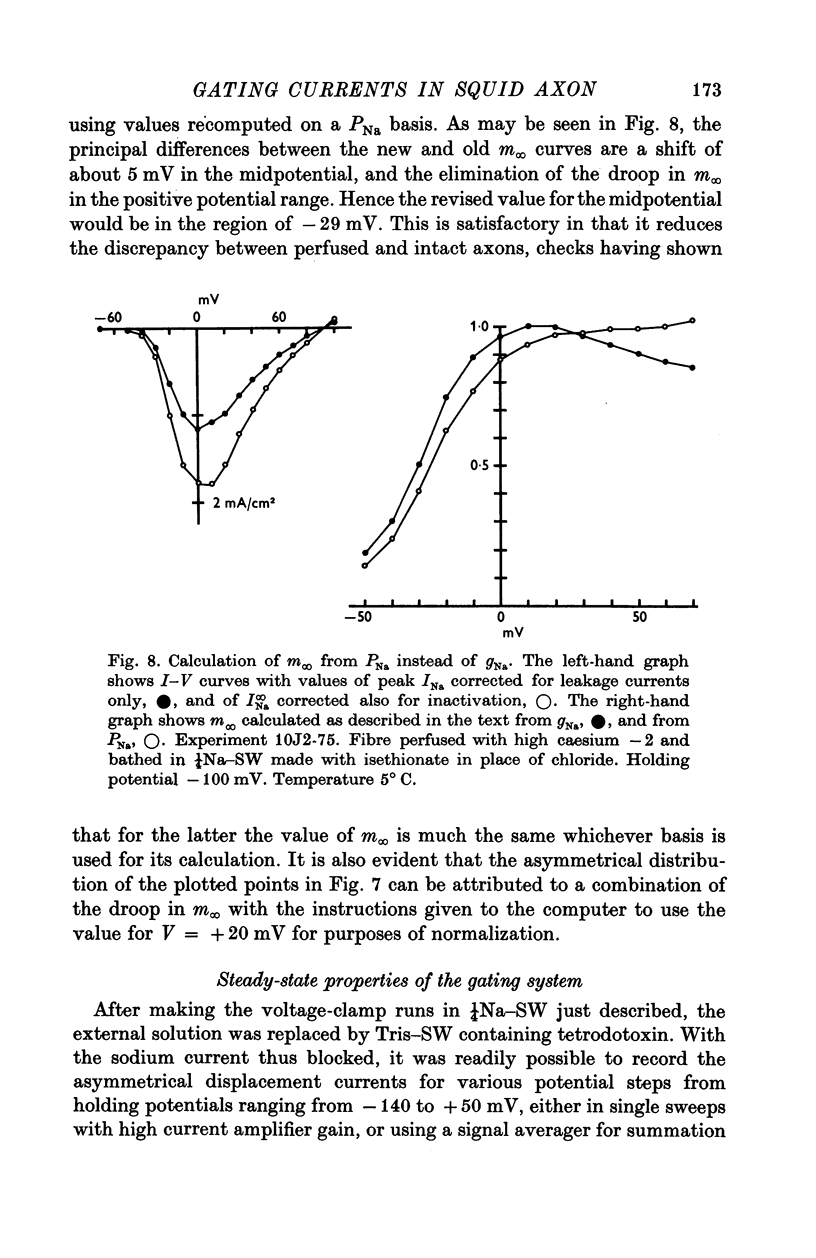
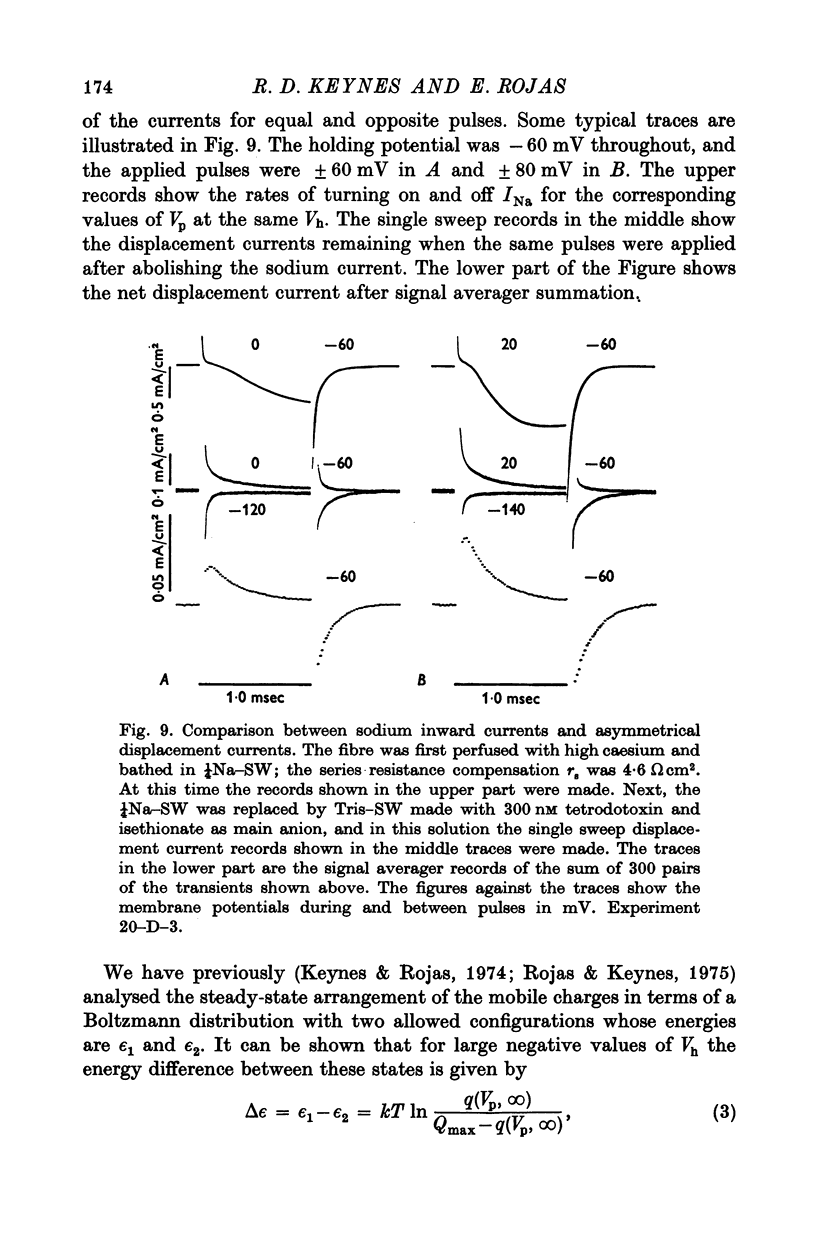
1. Comparisons were made between the kinetics and steady-state properties of the sodium conductance changes and of the sodium gating currents, in the squid giant axon perfused with caesium fluoride and maintained at a high membrane holding potential. The conductance measurements were made with reduced external sodium and as much electrical compensation as possible, in order to minimize errors caused by the series resistance. 2. After an initial delay of 10-150 musec whose size was a function of the holding potential and pulse amplitude, the conductance rise on depolarization followed cube law kinetics. 3. Values of the time constant taum, as defined by Hodgkin & Huxley (1952b), were determined for membrane potentials ranging between -140 and +70 mV. They lay on a nearly symmetrical bell-shaped curve with maximum (at 6-3 degrees C) of just under 500 musec at -36 mV. 4. Values of the gating current time constant tau(V) were determined over the same potential range, and found to lie on a very similar bell-shaped curve. A computed least-squares best fit gave the maximum as 460 musec, also falling at about -36 mV. 5. The midpoint of the minfinity curve lay at -34 mV, and its slope at this point was 0-0139 mV-1. Another series of measurements on intact axons gave a midpotential of -25 mV. In the perfused axons the state of the membrane was better described by the constant field equation than by gNa. Recalculation of minfinity from PNa shifted the curve about 15 mV in a positive direction.
Full text
PDF








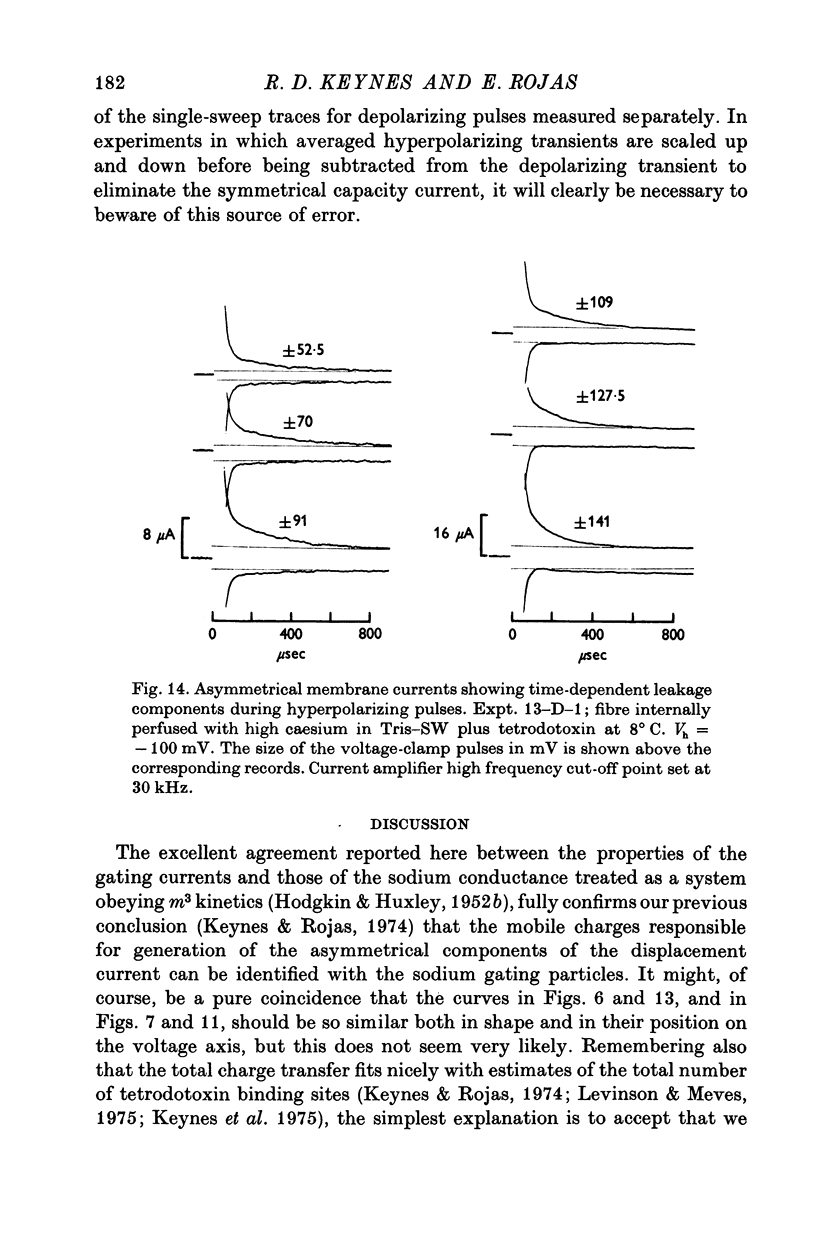


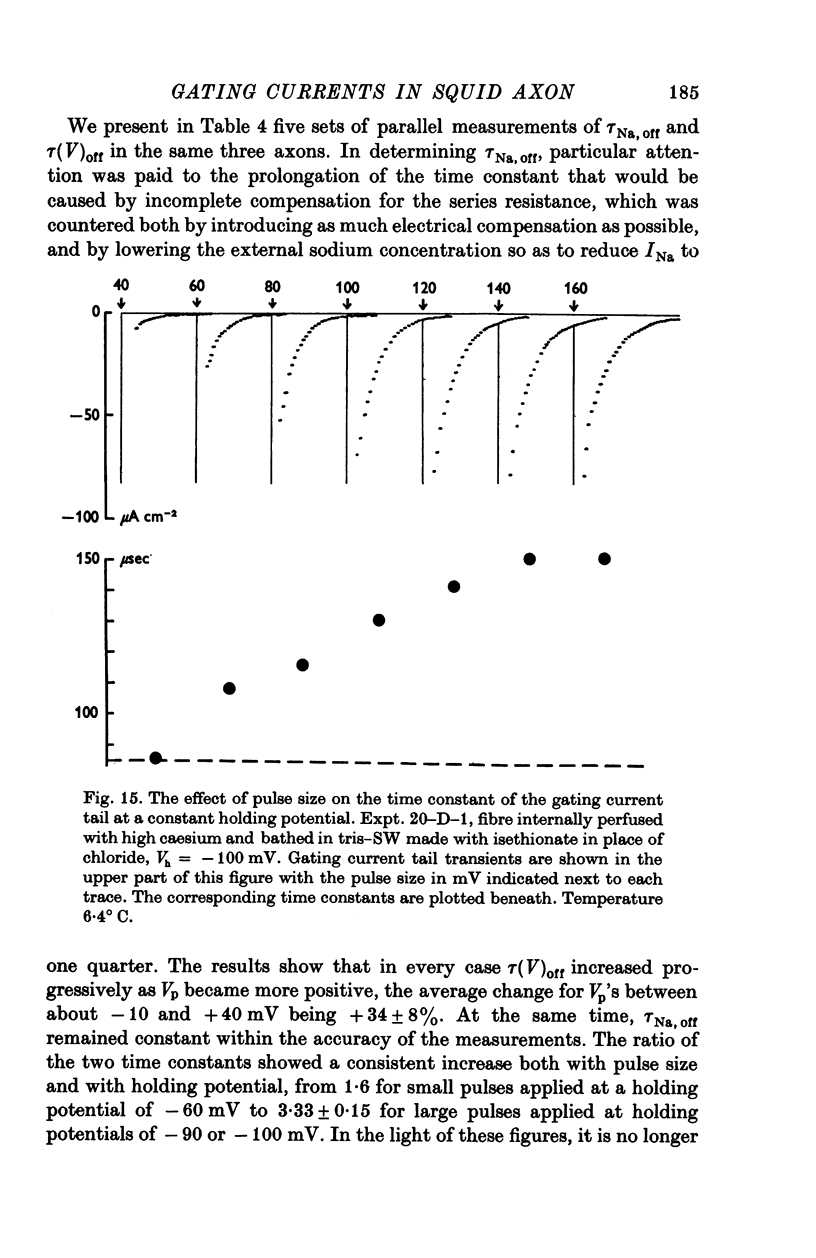
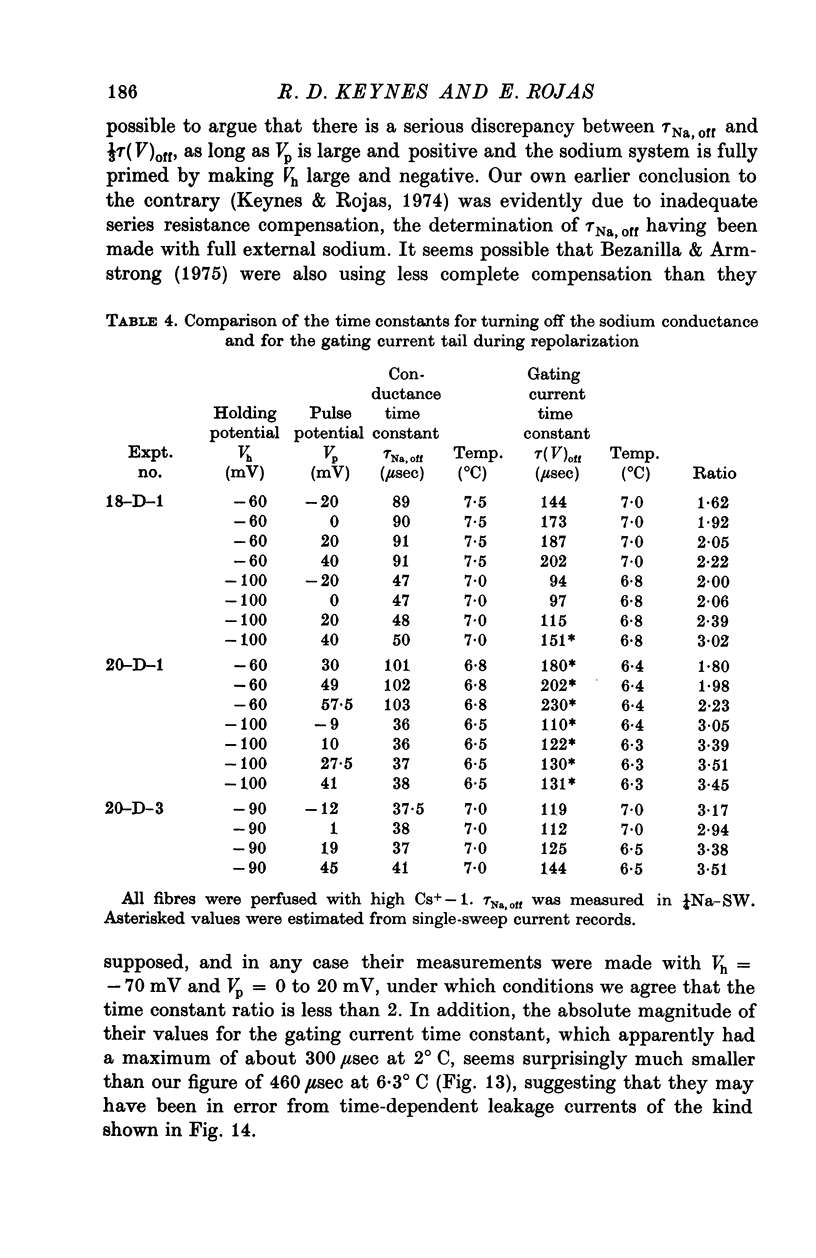



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Kinetic properties and inactivation of the gating currents of sodium channels in squid axon. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):449–458. doi: 10.1098/rstb.1975.0022. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D., Landowne D., Rojas E. Analysis of the potential-dependent changes in optical retardation in the squid giant axon. J Physiol. 1971 Oct;218(1):205–237. doi: 10.1113/jphysiol.1971.sp009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., De Felice L. J., Wanke E. Potassium and sodium ion current noise in the membrane of the squid giant axon. J Physiol. 1975 Jun;248(1):45–82. doi: 10.1113/jphysiol.1975.sp010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Quantitative description of sodium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1960 Jun;151:491–501. doi: 10.1113/jphysiol.1960.sp006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Bezanilla F., Taylor R. E., Rojas E. The rate of action of tetrodotoxin on sodium conductance in the squid giant axon. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):365–375. doi: 10.1098/rstb.1975.0016. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974 Jun;239(2):393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson S. R., Meves H. The binding of tritiated tetrodotoxin to squid giant axons. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):349–352. doi: 10.1098/rstb.1975.0014. [DOI] [PubMed] [Google Scholar]
- Meves H. The effect of holding potential on the asymmetry currents in squid gaint axons. J Physiol. 1974 Dec;243(3):847–867. doi: 10.1113/jphysiol.1974.sp010780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli H. Displacement currents in the node of Ranvier. Voltage and time dependence. Pflugers Arch. 1975;354(1):1–18. doi: 10.1007/BF00584499. [DOI] [PubMed] [Google Scholar]
- Rojas E., Keynes R. D. On the relation between displacement currents and activation of the sodium conductance in the squid giant axon. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):459–482. doi: 10.1098/rstb.1975.0023. [DOI] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E., Atwater I., Bezanilla F. Analysis of the effects of calcium or magnesium on voltage-clamp currents in perfused squid axons bathed in solutions of high potassium. J Gen Physiol. 1969 Oct;54(4):532–552. doi: 10.1085/jgp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]


