Abstract
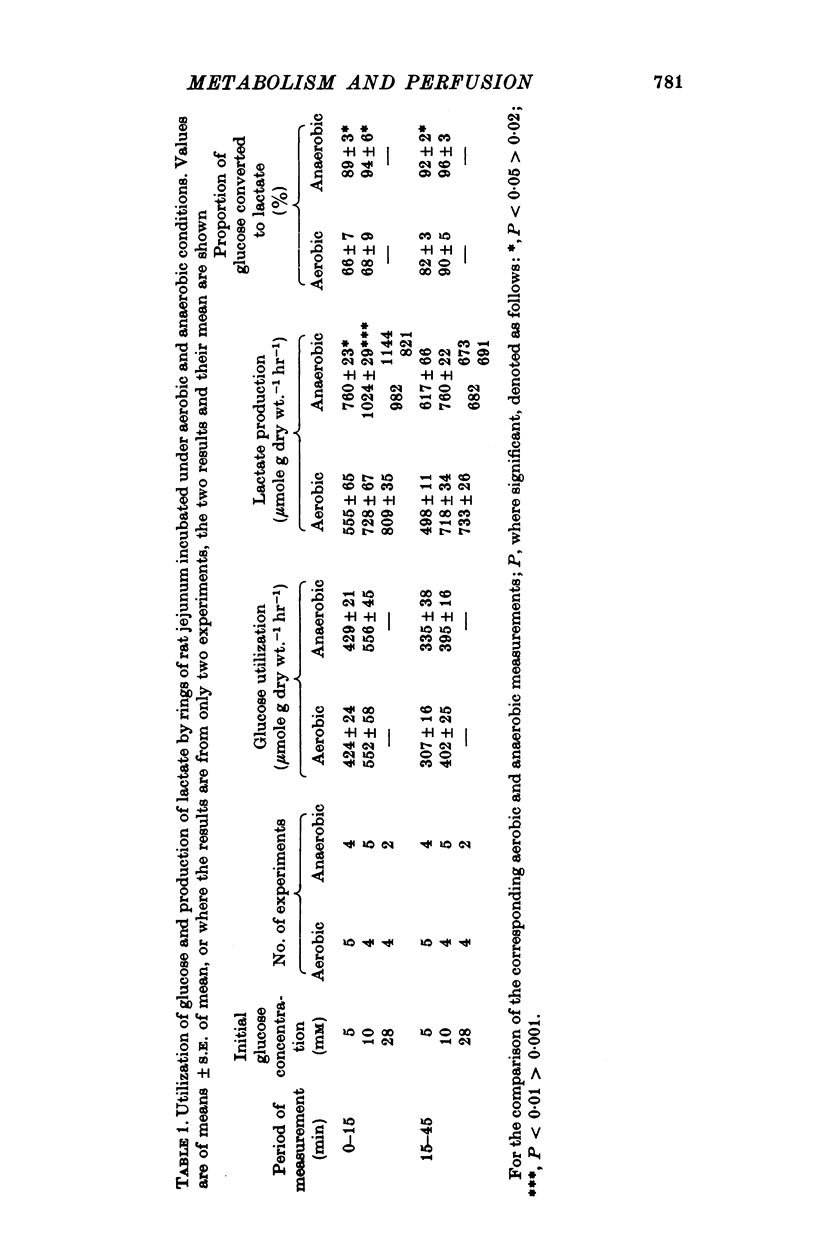
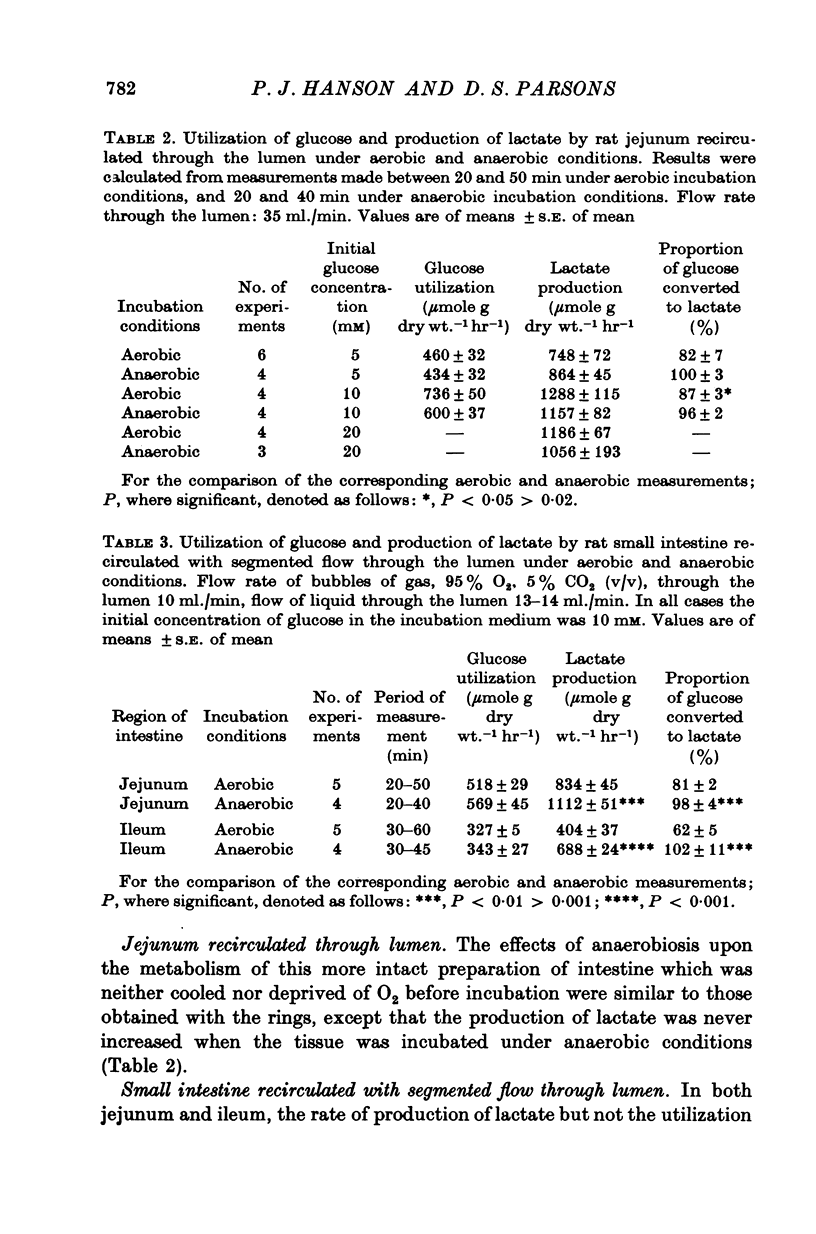
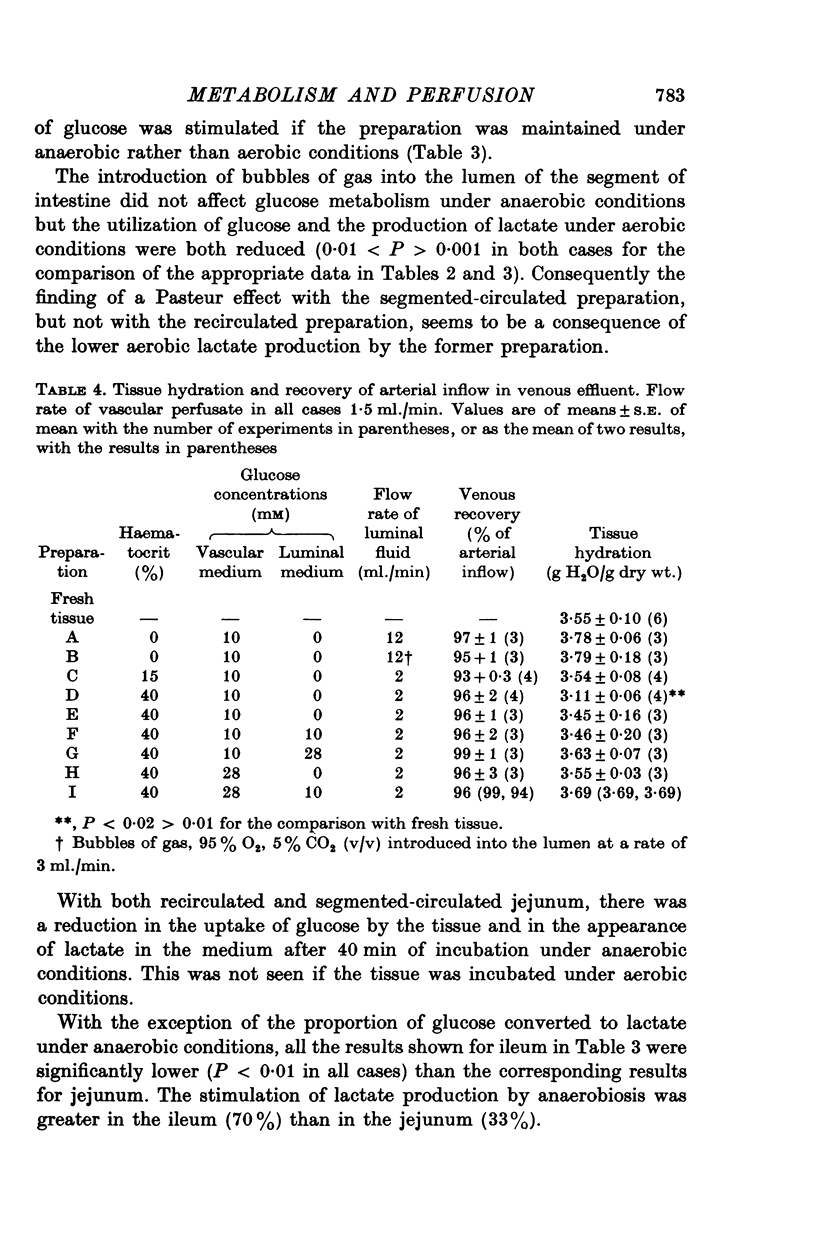
1. The rate of metabolism of glucose to lactate has been measured in a number of non-vascularly perfused preparations of rat jejunum in vitro. The glucose and lactate metabolism was measured simultaneously and under conditions such that the uptake of glucose and the appearance of lactate were linearly related to time. 2. It is found that there is no difference between the rates at which rings of rat jejunum utilize glucose during the first 45 min of anaerobic or aerobic incubation. During the first 15 min of incubation between 60-70% of the metabolized glucose was converted to lactate under aerobic conditions; this value increased to 80-90% during the subsequent 30 min. During the period 0-15 min of incubation, lactate production was found to be higher under anaerobic than under aerobic conditions but after this initial period the rate of lactate production was the same under aerobic and anaerobic conditions. 3. For segments of rat jejunum, maintained in vitro by the recirculation of nutrient fluid through the intestinal lumen, neither the rate of production of lactate, nor the utilization of glucose, was stimulated if the preparation was maintained under anaerobic rather than aerobic conditions. The direct delivery of gas into the intestinal lumen in the form of a stream of bubbles (segmented circulation) reduced both the utilization of glucose and the production of lactate under aerobic conditions. However, not effect on glucose metabolism was observed under anaerobic conditions. The finding of a Pasteur effect with the segmented-circulated preparation, but not with the simple recirculated preparation, is associated with lower rate of aerobic lactate production in the former preparation. Reasons are given for supposing that under conditions of segmented circulation, the luminal compartment is better stirred, thereby increasing access of O2 to the tissue. 4. A preparation of rat small intestine perfused through the vascular bed is described. With this preparation the rate of glucose utilization is significantly lower than that for recirculated preparations and the rate of lactate production is substantially less than that of the other preparations studied. 5. With the preparation perfused through the vascular bed, and with glucose, 10 mM, present only in the vascular medium the addition of erythrocytes to the vascular infusate causes a significant reduction in both glucose utilization and in the rate of lactate production. The addition of erythrocytes to produce an haematocrit of 40% (v/v) causes a greater reduction in glucose utilization and lactate production than is found for an haematrocrit of 15%. About 10% of the lactate produced appears in the luminal contents. With an haematocrit of 15%, the O2 consumption of the whole wall of the jejunum was found to be 6-4 mumole O2 g dry wt.-1 min-1, equivalent of a value for the Q02 of 8-6 mul. O2 mg druwy wt.-1 hr-1. The uptake of O2 was almost entirely from the vascular infusate. 6...
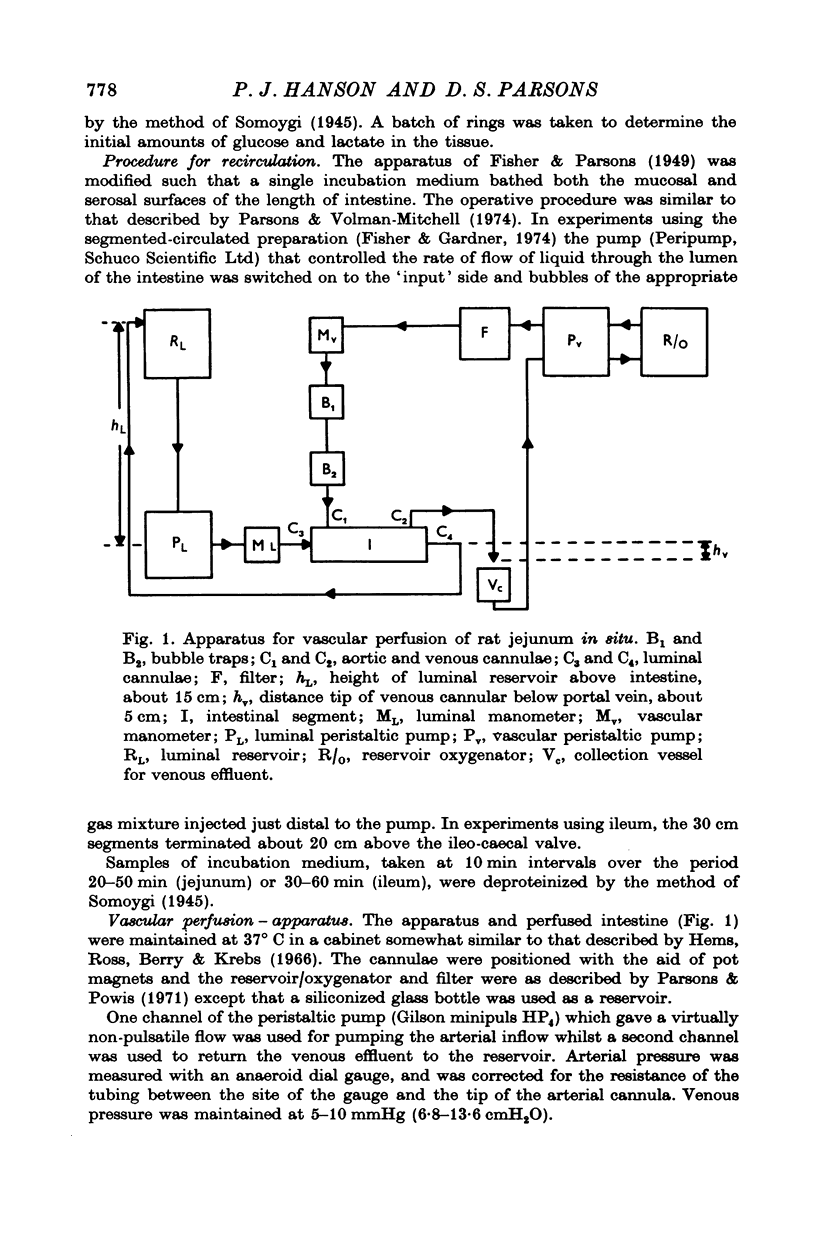
Full text
PDF





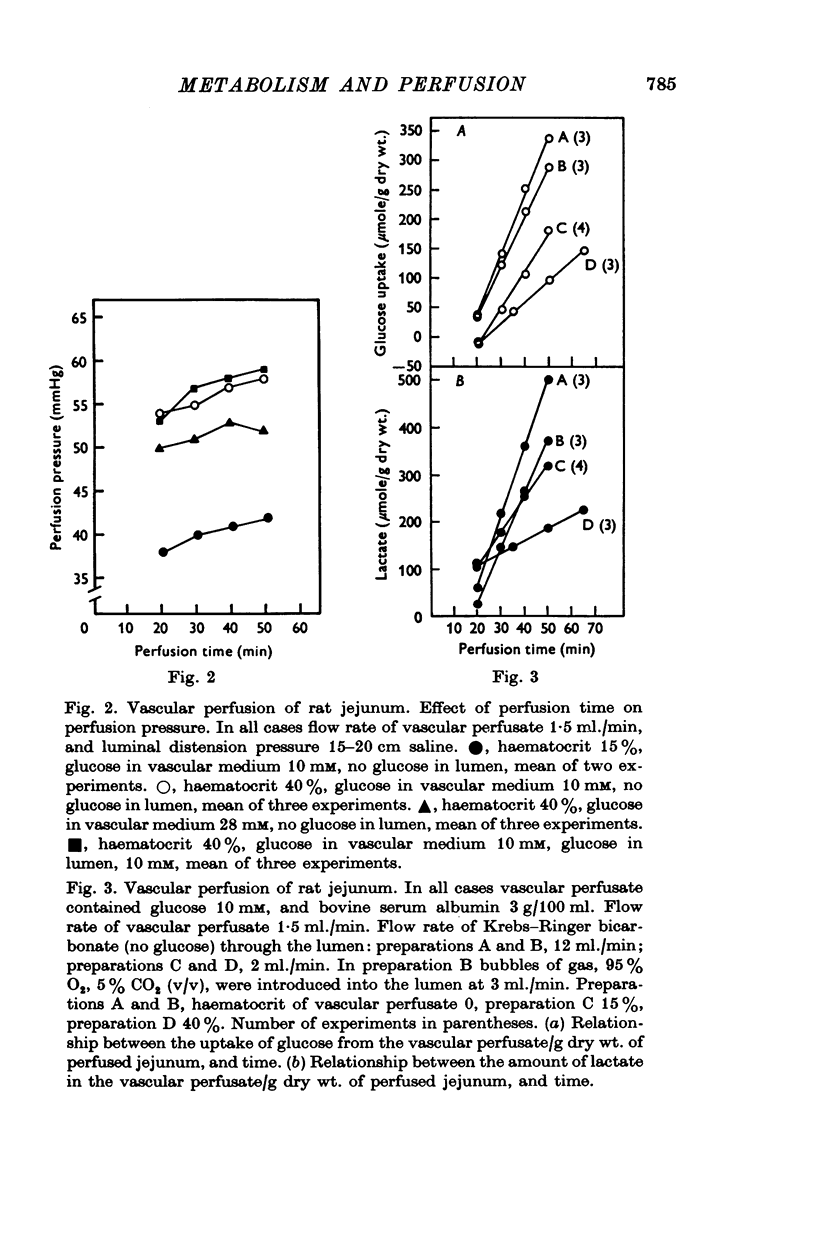
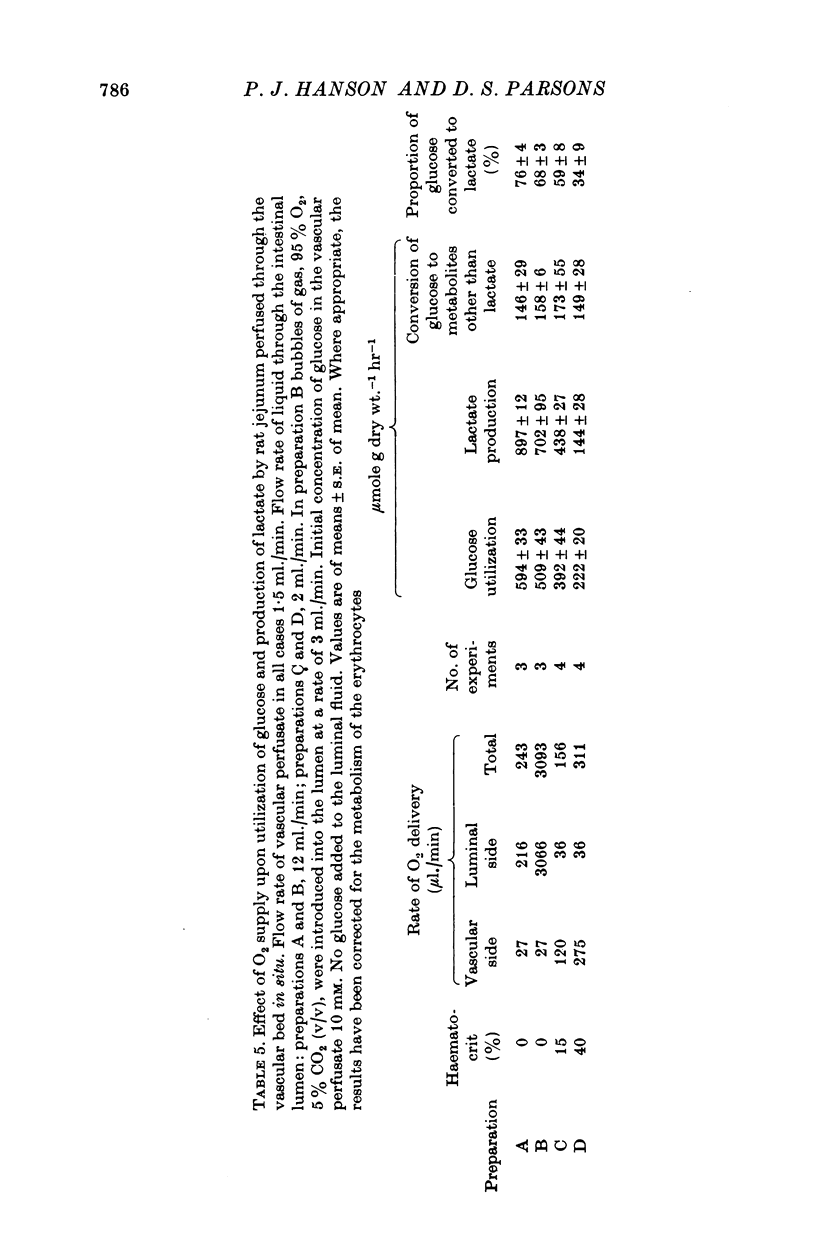

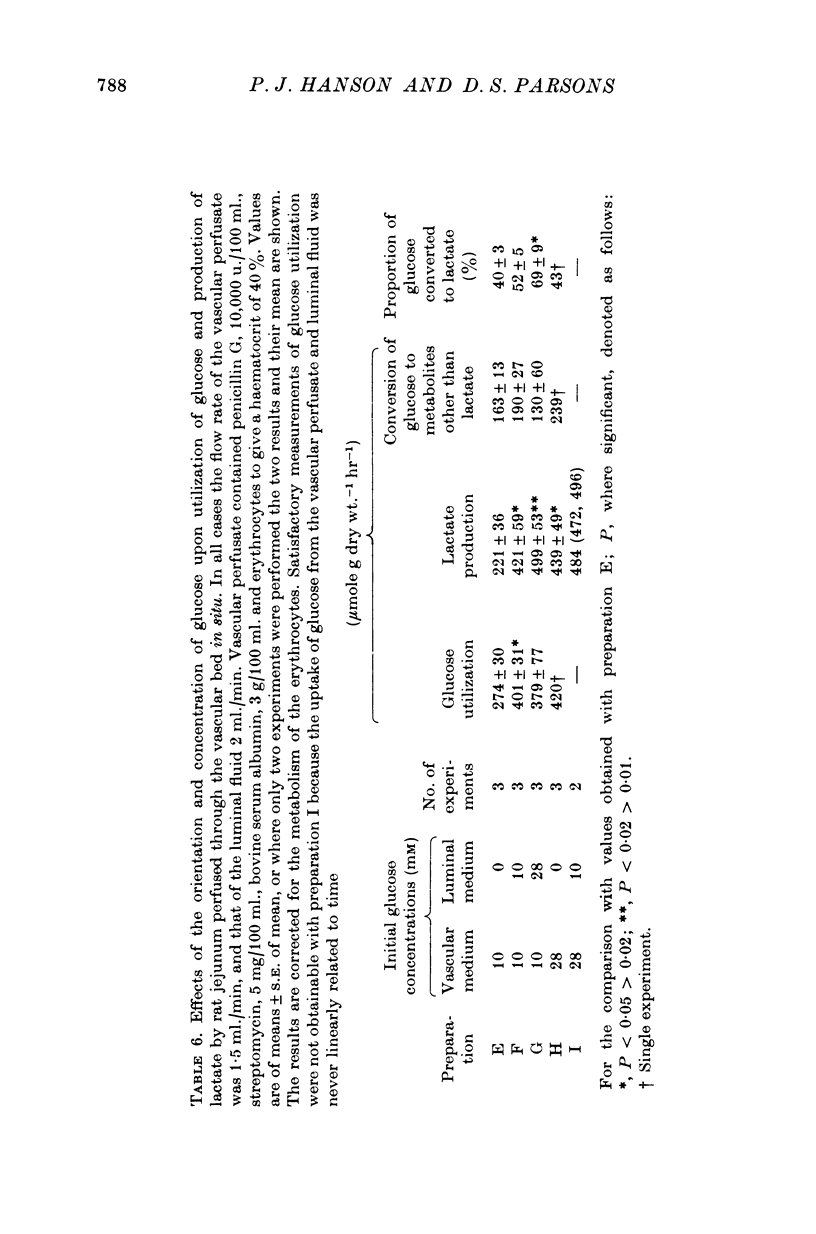


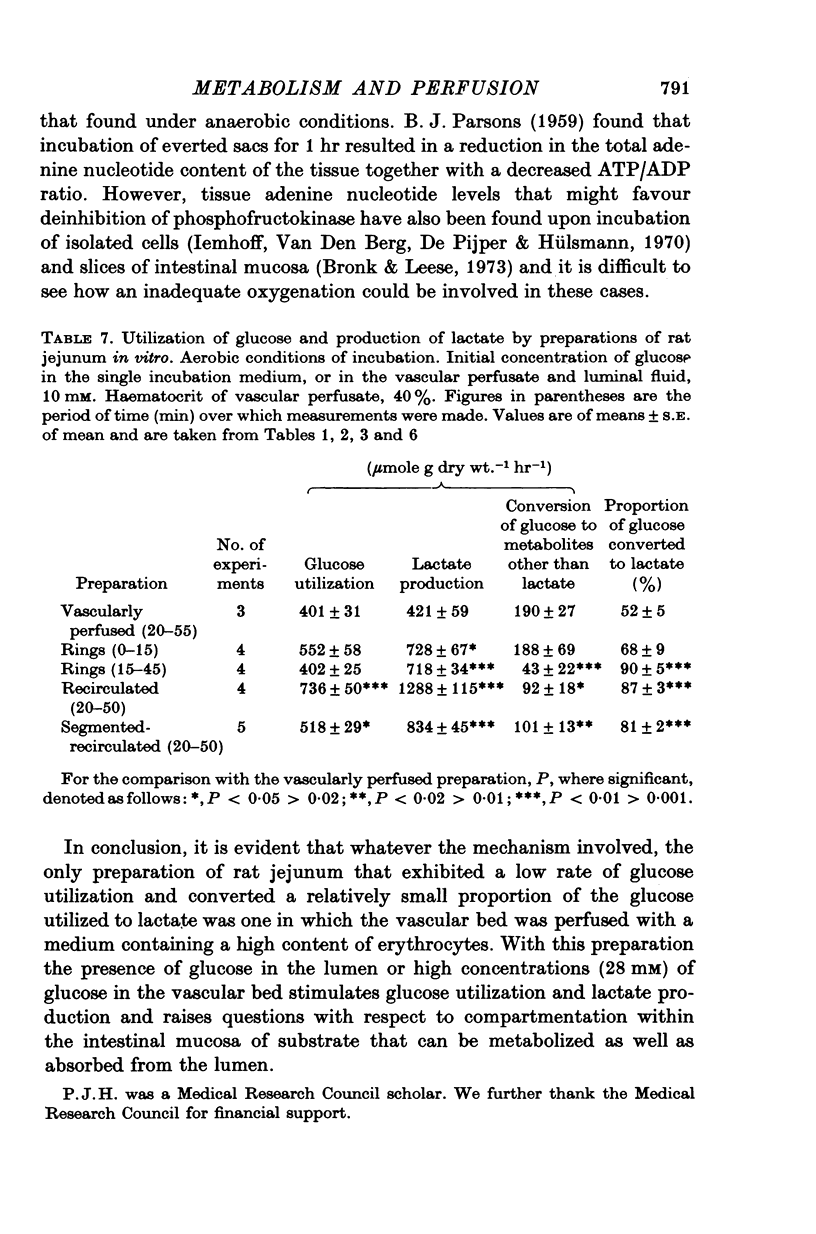



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronk J. R., Leese H. J. Changes in the adenine nucleotide content of preparations of the rat small intestine in vitro. J Physiol. 1973 Nov;235(1):183–196. doi: 10.1113/jphysiol.1973.sp010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. The polarographic determination of the respiration of the small intestine of the rat. Biochim Biophys Acta. 1965 Oct 18;107(3):397–404. doi: 10.1016/0304-4165(65)90183-2. [DOI] [PubMed] [Google Scholar]
- Csáky T. Z., Ho P. M. The effect of potassium on the intestinal transport of glucose. J Gen Physiol. 1966 Sep;50(1):113–128. doi: 10.1085/jgp.50.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F., Weil-Malherbe H. Metabolism of normal and tumour tissue: The metabolism of intestinal mucous membrane. Biochem J. 1941 Jan;35(1-2):7–15. doi: 10.1042/bj0350007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. A preparation of surviving rat small intestine for the study of absorption. J Physiol. 1949 Dec 15;110(1-2):36-46, pl. doi: 10.1113/jphysiol.1949.sp004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. Glucose movements across the wall of the rat small intestine. J Physiol. 1953 Feb 27;119(2-3):210–223. doi: 10.1113/jphysiol.1953.sp004839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. B., Gardner M. L. A kinetic approach to the study of absorption of solutes by isolated perfused small intestine. J Physiol. 1974 Aug;241(1):211–234. doi: 10.1113/jphysiol.1974.sp010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster H., Meyer E., Ziege M. The intestinal absorption of glucose with the simultaneous determination of the arterioportal glucose concentration difference. Rev Eur Etud Clin Biol. 1972 Dec;17(10):958–964. [PubMed] [Google Scholar]
- Gerber G. B., Remy-Defraigne J. DNA metabolism in perfused organs. II. Incorporation into DNA and catabolism of thymidine at different levels of substrate by normal and x-irradiated liver and intestine. Arch Int Physiol Biochim. 1966 Nov;74(5):785–806. doi: 10.3109/13813456609059952. [DOI] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemhoff W. G., van den Berg J. W., de Pijper A. M., Hülsmann W. C. Metabolic aspects of isolated cells from rat small intestinal epithelium. Biochim Biophys Acta. 1970 Aug 14;215(2):229–241. doi: 10.1016/0304-4165(70)90020-6. [DOI] [PubMed] [Google Scholar]
- Jodal M., Lundgren O. Plasma skimming in the intestinal tract. Acta Physiol Scand. 1970 Sep;80(1):50–60. doi: 10.1111/j.1748-1716.1970.tb04769.x. [DOI] [PubMed] [Google Scholar]
- Lamers J. M., Hülsmann W. C. Pasteur effect in the in vitro vascularly perfused rat small intestine. Biochim Biophys Acta. 1972 Sep 20;275(3):491–495. doi: 10.1016/0005-2728(72)90234-4. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Bronk J. R. Lactate formation by rat small intestine in vitro. Biochim Biophys Acta. 1975 Sep 8;404(1):40–48. doi: 10.1016/0304-4165(75)90145-2. [DOI] [PubMed] [Google Scholar]
- PARSONS B. J. Studies of the effect of triethyltin sulphate on transport and metabolism in the small intestine of the rat. J Physiol. 1959 Oct;148:117–126. doi: 10.1113/jphysiol.1959.sp006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. S., Powis G. Some properties of a preparation of rat colon perfused in vitro through the vascular bed. J Physiol. 1971 Sep;217(3):641–663. doi: 10.1113/jphysiol.1971.sp009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. S., Volman-Mitchell H. The transamination of glutamate and aspartate during absorption in vitro by small intestine of chicken, guinea-pig and rat. J Physiol. 1974 Jun;239(3):677–694. doi: 10.1113/jphysiol.1974.sp010589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt H. S. The metabolism of the small intestine. Oxygen uptake and L-lactate production along the length of the small intestine of the rat and guinea pig. Comp Biochem Physiol. 1968 Mar;24(3):745–761. doi: 10.1016/0010-406x(68)90787-1. [DOI] [PubMed] [Google Scholar]
- WILSON T. H. The role of lactic acid production in glucose absorption from the intestine. J Biol Chem. 1956 Oct;222(2):751–763. [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. Metabolic activity of the small intestine of the rat and golden hamster (Mesocricetus auratus). J Physiol. 1954 Jan;123(1):126–130. doi: 10.1113/jphysiol.1954.sp005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Fat transport and lymph and plasma lipoprotein biosynthesis by isolated intestine. J Lipid Res. 1972 Jan;13(1):92–105. [PubMed] [Google Scholar]
- Winne D. Unstirred layer, source of biased Michaelis constant in membrane transport. Biochim Biophys Acta. 1973 Feb 27;298(1):27–31. doi: 10.1016/0005-2736(73)90005-9. [DOI] [PubMed] [Google Scholar]


