Abstract
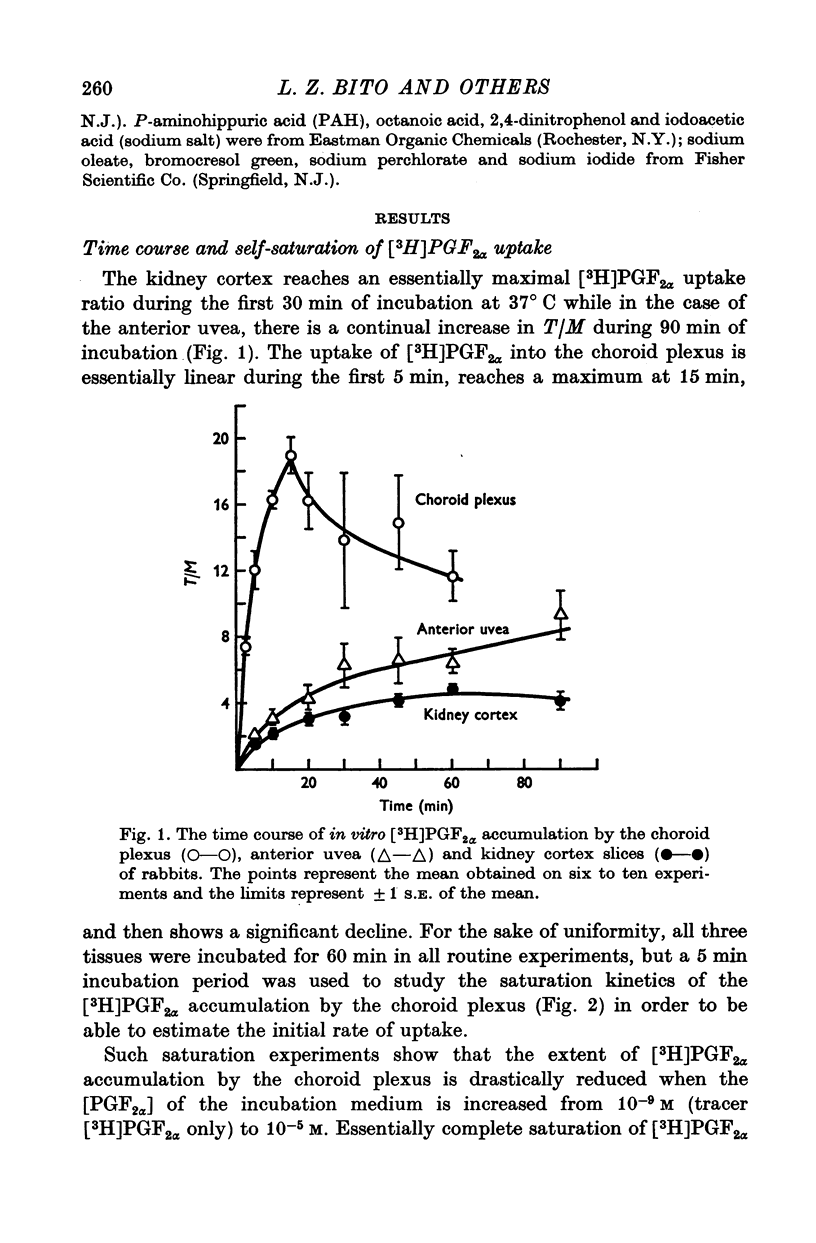
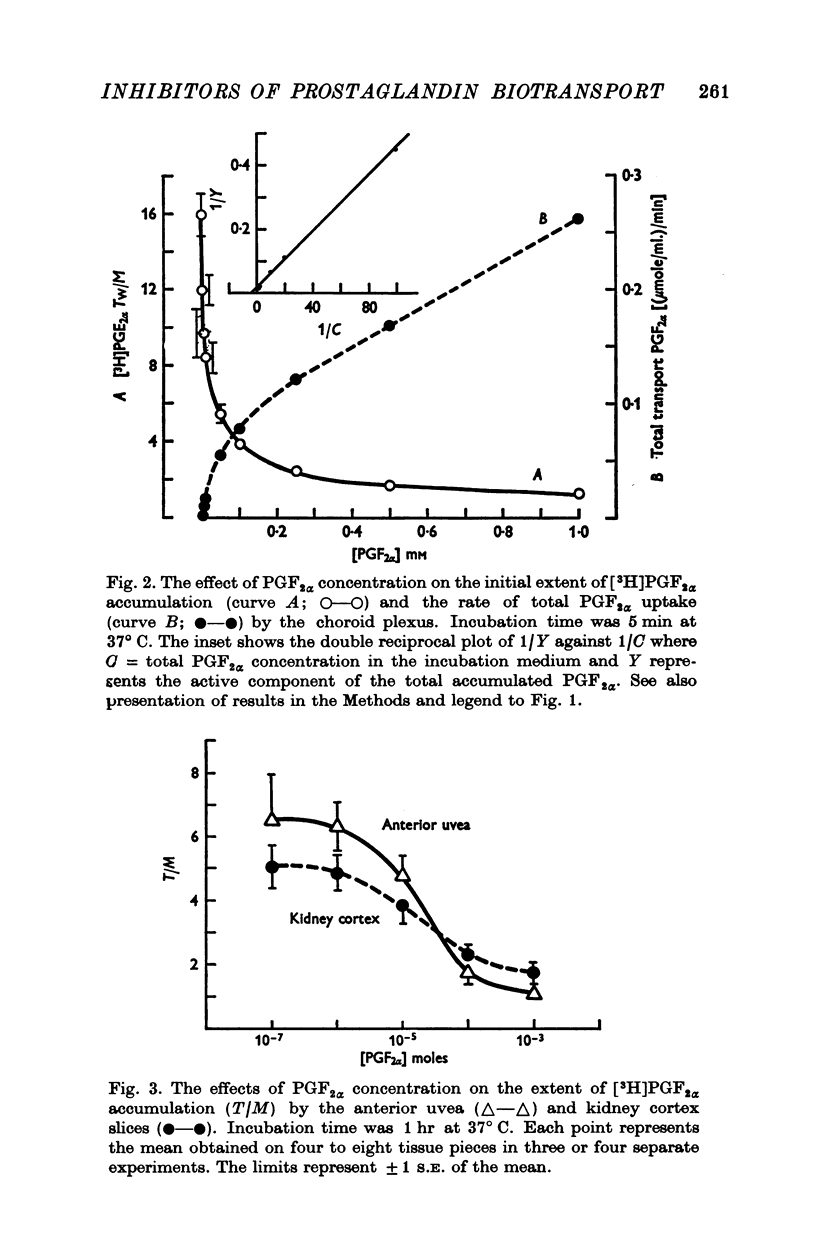
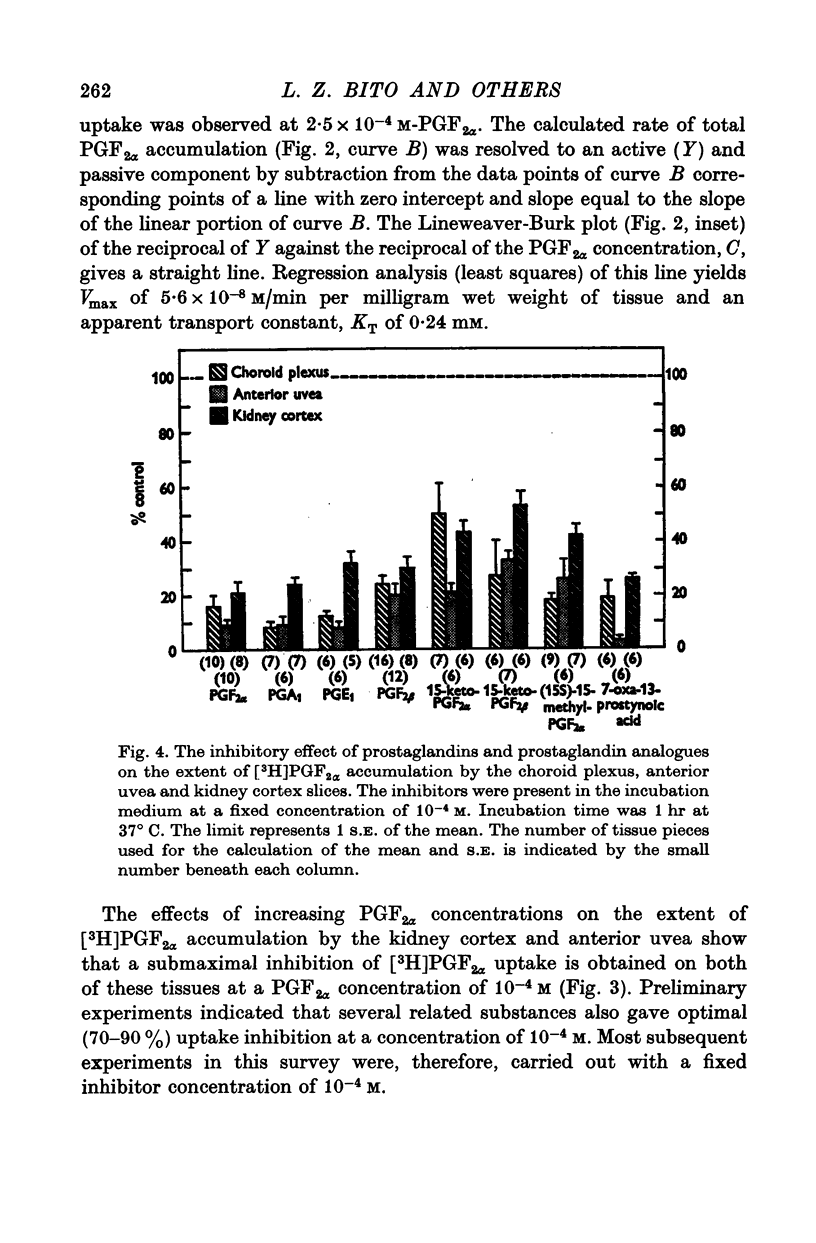
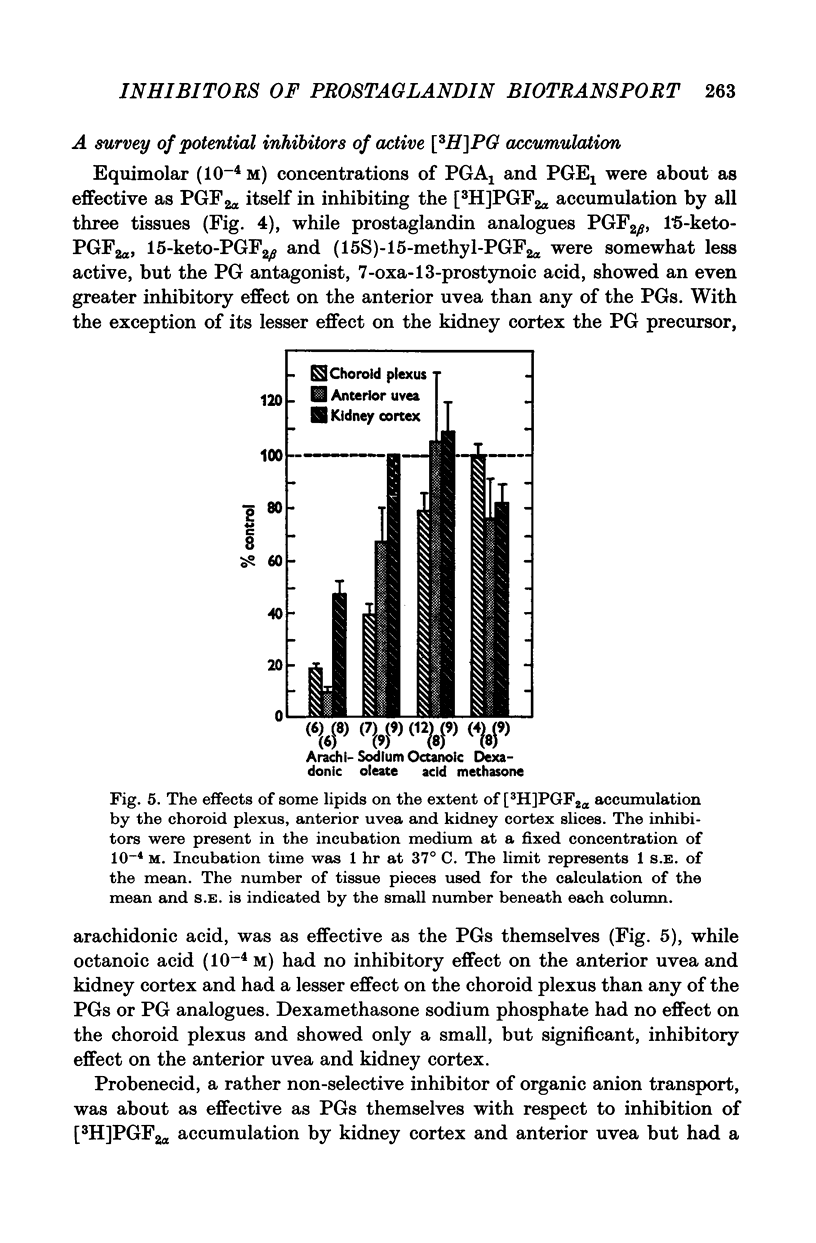
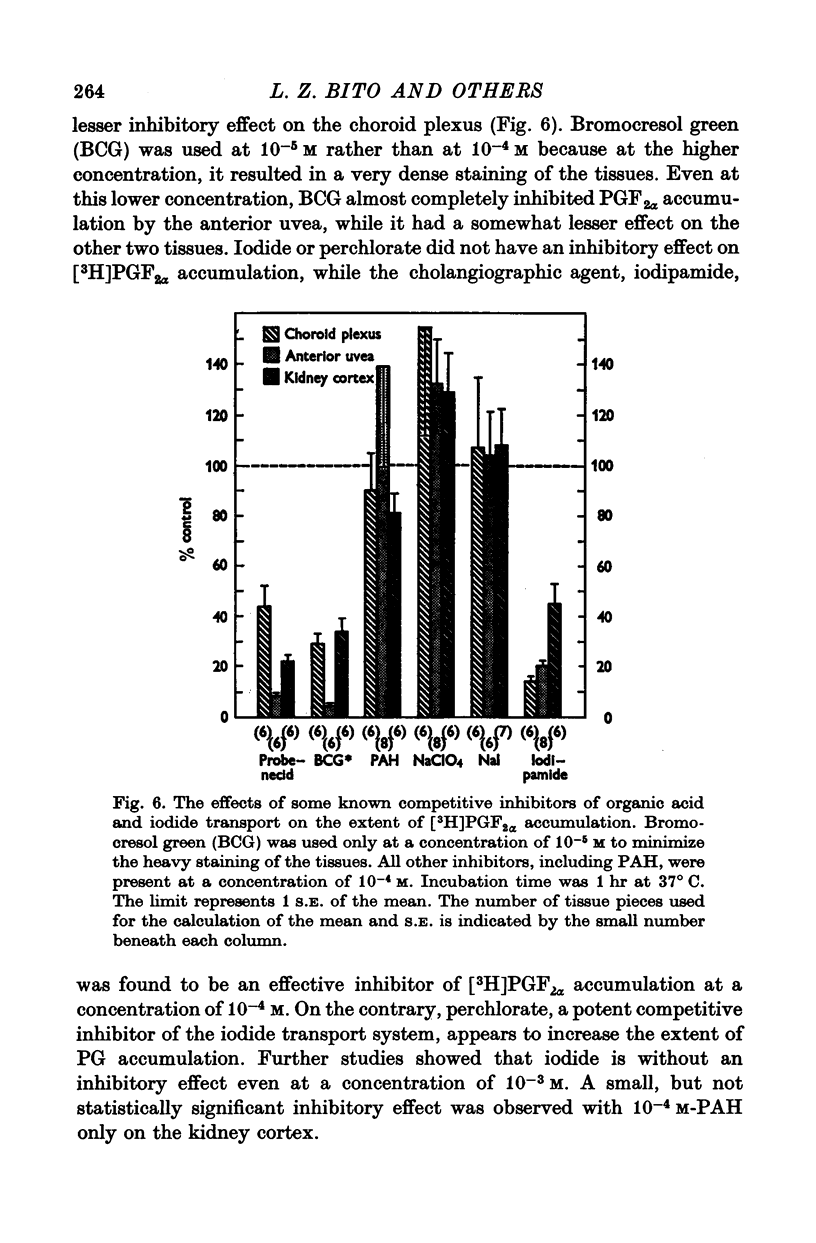
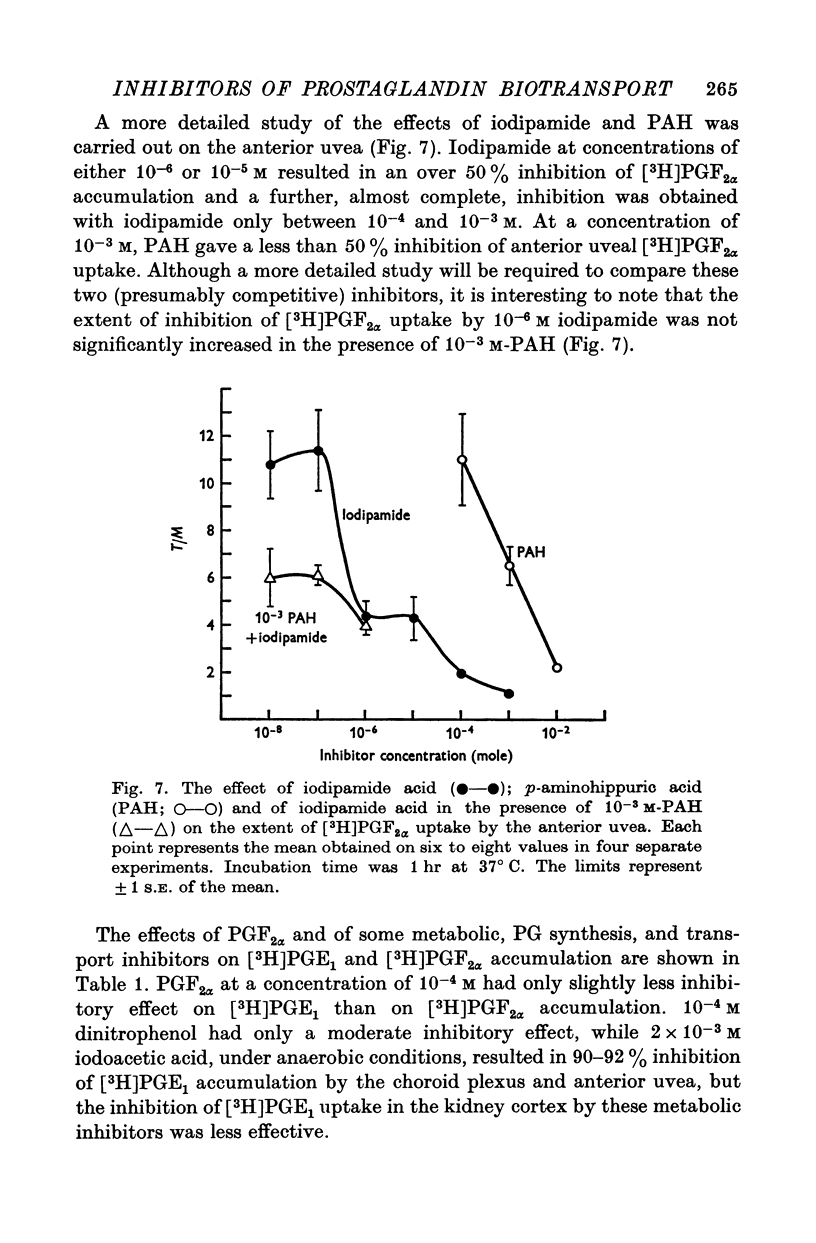
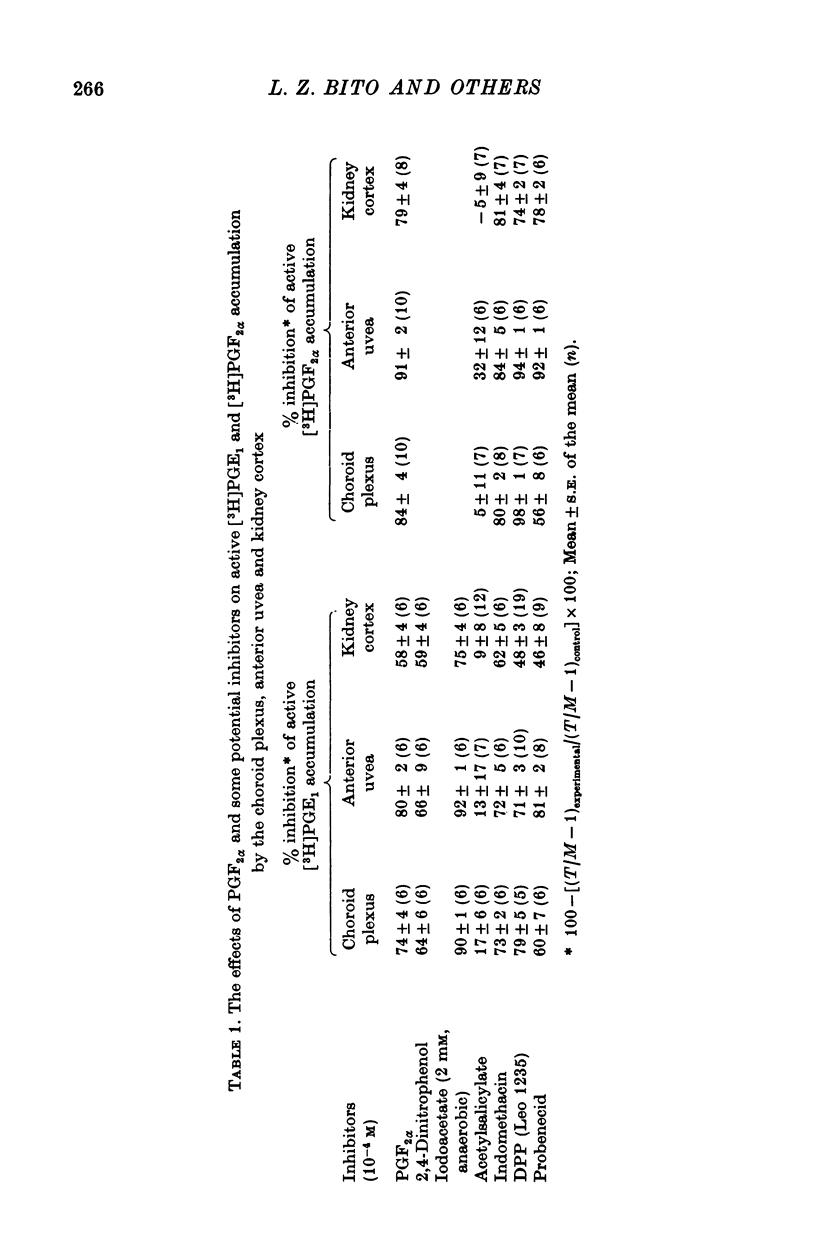
1. Incubation of rabbit choroid plexus, anterior uvea (iris-ciliary body complex) or slices of kidney cortex in a medium containing tritium-labelled prostaglandin F(2alpha) ([3H]PGF(2alpha) or E1 ([3H]PGE1) results in a four- to thirteenfold concentrative accumulation of 3H activity. 2. Addition of PGF(2alpha, PGF(1) or PGA(1), any one of five PG analogues or a PG precursor, arachidonic acid, at a concentration of 10(-4) M reduced the active accumulation of [3H]PGs by 47-97%. Octanoic acid, at the same concentration, had only a moderate effect on the choroid plexus and no significant inhibitory effect on [3H]PFG(2alpha) accumulation by anterior uvea or kidney cortex. 3. Inhibition was also obtained with 2 mM iodoacetate (under anaerobic conditions) and with 10(-4) M diploretin phosphate, probenecid, iodipamide, indomethacin or dinitrophenol. Perchlorate (10(-4) M) and iodide (10(-4) or 10(-3) M) had no inhibitory effect while 10(-4) M p-aminohippuric acid had a significant inhibitory effect on the kidney cortex at a concentration of 10(-4) M and on the anterior uvea at 10(-3) M. 4. It is concluded that the apparent carrier mediated PG transport systems of the choroid plexus, anterior uvea and kidney cortex are not related to the iodide transport system, but may represent a subcomponent of the iodipamide transport system of these tissues. 5. These results suggest that the systemic distribution and the rate of renal excretion of PGs could be altered by high concentrations of PGs, pharmacologically less active PG analogues, some inhibitors of organic acid transport, and by some inhibitors of PG synthesis and PG action.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER B. Iodide transport by the rabbit eye. Am J Physiol. 1961 Apr;200:804–806. doi: 10.1152/ajplegacy.1961.200.4.804. [DOI] [PubMed] [Google Scholar]
- Berndt W. O., Mudge G. H. Renal excretion of iodipamide. Comparative study in the dog and rabbit. Invest Radiol. 1968 Nov-Dec;3(6):414–426. [PubMed] [Google Scholar]
- Bito L. Z. Accumulation and apparent active transport of prostaglandins by some rabbit tissues in vitro. J Physiol. 1972 Mar;221(2):371–387. doi: 10.1113/jphysiol.1972.sp009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito L. Z., Baroody R. Concentrative accumulation of 3H-prostaglandins by some rabbit tissues in vitro: the chemical nature of the accumulated 3H-labelled substances. Prostaglandins. 1974 Jul 25;7(2):131–140. doi: 10.1016/0090-6980(74)90133-6. [DOI] [PubMed] [Google Scholar]
- Bito L. Z., Davson H., Hollingsworth J. R. Facilitated transport of prostaglandins across the blood-cerebrospinal fluid and blood-brain barriers. J Physiol. 1976 Apr;256(2):273–285. doi: 10.1113/jphysiol.1976.sp011325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito L. Z., Davson H. Proceedings: Carrier-mediated removal of prostaglandins from cerebrospinal fluid. J Physiol. 1974 Jan;236(1):39P–40P. [PubMed] [Google Scholar]
- Bito L. Z., Salvador E. V. Intraocular fluid dynamics. 3. The site and mechanism of prostaglandin transfer across the blood intraocular fluid barriers. Exp Eye Res. 1972 Nov;14(3):233–241. doi: 10.1016/0014-4835(72)90008-5. [DOI] [PubMed] [Google Scholar]
- Bito L. Z., Spellane P. J. Saturable, "carrier-mediated", absorption of prostaglandin F2 alpha from the in vivo rabbit vagina and its inhibition by prostaglandin F2 beta. Prostaglandins. 1974 Nov 25;8(4):345–352. doi: 10.1016/s0090-6980(74)80100-0. [DOI] [PubMed] [Google Scholar]
- Bárány E. H. The liver-like anion transport system in rabbit kidney, uvea and choroid plexus. I. Selectivity of some inhibitors, direction of transport, possible physiological substrates. Acta Physiol Scand. 1973 Jul;88(3):412–429. doi: 10.1111/j.1748-1716.1973.tb05470.x. [DOI] [PubMed] [Google Scholar]
- Bárány E. H. The liver-like anion transport system in rabbit kidney, uvea and choroid plexus. II. Efficiency of acidic drugs and other anions as inhibitors. Acta Physiol Scand. 1973 Aug;88(4):491–504. doi: 10.1111/j.1748-1716.1973.tb05478.x. [DOI] [PubMed] [Google Scholar]
- CROSS R. J., TAGGART J. V. Renal tubular transport: accumulation of p-aminohippurate by rabbit kidney slices. Am J Physiol. 1950 Apr 1;161(1):181–190. doi: 10.1152/ajplegacy.1950.161.1.181. [DOI] [PubMed] [Google Scholar]
- Cserr H. F. Physiology of the choroid plexus. Physiol Rev. 1971 Apr;51(2):273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- Fried J., Lin C., Mehra M., Kao W., Dalven P. Synthesis and biological activity of prostaglandins and prostaglandin antagonists. Ann N Y Acad Sci. 1971 Apr 30;180:38–63. doi: 10.1111/j.1749-6632.1971.tb53184.x. [DOI] [PubMed] [Google Scholar]
- Kloeze J. Relationship between chemical structure and platelet-aggregation activity of prostaglandins. Biochim Biophys Acta. 1969 Oct 28;187(3):285–292. doi: 10.1016/0005-2760(69)90001-0. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L. Direct evidence for a prostaglandin receptor and its application to prostaglandin measurements (rat-adipocytes-antagonists-analogues-mouse ovary assay). Proc Natl Acad Sci U S A. 1972 Feb;69(2):480–484. doi: 10.1073/pnas.69.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling A. E., Kirton K. T. Prostaglandin receptors in the hamster uterus during the estrous cycle. Prostaglandins. 1973 Jul;4(1):1–8. doi: 10.1016/0090-6980(73)90050-6. [DOI] [PubMed] [Google Scholar]


