Abstract
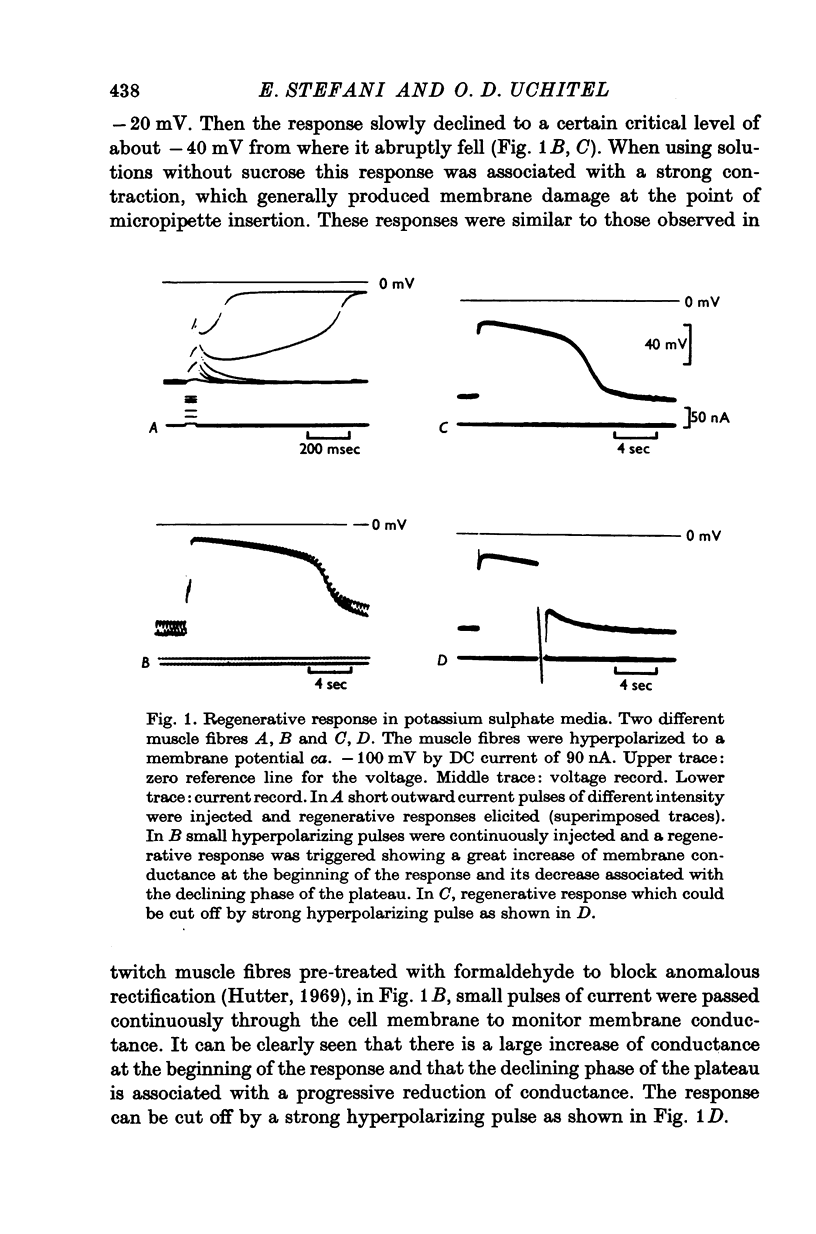
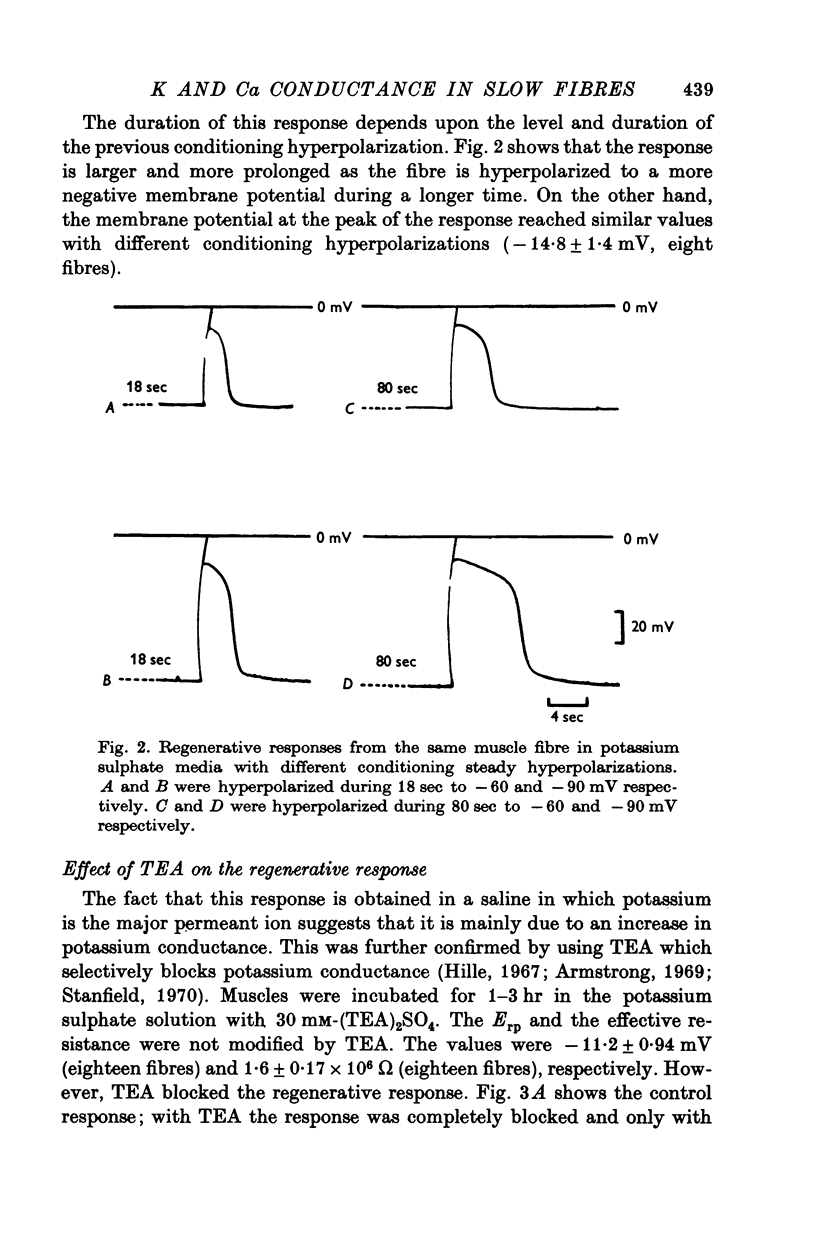
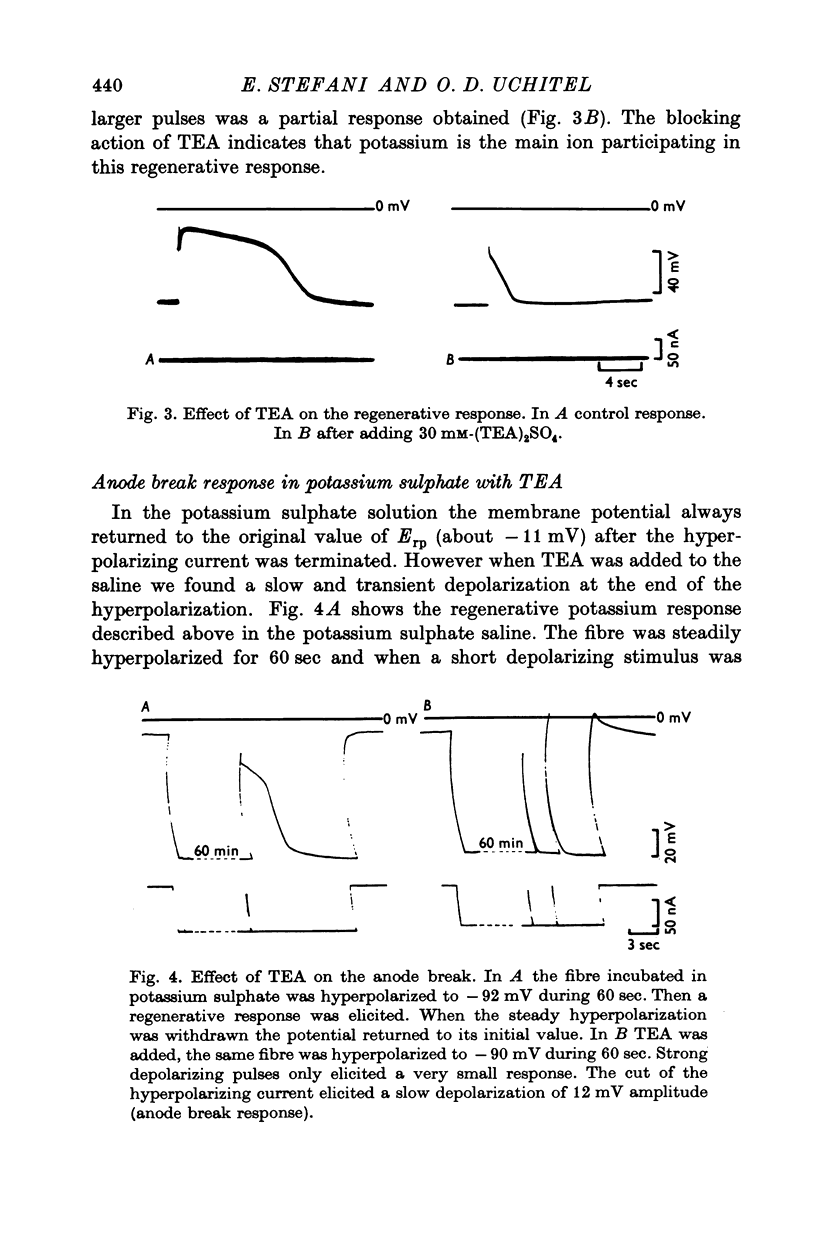
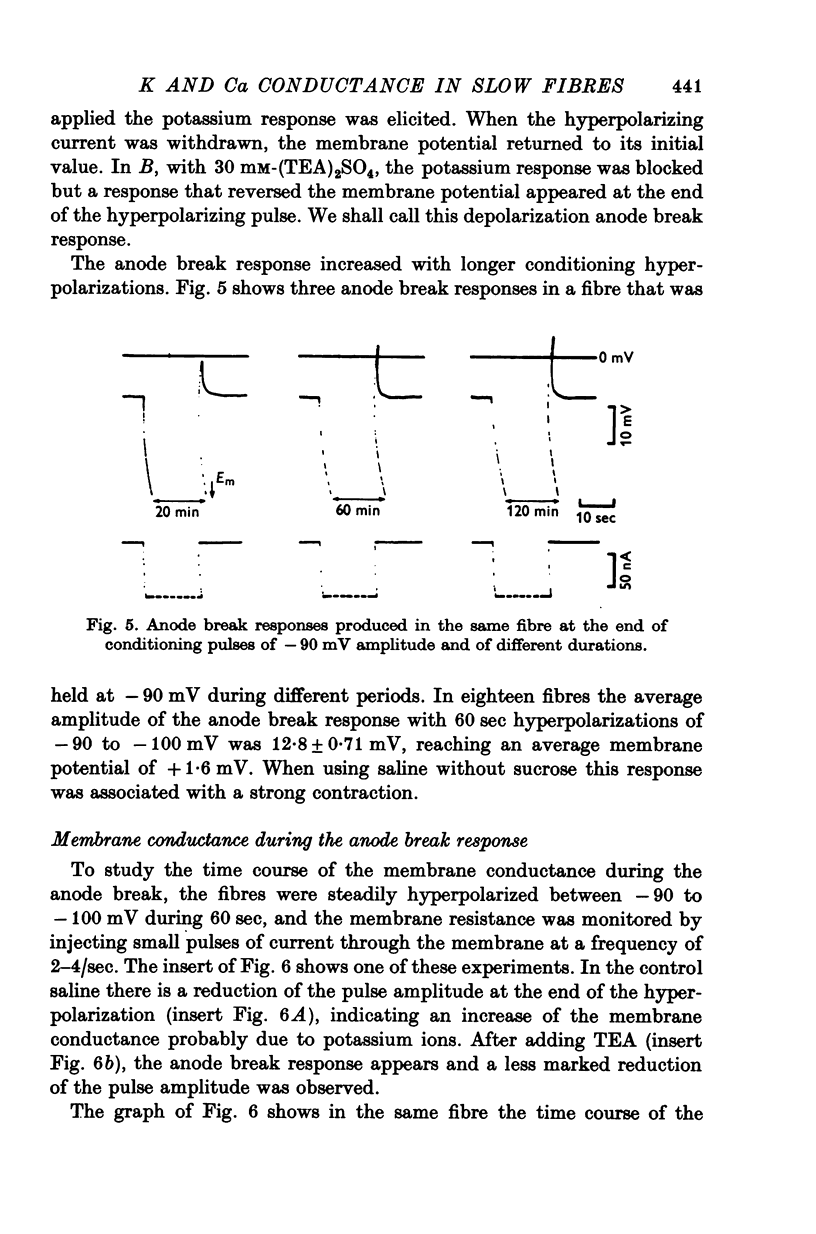
Slow muscle fibres in isotonic potassium sulphate saline could be easily repolarized to -90 mV. From this membrane potential a regenerative response could be elicited with short depolarizing pulses. 2. This response is blocked by TEA, suggesting that potassium is the main ion involved. 3. In the presence of TEA, a transient depolarization is recorded when the steady hyperpolarization is withdrawn. This anode break response is dependent upon the external calcium and is blocked by cobalt, suggesting that it is due to a calcium conductance. 4. The membrane conductance change was continuously recorded with short pulses at the end of the hyperpolarization. The membrane conductance decayed with at least two components with an average t1/2 of 1-2 and 6-8 sec. TEA blocked the slow component, and the fast one was dependent upon calcium and was blocked by cobalt.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK F. The role of calcium ions in neural processes. Pharmacol Rev. 1954 Sep;6(3):243–298. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Intracellular calcium movements in skinned muscle fibres. J Physiol. 1972 May;223(1):21–33. doi: 10.1113/jphysiol.1972.sp009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDFEST H. Ionic mechanisms in electrogenesis. Ann N Y Acad Sci. 1961 Sep 6;94:405–457. doi: 10.1111/j.1749-6632.1961.tb35554.x. [DOI] [PubMed] [Google Scholar]
- HOWARTH J. V. The behaviour of frog muscle in hypertonic solutions. J Physiol. 1958 Nov 10;144(1):167–175. doi: 10.1113/jphysiol.1958.sp006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Hayashi H., Takahashi K. Calcium and potassium currents of the membrane of a barnacle muscle fibre in relation to the calcium spike. J Physiol. 1969 Nov;205(1):115–129. doi: 10.1113/jphysiol.1969.sp008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F. Potassium conductance of skeletal muscle treated with formaldehyde. Nature. 1969 Dec 20;224(5225):1215–1217. doi: 10.1038/2241215a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Selective inhibition of the transmembrane Ca conductivity of mammalian myocardial fibres by Ni, Co and Mn ions. Pflugers Arch. 1973 Jan 22;338(2):115–123. doi: 10.1007/BF00592747. [DOI] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE J. W. Excitation of the squid axon membrane in isosmotic potassium chloride. Nature. 1959 Jan 24;183(4656):265–266. doi: 10.1038/183265b0. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kusano K. Behavior of delayed current under voltage clamp in the supramedullary neurons of puffer. J Gen Physiol. 1966 Mar;49(4):613–628. doi: 10.1085/jgp.49.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Chiarandini D. J. Skeletal muscle: dependence of potassium contractures on extracellular calcium. Pflugers Arch. 1973 Oct 17;343(2):143–150. doi: 10.1007/BF00585709. [DOI] [PubMed] [Google Scholar]
- Stefani E., Schmidt H. A convenient method for repeated intracellular recording of action potentials from the same muscle fibre without membrane damage. Pflugers Arch. 1972;334(3):276–278. doi: 10.1007/BF00626229. [DOI] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. B. Resting potential and electrical properties of frog slow muscle fibres. Effect of different external solutions. J Physiol. 1969 Aug;203(2):383–401. doi: 10.1113/jphysiol.1969.sp008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Kurtz G., Reuben J. P., Brandt P. W., Grundfest H. Membrane calcium activation in excitation-contraction coupling. J Gen Physiol. 1972 Jun;59(6):676–688. doi: 10.1085/jgp.59.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]


