Abstract
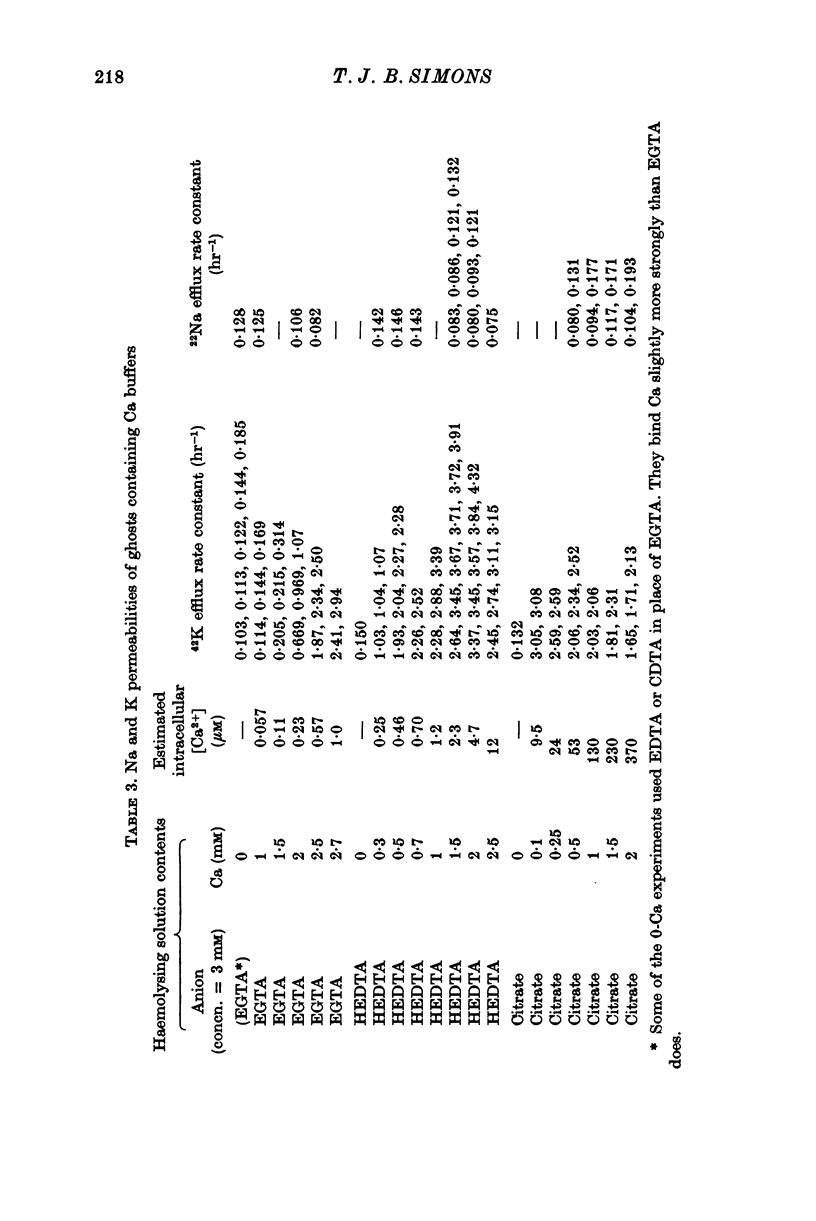
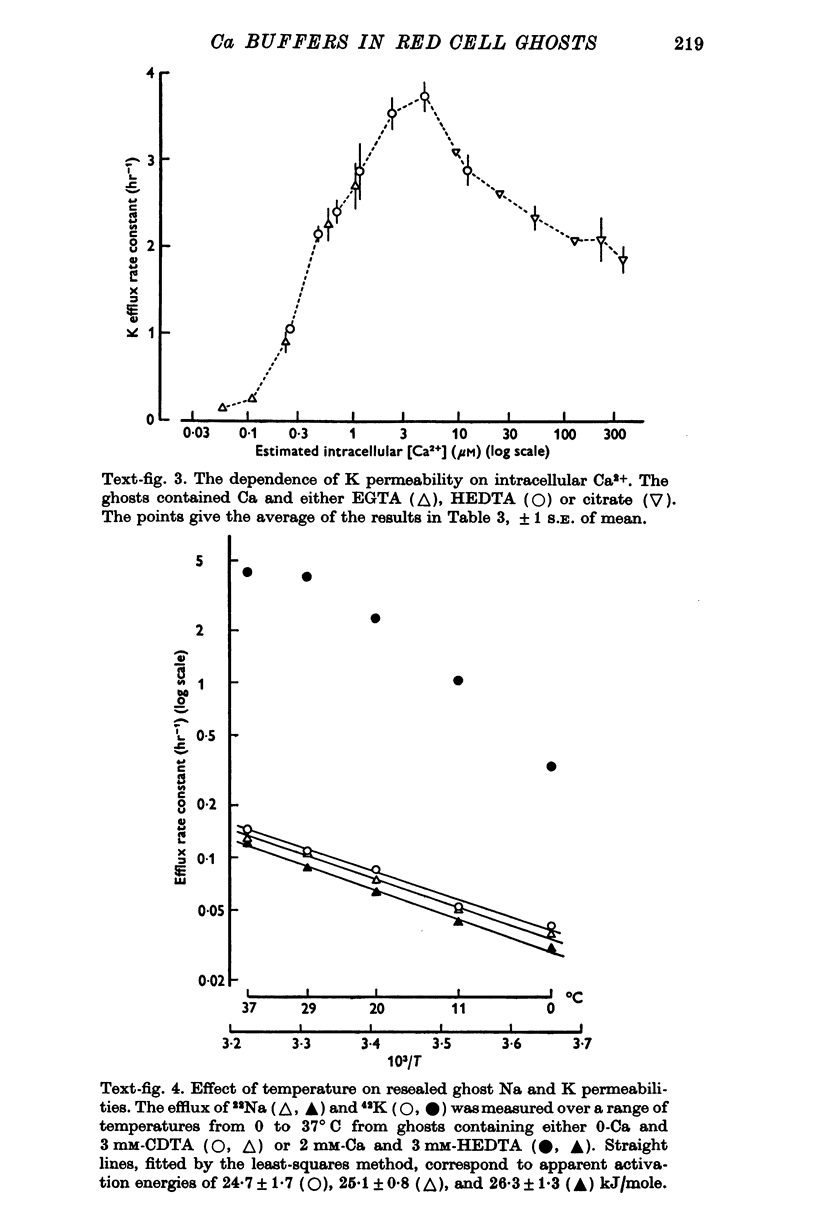
1. Ca buffers may be introduced into human red cells by reversible haemolysis. The resealed ghosts retain Ca and chelating anions in the same ratio as in the haemolysing solution, enabling the intracellular Ca2+ concentration to be calculated simply. 2. The passive permeability of the ghosts to Na and Cl is unaffected by intracellular Ca2+ concentrations in the 10(-8)-10(-4) M range, whereas the K permeability is greatly increased at concentrations above 10(-7) M. 3. These preparations enable Ca-dependent K movements to be studied under stable conditions. When the ghosts contain about 5 X 10(-6) M-Ca2+, over 96% of K transport occurs via the Ca-sensitive route.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum R. M., Hoffman J. F. Ca-induced K transport in human red cells: localization of the Ca-sensitive site to the inside of the membrane. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1146–1152. doi: 10.1016/s0006-291x(72)80094-9. [DOI] [PubMed] [Google Scholar]
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Colombe B. W., Macey R. I. Effects of calcium on potassium and water transport in human erythrocyte ghosts. Biochim Biophys Acta. 1974 Sep 6;363(2):226–239. doi: 10.1016/0005-2736(74)90062-5. [DOI] [PubMed] [Google Scholar]
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J., Manery J. F. Calcium binding by human erythrocyte membranes. Biochem J. 1971 Sep;124(3):563–571. doi: 10.1042/bj1240563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Hoffman J. F. Nucleotide requirements for sodium-sodium exchange catalysed by the sodium pump in human red cells. J Physiol. 1971 Oct;218(1):239–256. doi: 10.1113/jphysiol.1971.sp009612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L. Effect of intracellular calcium on the potassium permeability of human red cells. J Physiol. 1970 Feb;206(2):35P–36P. [PubMed] [Google Scholar]
- Ogawa Y. The apparent binding constant of glycoletherdiaminetetraacetic acid for calcium at neutral pH. J Biochem. 1968 Aug;64(2):255–257. doi: 10.1093/oxfordjournals.jbchem.a128887. [DOI] [PubMed] [Google Scholar]
- Porzig H. Comparative study of the effects of propranolol and tetracaine on cation movements in resealed human red cell ghosts. J Physiol. 1975 Jul;249(1):27–49. doi: 10.1113/jphysiol.1975.sp011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. Calcium-dependent potassium exchange in human red cell ghosts. J Physiol. 1976 Mar;256(1):227–244. doi: 10.1113/jphysiol.1976.sp011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons T. J. Potassium: potassium exchange catalysed by the sodium pump in human red cells. J Physiol. 1974 Feb;237(1):123–155. doi: 10.1113/jphysiol.1974.sp010474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]