Abstract
1. The electroretinogram (e.r.g.) of the isolated rat retina has been investigated by recording potential differences developed between two micropipettes.
2. In the uniformly illuminated receptor layer, voltage gradients at 90° to the long axes of the receptors are negligible in comparison with the radial voltage gradients.
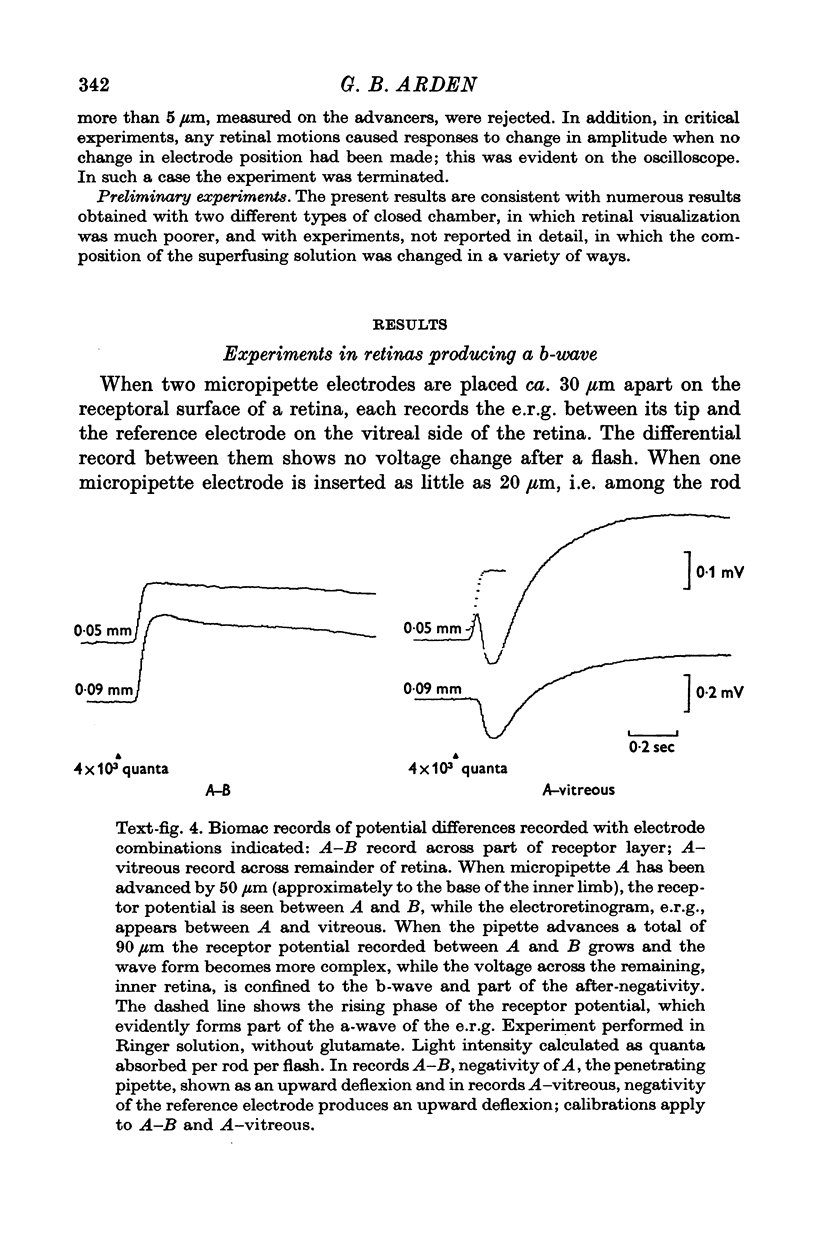
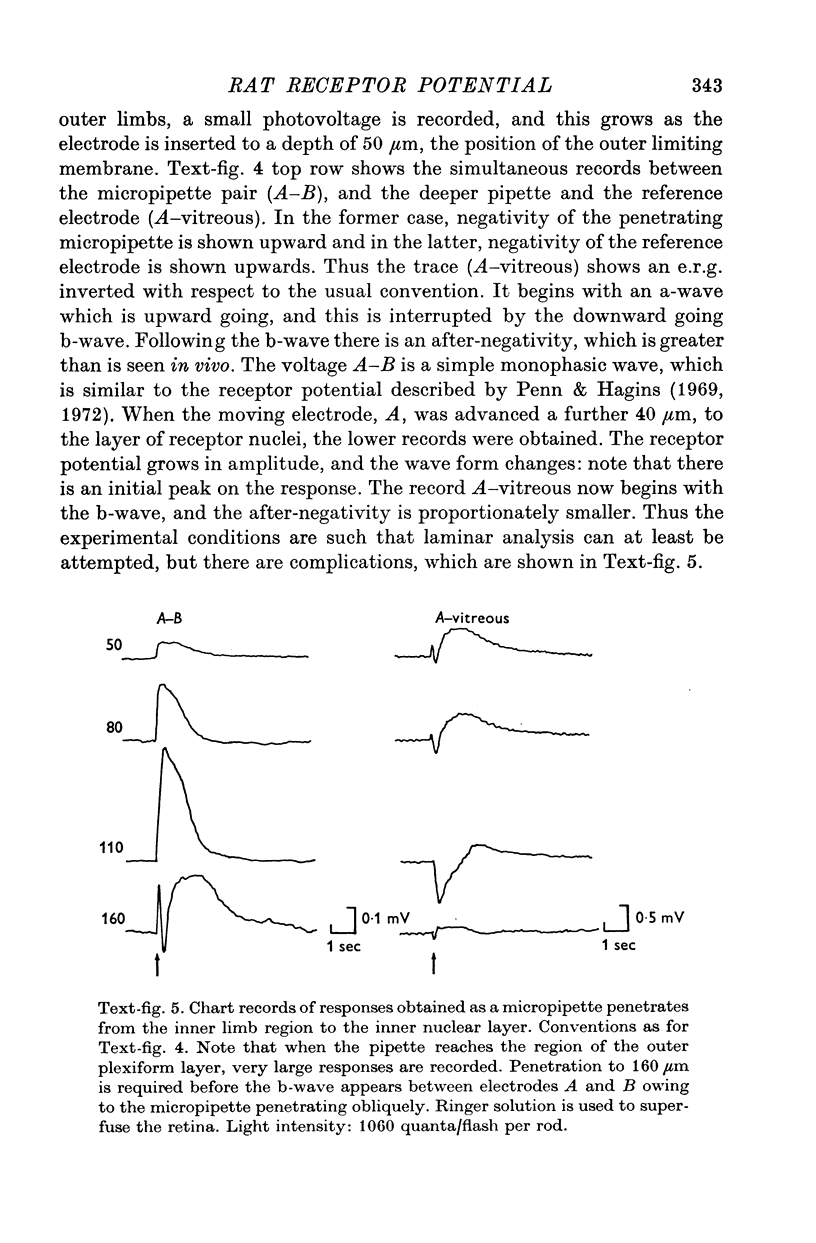
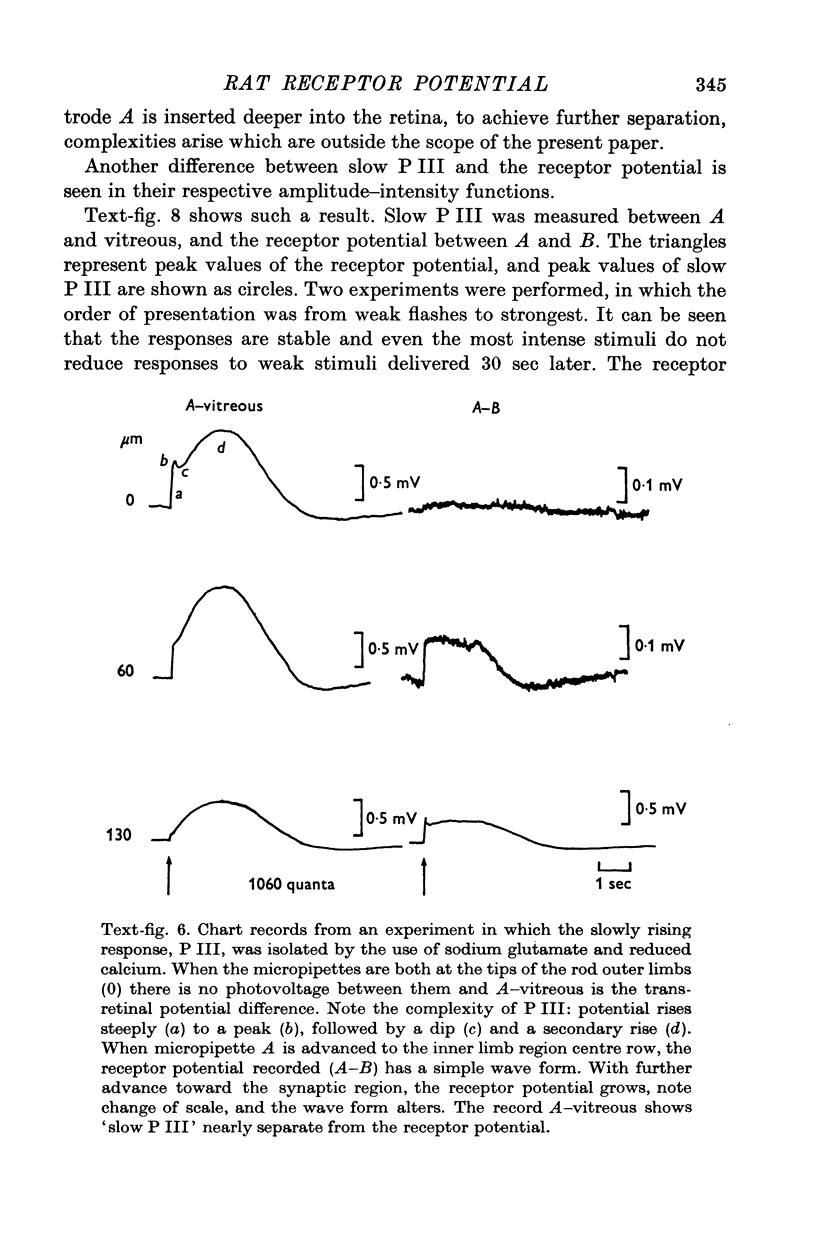
3. When all transsynaptic neural activity has been abolished, the photoresponse recorded across the receptor layer is very different from the photoresponse recorded across the inner retinal layer.
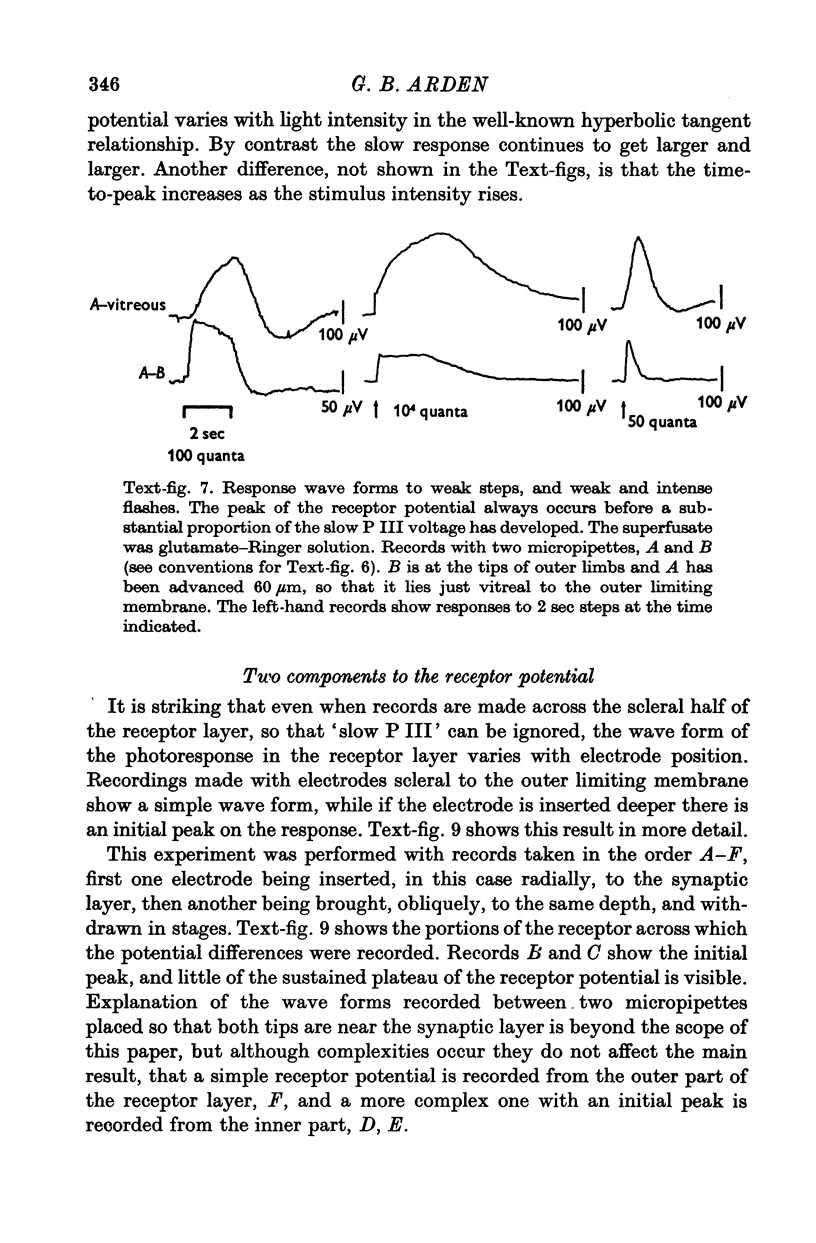
4. The photoresponse developed across the inner retinal layers, slow P III, develops slowly and the peak voltage is approximately proportional to log. flash energy.
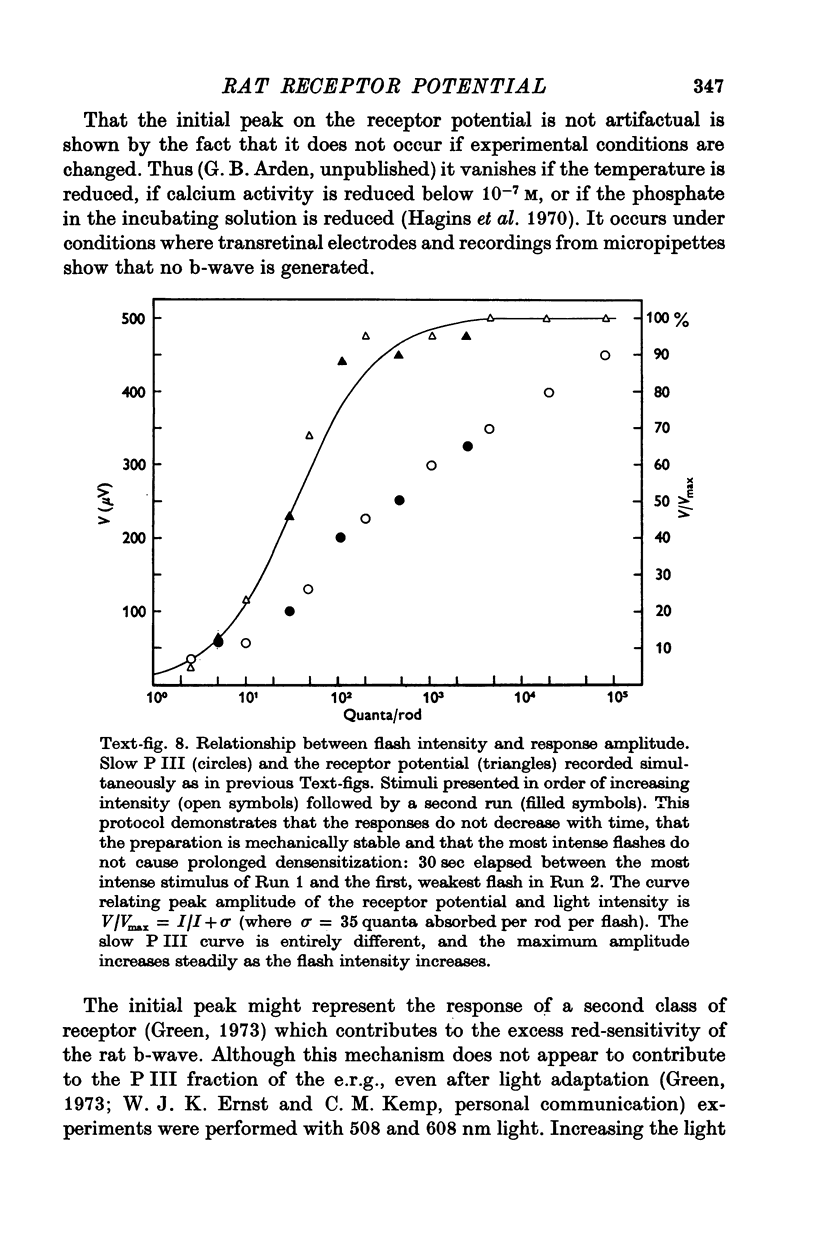
5. The photovoltage across the receptor layer rises rapidly to its peak, before a significant fraction of slow P III has developed.
6. The faster photovoltage (receptor potential) increases with flash intensity according to the hyperbolic function characteristic of photo-receptors.
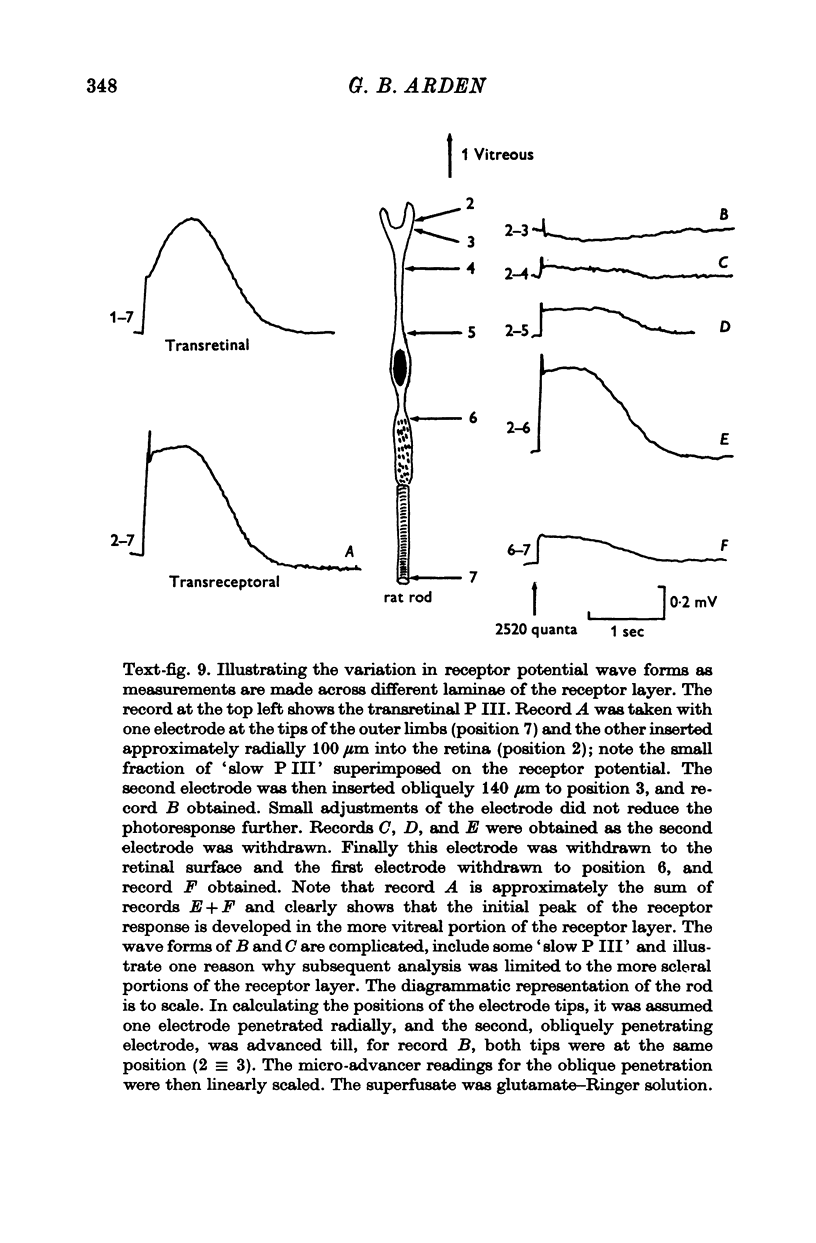
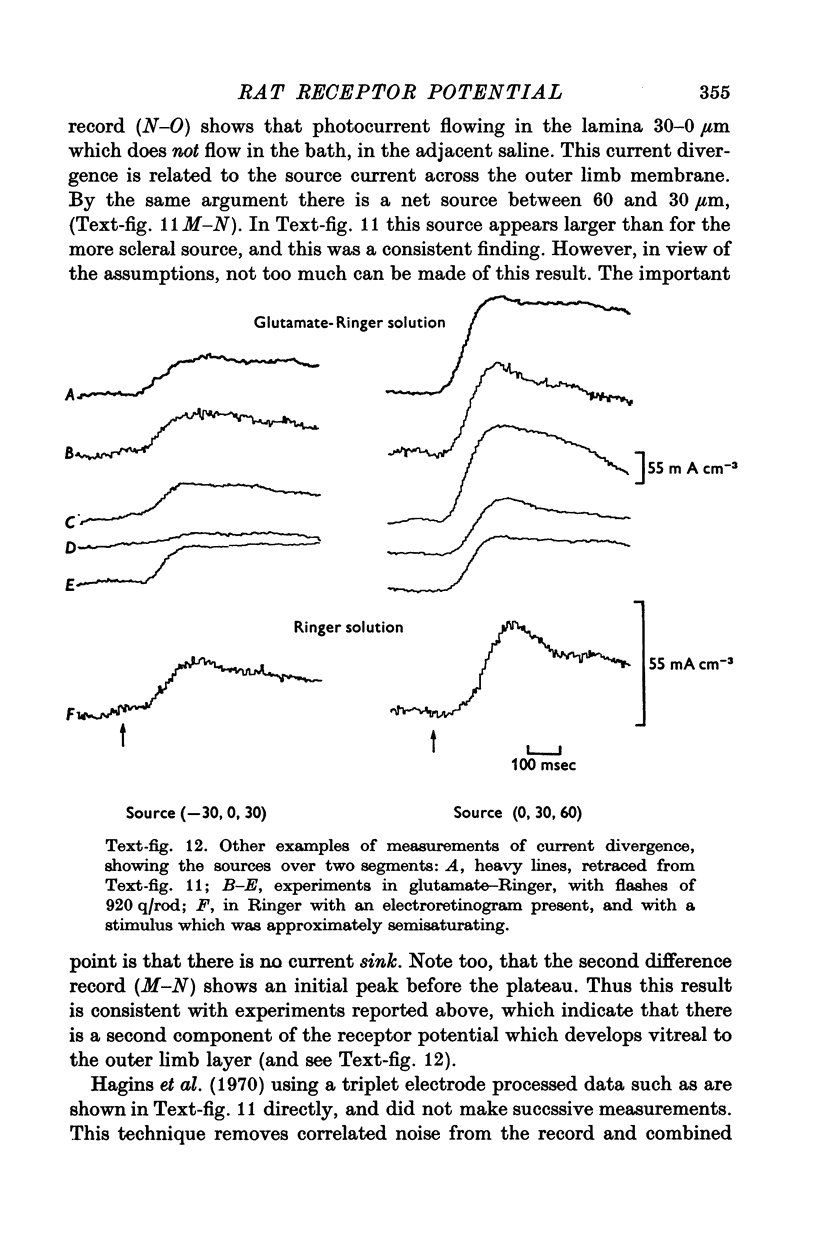
7. The faster photovoltage can be split into two components. Between the tips of the outer limbs and the bases of the inner limbs, it has a simple wave form. In the region between the bases of the inner limbs and the receptor synapses, there is an additional peak (nose) to the photovoltage.
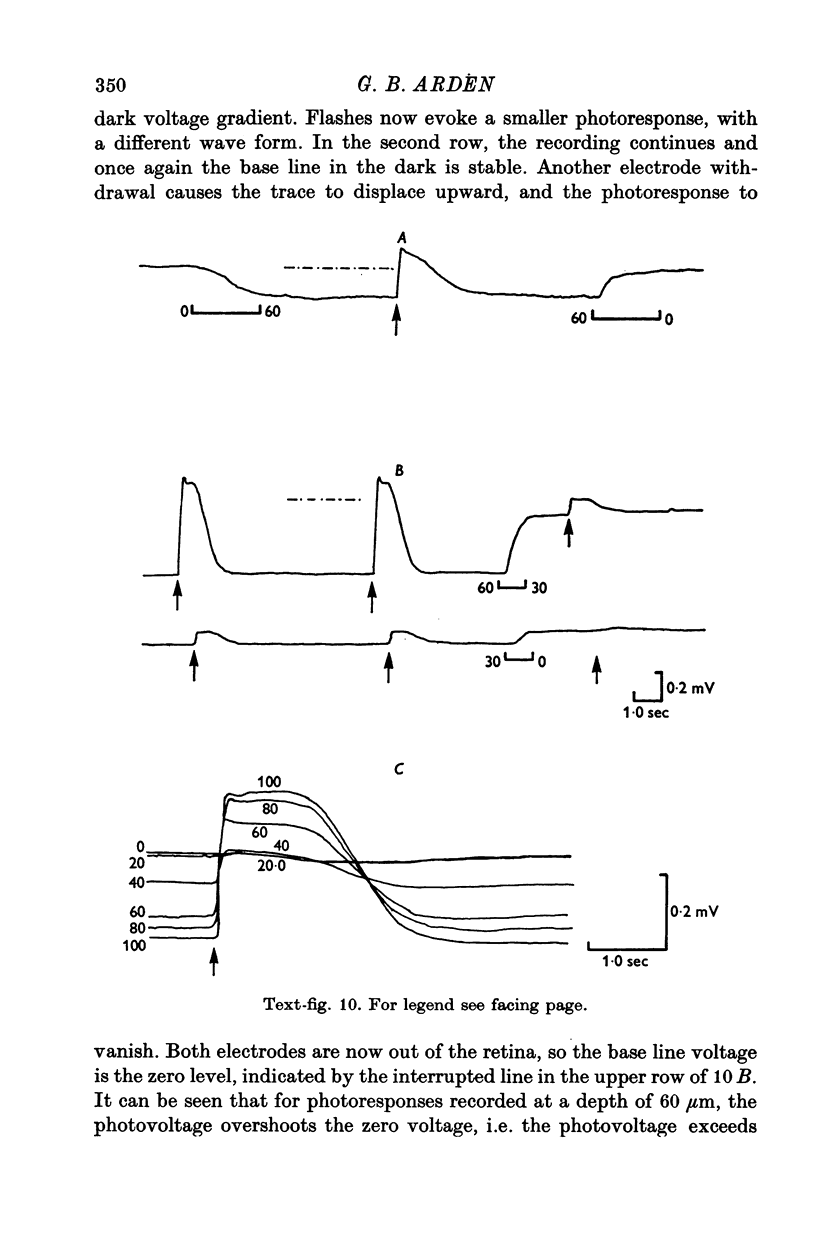
8. In the scleral portion of the receptor layer, the photovoltage approximately equals the dark voltage. In the remaining, vitreal portion of the receptor layer the photovoltage exceeds the dark voltage.
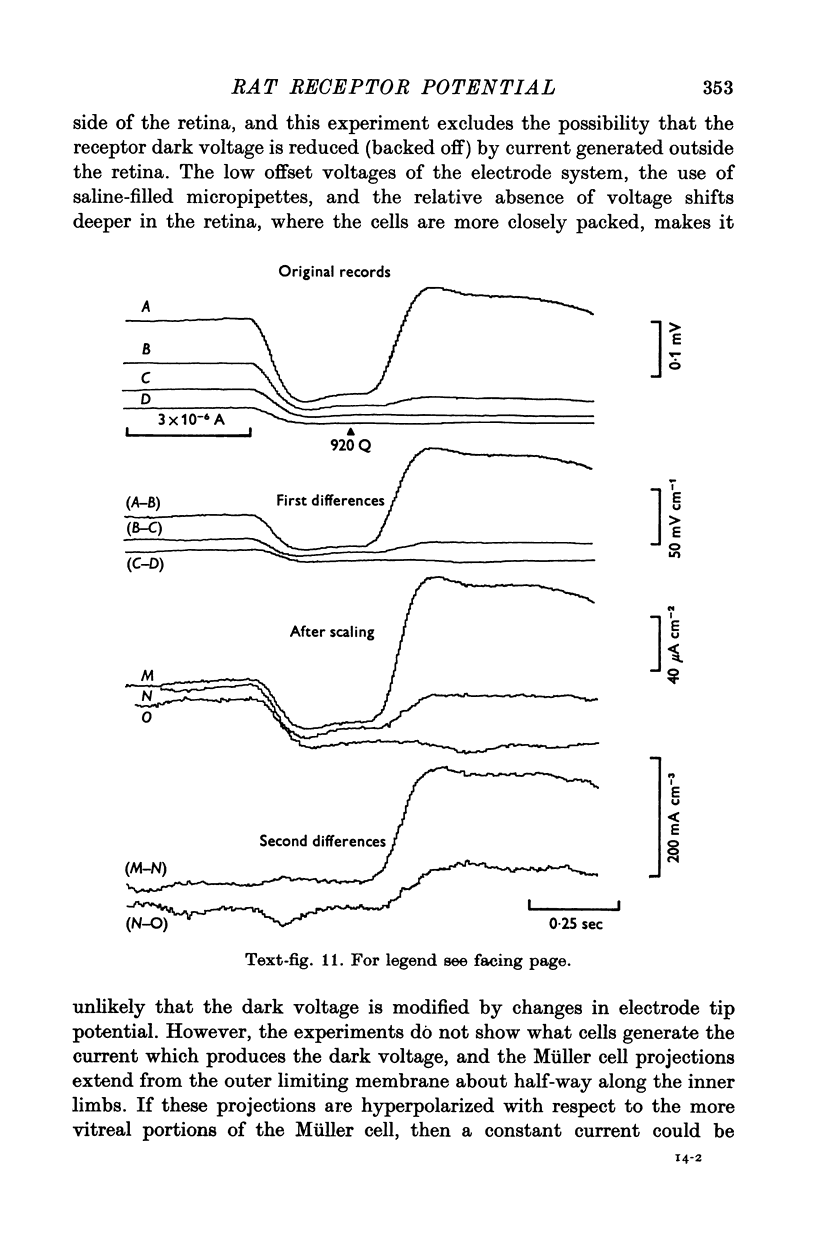
9. Photocurrent divergence has been measured and the results indicate that the source of photocurrent extends further vitreally than the base of the outer limb.
10. The results suggest that the photoresponse generated in the outer limb is modified by an active process which occurs in portions of the rods which are nearer the synapse.
Full text
PDF

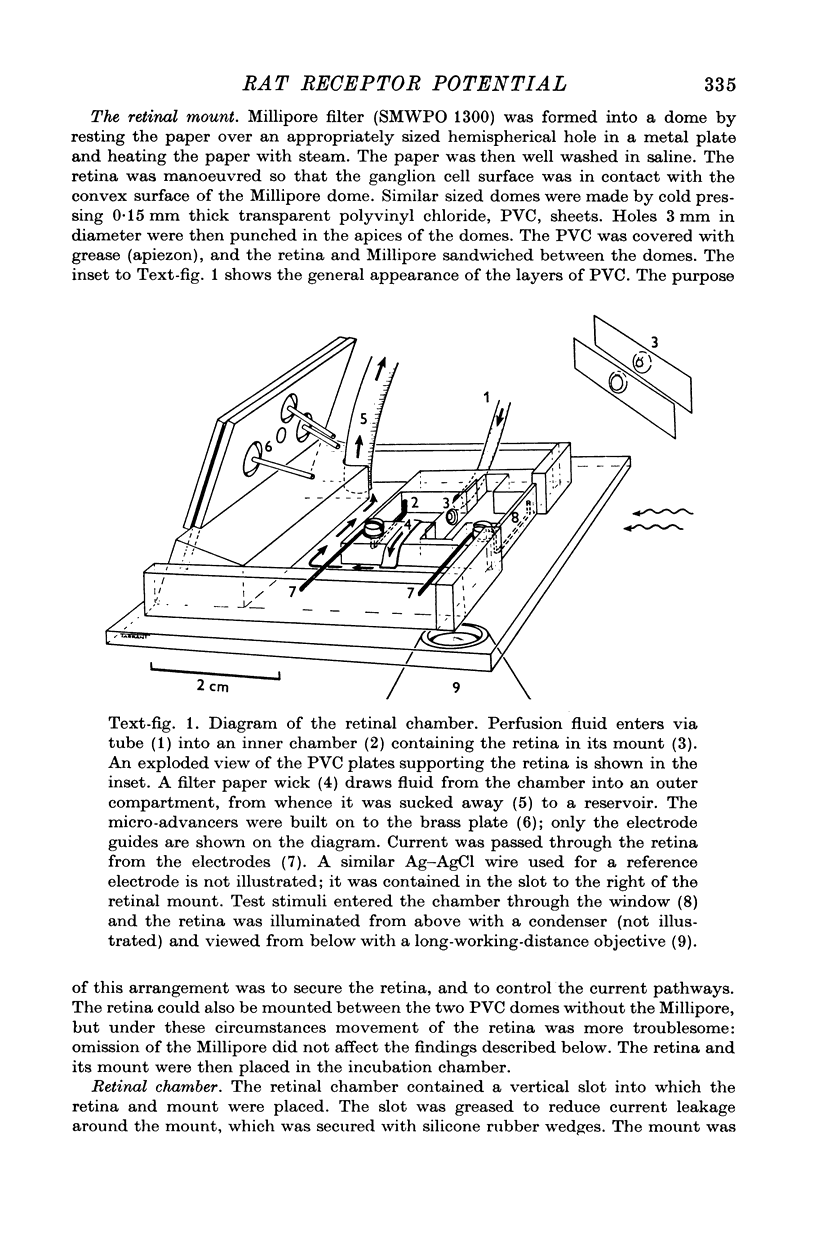



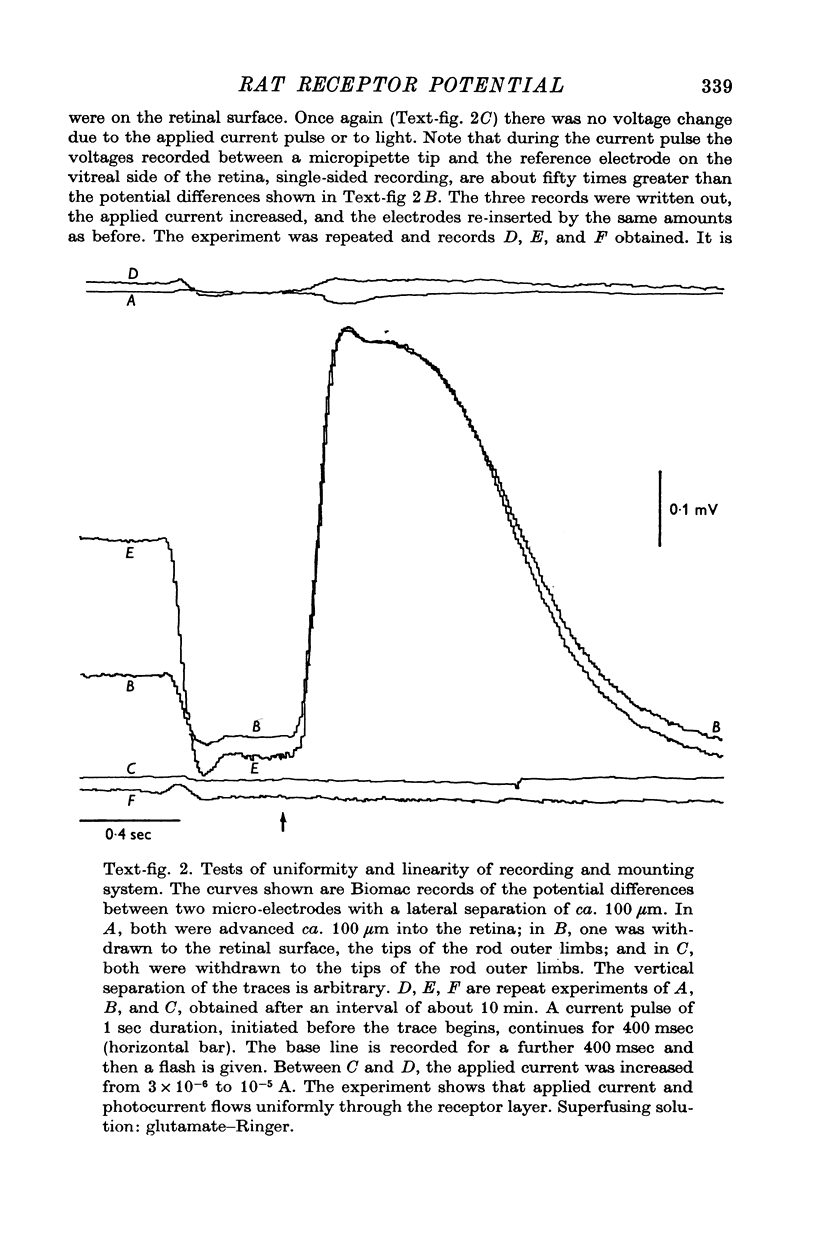

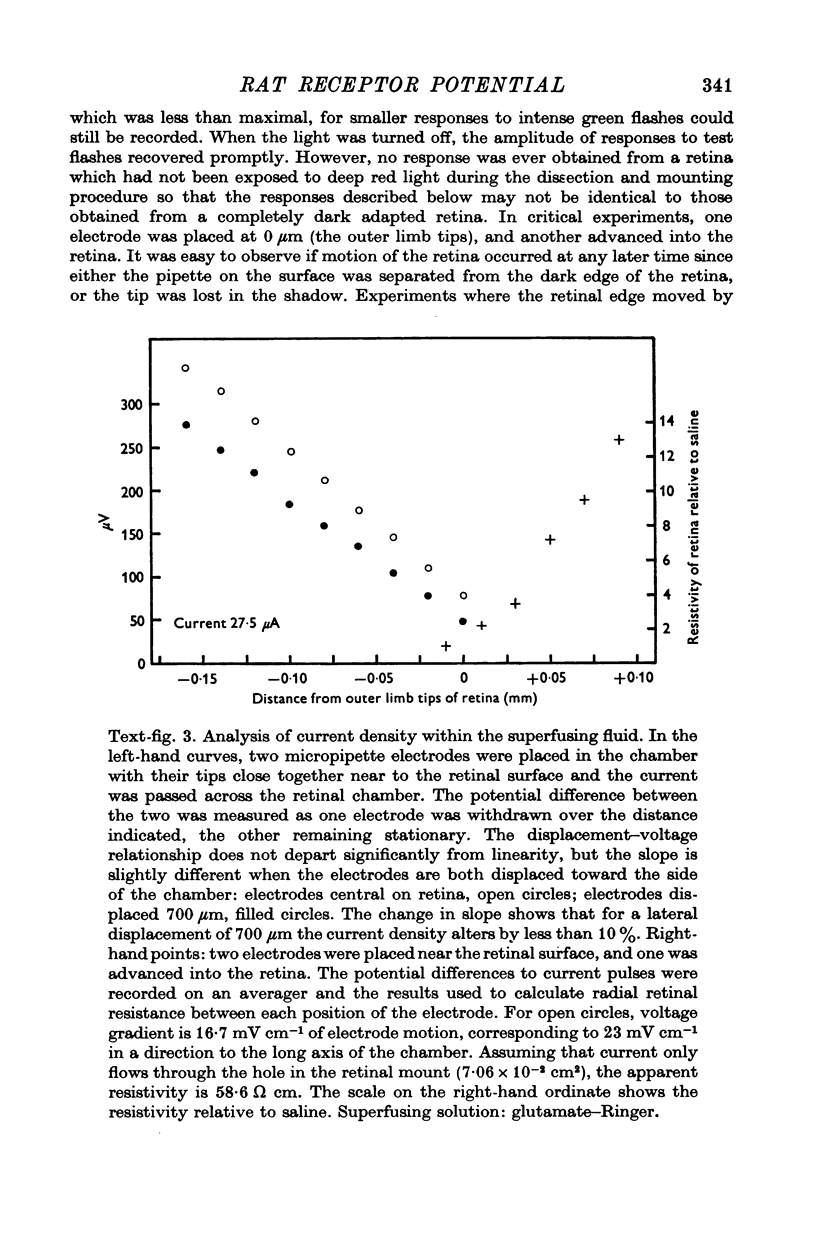










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden G. B., Ernst W. A comparison of the behaviour to ions of the P3 component of the pigeon cone and rat rod electroretinogram. J Physiol. 1972 Jan;220(2):479–497. doi: 10.1113/jphysiol.1972.sp009717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden G. B., Ernst W. The effect of ions on the photoresponses of pigeon cones. J Physiol. 1970 Dec;211(2):311–339. doi: 10.1113/jphysiol.1970.sp009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN K. T. OPTICAL STIMULATOR, MICROELECTRODE ADVANCER, AND ASSOCIATED EQUIPMENT FOR INTRARETINAL NEUROPHYSIOLOGY IN CLOSED MAMMALIAN EYES. J Opt Soc Am. 1964 Jan;54:101–109. doi: 10.1364/josa.54.000101. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., MacNichol E. F., Jr Inactivation of horizontal cells in turtle retina by glutamate and aspartate. Science. 1972 Nov 17;178(4062):767–768. doi: 10.1126/science.178.4062.767. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Visual adaptation in the retina of the skate. J Gen Physiol. 1970 Oct;56(4):491–520. doi: 10.1085/jgp.56.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst W., Arden G. B. Separation of two P3 components in the rat electroretinogram by a flicker method. Vision Res. 1972 Oct;12(10):1759–1761. doi: 10.1016/0042-6989(72)90047-8. [DOI] [PubMed] [Google Scholar]
- Ernst W., Jagger W. S., Baumann C. Extracellular currents from frog photoreceptors. Nature. 1974 Mar 15;248(445):253–255. doi: 10.1038/248253a0. [DOI] [PubMed] [Google Scholar]
- Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol. 1933 Feb 8;77(3):207–239. doi: 10.1113/jphysiol.1933.sp002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G. Scotopic and photopic components of the rat electroetinogram. J Physiol. 1973 Feb;228(3):781–797. doi: 10.1113/jphysiol.1973.sp010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Proceedings: A role for Ca2+ in excitation of retinal rods and cones. Exp Eye Res. 1974 Mar;18(3):299–305. doi: 10.1016/0014-4835(74)90157-2. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Recording site of the single cone response determined by an electrode marking technique. Vision Res. 1967 Nov;7(11):847–851. doi: 10.1016/0042-6989(67)90005-3. [DOI] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Otsuka T., Shimazaki H. Effects of aspartate and glutamate on the bipolar cells in the carp retina. Vision Res. 1975 Mar;15(3):456–458. doi: 10.1016/0042-6989(75)90101-7. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969 Jul 12;223(5202):201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vision Res. 1969 Dec;9(12):1435–1442. doi: 10.1016/0042-6989(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Negishi K. Effects of some amino acids on light-induced responses in the isolated carp retina. Vision Res. 1973 Dec;13(12):2479–2489. doi: 10.1016/0042-6989(73)90245-9. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical activity of vertebrate photoreceptors. Q Rev Biophys. 1970 May;3(2):179–222. doi: 10.1017/s0033583500004571. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler B. S. The electroretinogram of the isolated rat retina. Vision Res. 1972 Jun;12(6):1183–1198. doi: 10.1016/0042-6989(72)90106-x. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dudek F. E., Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol. 1975 Feb;65(2):119–134. doi: 10.1085/jgp.65.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]