Abstract
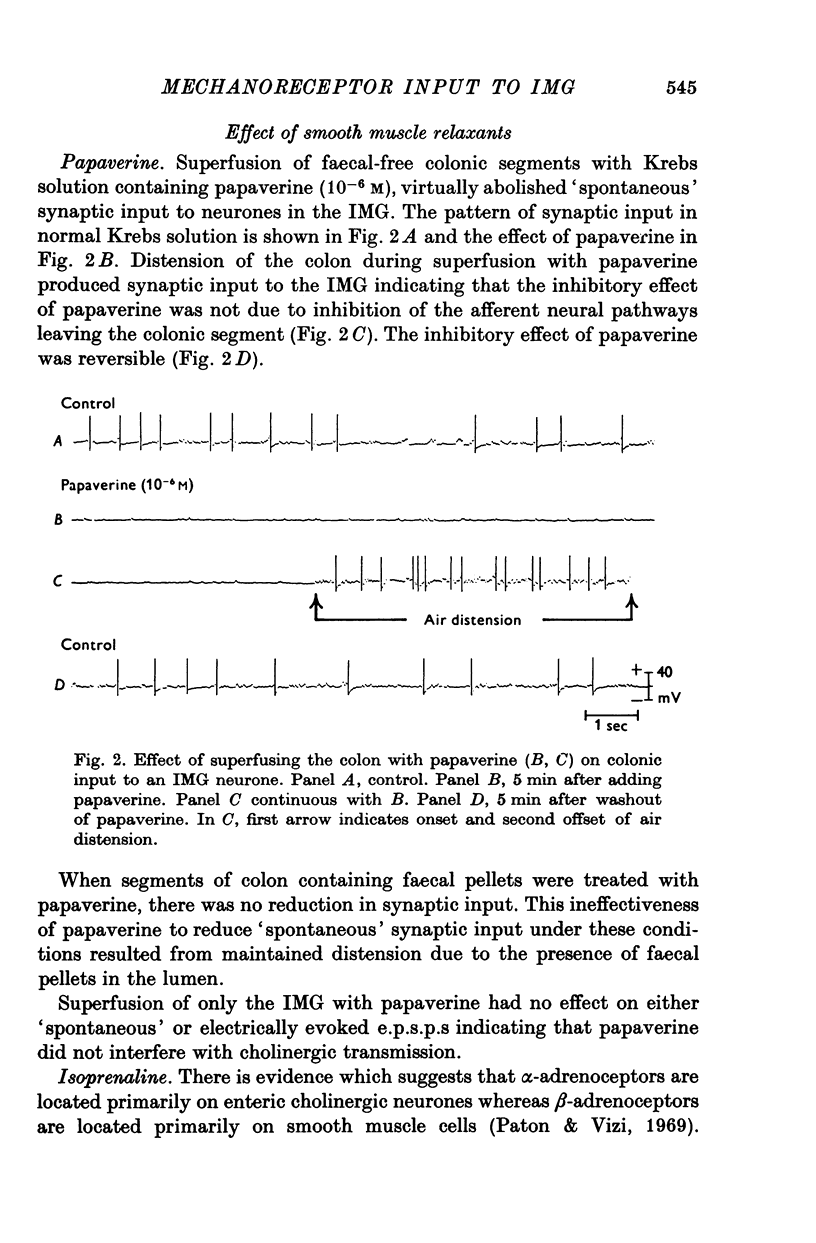
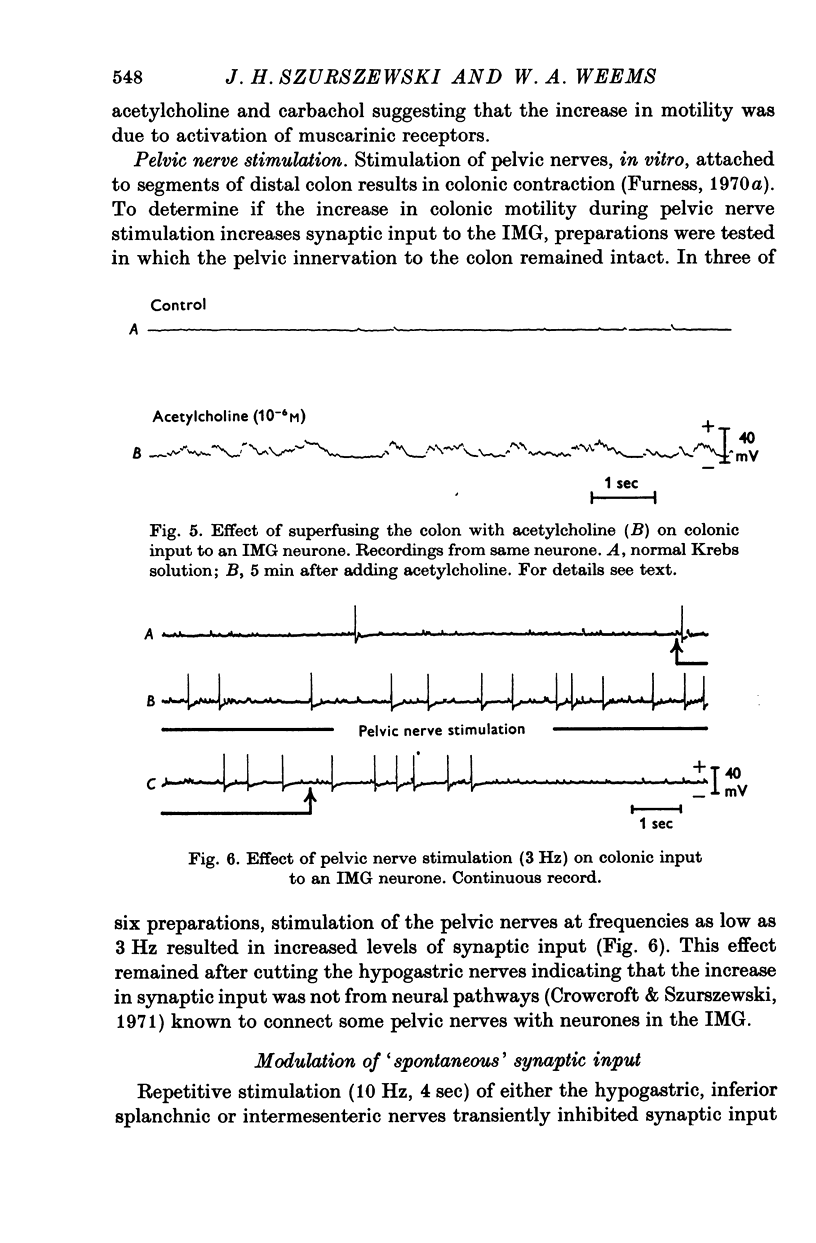
1. Intracellular recordings were made, in vitro, from neurones of guinea-pig inferior mesenteric ganglia (IMG) attached, via the lumbar colonic nerves, to segments of distal colon. 2. 'Spontaneous' synaptic input from colonic afferent fibres was observed in 79% of the neurones tested. In any given preparation, the level and pattern of this synaptic input to different neurones varied considerably. 3. Superfusion of colonic segments with drugs (papaverine, isoprenaline, and adenosine triphosphate) which reduce colonic motility decreased colonic afferent input to IMG neurones. 4. Superfusion of colonic segments with acetylcholine or stimulation of pelvic nerves, both of which increase colonic motility, increased colonic afferent input to IMG neurones. 5. Superfusion of colonic segments with either atropine or tubocurarine reduced the level of 'spontaneous', colonic afferent input. However, distension of these relaxed segments increased the colonic afferent input. 6. Repetitive stimulation of preganglionic inputs to the IMG inhibited afferent input from drug relaxed segments of colon that were moderately distended by the injection of air into the lumen. Superfusion of the colon with phentolamine blocked this inhibition. 7. The results of this study suggest that IMG neurones receive afferent input from mechanoreceptors located in the distal colon and that the mechanosensitivity of this afferent pathway is in part controlled by efferent noradrenergic neurones of the IMG. The IMG-colon neural circuitry can therefore be considered to form a feed-back control system which participates in the regulation of colonic motility.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTAMIRANO-ORREGO R., LOEWENSTEIN W. R. Enhancement of activity in a Pacinian corpuscle by sympathomimetic agents. Nature. 1956 Dec 8;178(4545):1292–1293. doi: 10.1038/1781292a0. [DOI] [PubMed] [Google Scholar]
- Axelsson J., Holmberg B. The effects of extracellularly applied ATP and related compounds on electrical and mechanical activity of the smooth muscle taenia coli from the guinea-pig. Acta Physiol Scand. 1969 Jan-Feb;75(1):149–156. doi: 10.1111/j.1748-1716.1969.tb04366.x. [DOI] [PubMed] [Google Scholar]
- Beani L., Bianchi C., Crema A. The effect of catecholamines and sympathetic stimulation on the release of acetylcholine from the guinea-pig colon. Br J Pharmacol. 1969 May;36(1):1–17. doi: 10.1111/j.1476-5381.1969.tb08298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- CHERNETSKI K. E. SYMPATHETIC ENHANCEMENT OF PERIPHERAL SENSORY INPUT IN THE FROG. J Neurophysiol. 1964 May;27:493–515. doi: 10.1152/jn.1964.27.3.493. [DOI] [PubMed] [Google Scholar]
- Cantino D., Mugnaini E. Adrenergic innervation of the parasympathetic ciliary ganglion in the chick. Science. 1974 Jul 19;185(4147):279–281. doi: 10.1126/science.185.4147.279. [DOI] [PubMed] [Google Scholar]
- Crowcroft P. J., Holman M. E., Szurszewski J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol. 1971 Dec;219(2):443–461. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. An electrophysiological study of the innervation of the smooth muscle of the colon. J Physiol. 1969 Dec;205(3):549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. An examination of nerve-mediated, hyoscine-resistant excitation of the guinea-pig colon. J Physiol. 1970 May;207(3):803–821. doi: 10.1113/jphysiol.1970.sp009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. The origin and distribution of adrenergic nerve fibres in the guinea-pig colon. Histochemie. 1970;21(4):295–306. doi: 10.1007/BF00280899. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., McKirdy H. C. A nervous mechanism for descending inhibition in guinea-pig small intestine. J Physiol. 1974 Apr;238(1):129–143. doi: 10.1113/jphysiol.1974.sp010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955 Jun 28;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek B. F. Reticulo-ruminal mechanoreceptors in sheep. J Physiol. 1969 Jun;202(3):585–609. doi: 10.1113/jphysiol.1969.sp008829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Prosser C. L. Electrical activity in myenteric and submucous plexuses of cat intestine. Am J Physiol. 1972 Jun;222(6):1412–1419. doi: 10.1152/ajplegacy.1972.222.6.1412. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell D. G., Burnstock G. Quantitative studies of the release of purine compounds following stimulation of non-adrenergic inhibitory nerves in the stomach. Biochem Pharmacol. 1971 Jul;20(7):1694–1697. doi: 10.1016/0006-2952(71)90299-1. [DOI] [PubMed] [Google Scholar]
- Schiff J. D. Role of the sympathetic innervation of the pacinian corpuscle. J Gen Physiol. 1974 May;63(5):601–608. doi: 10.1085/jgp.63.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Bevan J. A., Burnstock G. [3H]adenosine triphosphate: release during stimulation of enteric nerves. Science. 1971 Jul 23;173(3994):336–338. doi: 10.1126/science.173.3994.336. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Electrical activity from single neurons in Auerbach's plexus. Am J Physiol. 1970 Jul;219(1):159–169. doi: 10.1152/ajplegacy.1970.219.1.159. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Electrical discharge of single enteric neurons of guinea pig small intestine. Am J Physiol. 1973 Nov;225(5):1107–1113. doi: 10.1152/ajplegacy.1973.225.5.1107. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Patterned discharge of six different neurons in a single enteric ganglion. Pflugers Arch. 1973 Feb 6;338(3):247–256. doi: 10.1007/BF00587390. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Neurophysiology of Auerbach's plexus and control of intestinal motility. Physiol Rev. 1975 Apr;55(2):307–324. doi: 10.1152/physrev.1975.55.2.307. [DOI] [PubMed] [Google Scholar]


