Abstract
1. The effects of acute changes in plasma Na concentration (PNa) on renal blood flow (RBF) and glomerular filtration rate (GFR) were studied in anaesthetized greyhounds. Saline was infused at a constant rate (0·1 ml. kg-1 min-1) either into a renal artery or into a systemic vein. Plasma Na concentration was altered by varying the Na concentration of the infused saline from 0·154 to 0·077, 0·616 or 1·232 M.
2. Blood pressure (B.P.), packed cell volume (PCV), concentration of plasma solids (PS) and the plasma concentration of H+ and K (PK) ions were measured but no attempt was made to contain their fluctuation.
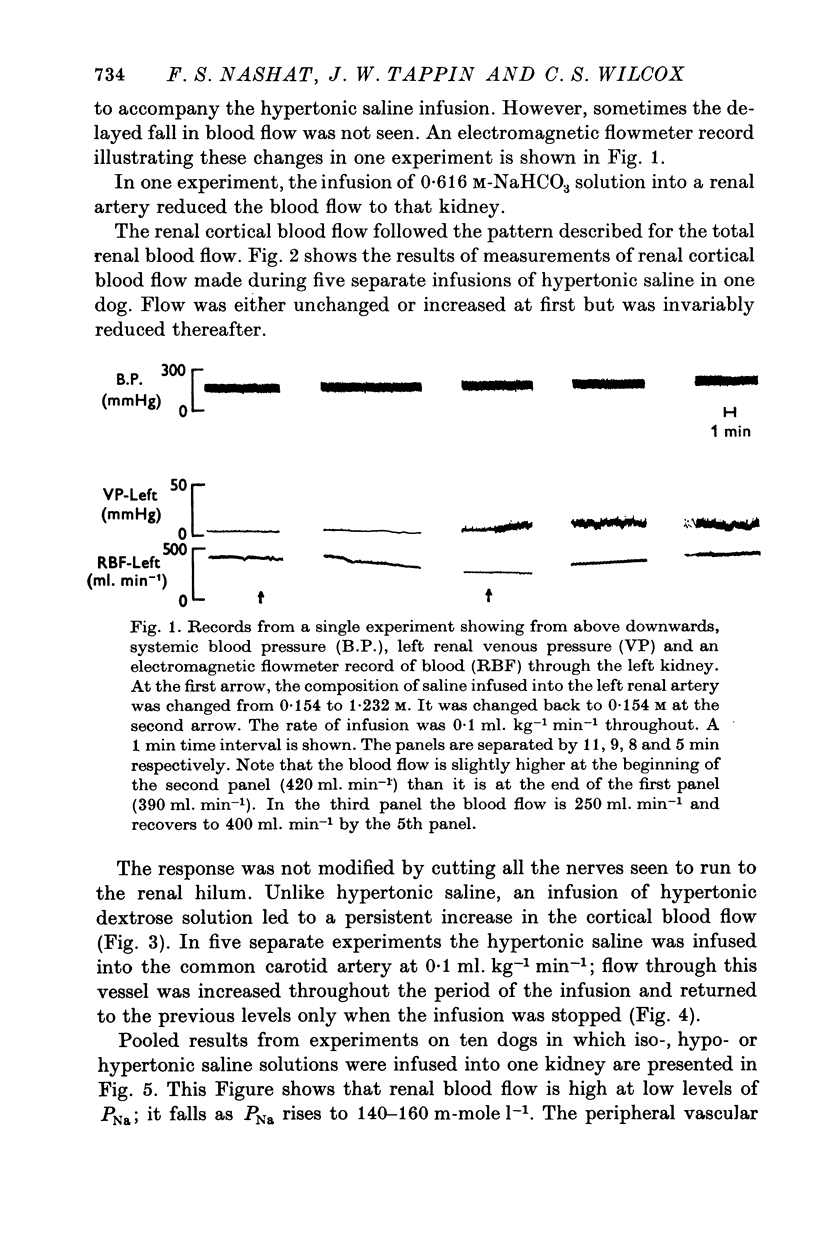
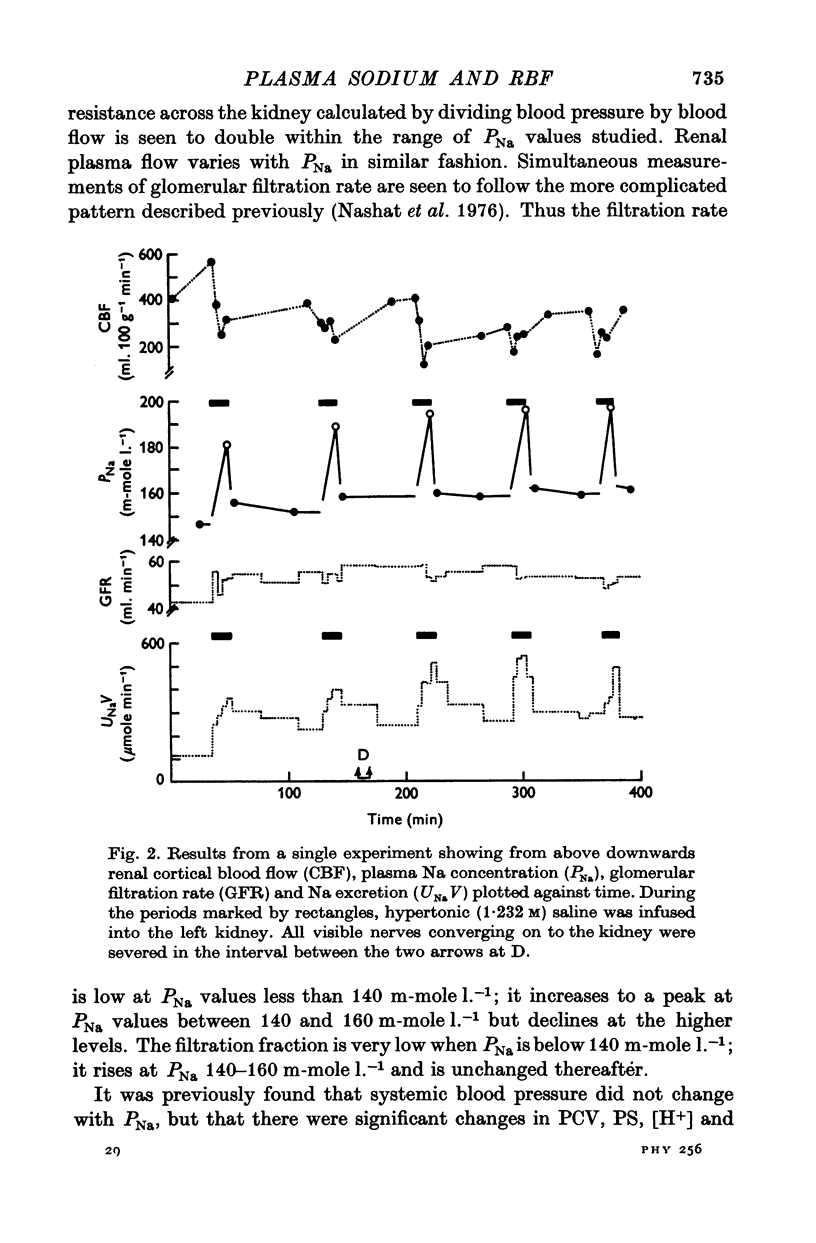
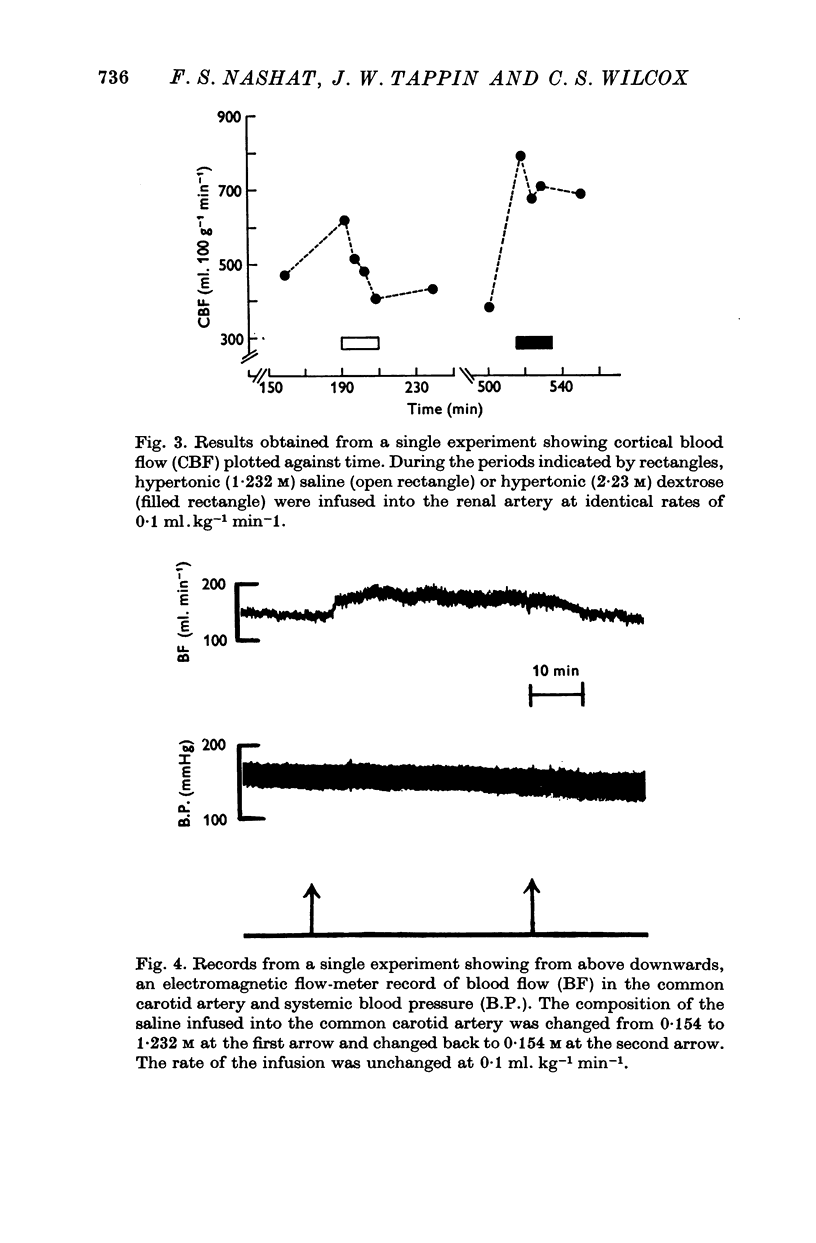
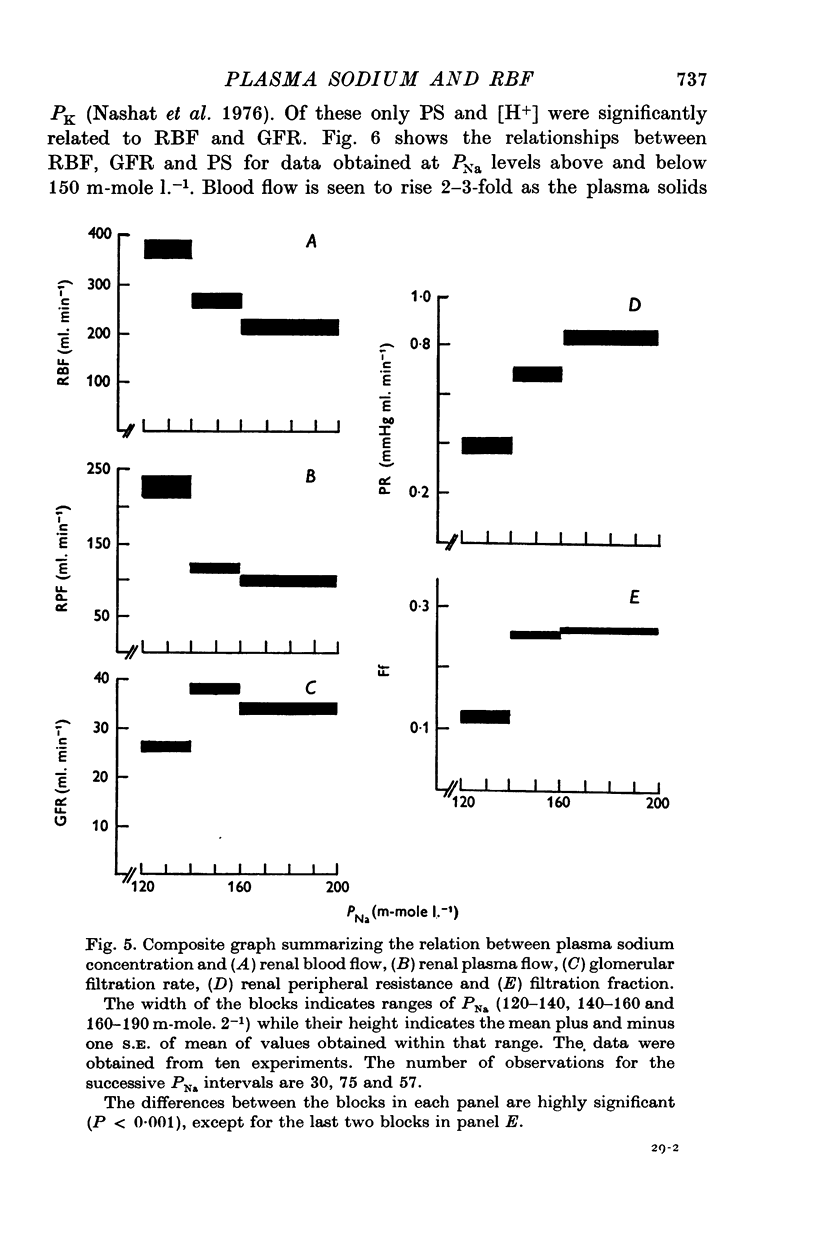
3. An infusion of hypertonic saline into a renal artery usually led to an ipsilateral increase in RBF for 5-15 min, followed by a progressive fall. Over-all, mean values of RBF fell with PNa throughout the range studied (120-190 m-mole l.-1). Glomerular filtration rate rose with PNa to reach maximal values at PNa levels of 140-160 m-mole l.-1, but fell thereafter. The combined fall in RBF and GFR, without change in filtration fraction, at PNa values above 160 m-mole l.-1 is consistent with an alteration in afferent arteriolar resistance. The fall in GFR despite a rise in RBF noted when PNa was reduced below 140 m-mole l.-1 requires an additional explanation.
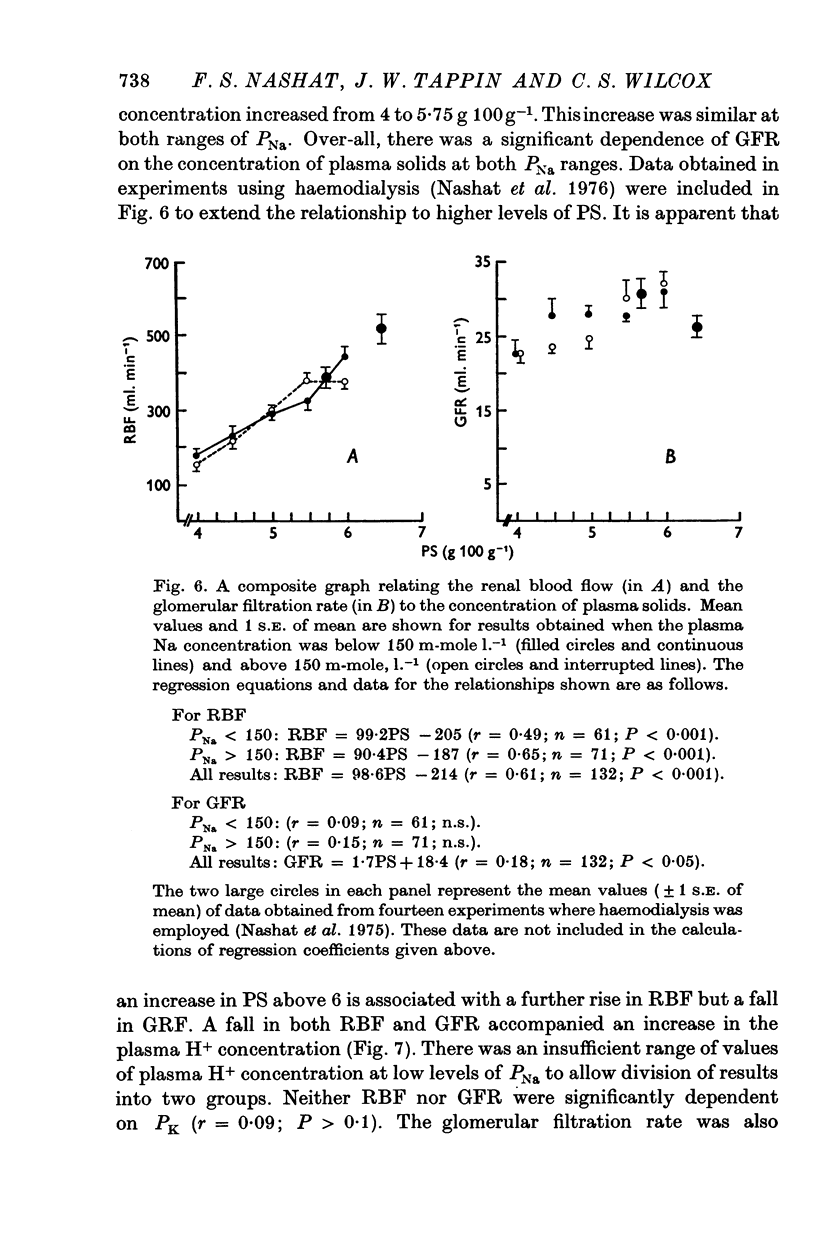
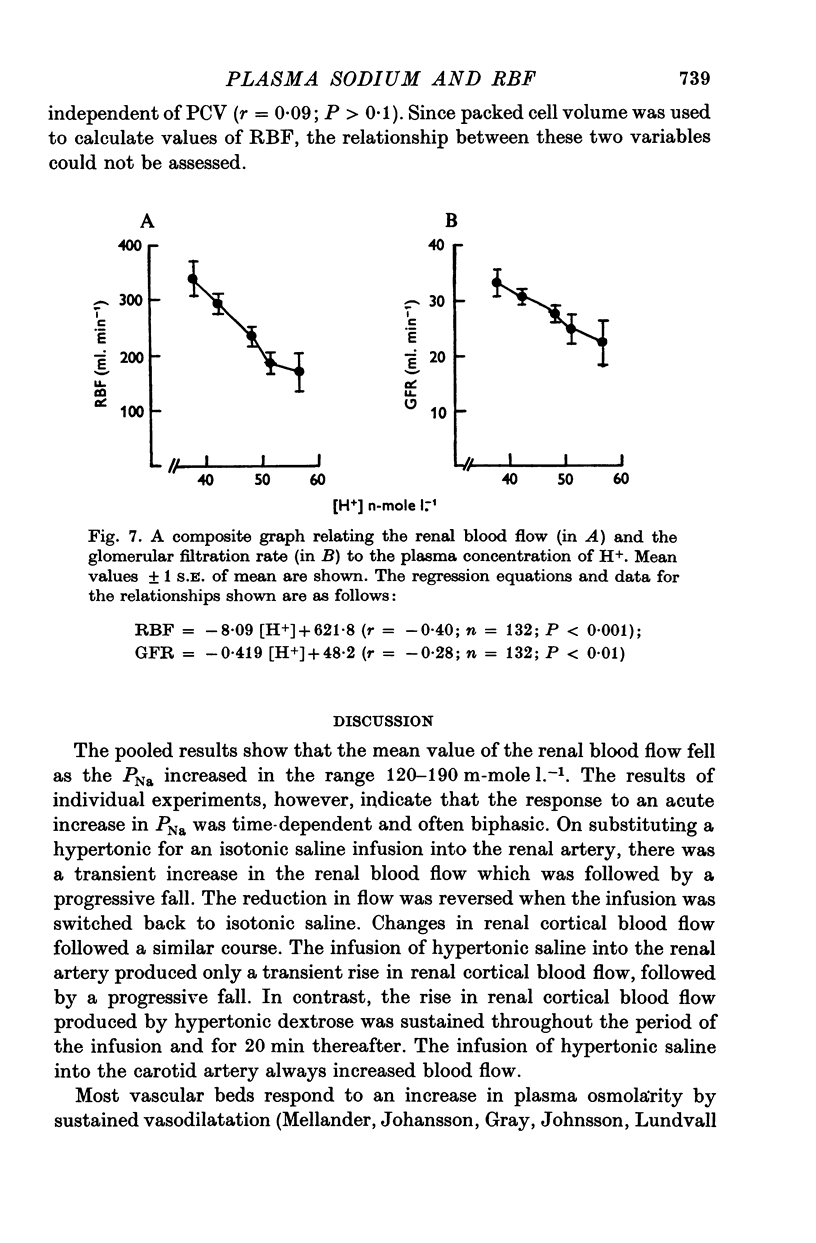
4. Renal blood flow was independent of PK; it was inversely related to [H+] and directly related to PS. Glomerular filtration rate was independent of PCV and PK. It was also inversely related to [H+] and directly related to PS up to a value of 6 g 100 g-1 plasma, after which the relationship was reversed. These results suggest that the renal vascular responses to acute changes in PNa may be mediated in part, at least, by concurrent change in PS and [H+].
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKER H. G., CLARK J. K. The effect of salt poor human albumin on renal oxygen consumption in man. Am J Med Sci. 1949 Dec;218(6):715–715. [PubMed] [Google Scholar]
- Blantz R. C., Rector F. C., Jr, Seldin D. W. Effect of hyperoncotic albumin expansion upon glomerular ultrafiltration in the rat. Kidney Int. 1974 Oct;6(4):209–221. doi: 10.1038/ki.1974.102. [DOI] [PubMed] [Google Scholar]
- Epstein M., Berk D. P., Hollenberg N. K., Adams D. F., Chalmers T. C., Abrams H. L., Merrill J. P. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970 Aug;49(2):175–185. doi: 10.1016/s0002-9343(70)80073-0. [DOI] [PubMed] [Google Scholar]
- Forgács I., Châtel R., Visy M. The effect of hypertonic sodium chloride infused into the renal artery. Acta Physiol Acad Sci Hung. 1969;35(3):219–229. [PubMed] [Google Scholar]
- GIEBISCH G., KLOSE R. M., WINDHAGER E. E. MICROPUNCTURE STUDY OF HYPERTONIC SODIUM CHLORIDE LOADING IN THE RAT. Am J Physiol. 1964 Apr;206:687–693. doi: 10.1152/ajplegacy.1964.206.4.687. [DOI] [PubMed] [Google Scholar]
- Gazitúa S., Scott J. B., Chou C. C., Haddy F. J. Effect of osmolarity on canine renal vascular resistance. Am J Physiol. 1969 Oct;217(4):1216–1223. doi: 10.1152/ajplegacy.1969.217.4.1216. [DOI] [PubMed] [Google Scholar]
- Kady N. N., Nashat F. S., Tappin J. W., Wilcox C. S. Proceedings: The effect of acute alterations in plasma sodium concentration on glomerular filtration rate and renal plasma flow in anaesthetized dogs. J Physiol. 1974 Jan;236(1):40P–42P. [PubMed] [Google Scholar]
- Klahr S., Alleyne G. A. Effects of chronic protein-calorie malnutrition on the kidney. Kidney Int. 1973 Mar;3(3):129–141. doi: 10.1038/ki.1973.21. [DOI] [PubMed] [Google Scholar]
- Little J. R., Cohen J. J. Effect of albumin concentration on function of isolated perfused rat kidney. Am J Physiol. 1974 Mar;226(3):512–517. doi: 10.1152/ajplegacy.1974.226.3.512. [DOI] [PubMed] [Google Scholar]
- Mellander S., Johansson B., Gray S., Jonsson O., Lundvall J., Ljung B. The effect of hyperosmolarity on intacet and isolated vascular smooth muscle. Possible role in exercise hyperemia. Angiologica. 1967;4(6):310–322. doi: 10.1159/000157715. [DOI] [PubMed] [Google Scholar]
- Nashat F. S., Scholefield F. R., Tappin J. W., Wilcox C. S. The effects of changes in haematocrit on the intrarenal distribution of blood flow in the dog's kidney. J Physiol. 1969 May;201(3):639–655. doi: 10.1113/jphysiol.1969.sp008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashat F. S., Tappin J. W., Wilcox C. S. Plasma sodium concentration and sodium excretion in the anaesthetized dog. J Physiol. 1976 Jan;254(1):183–202. doi: 10.1113/jphysiol.1976.sp011228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar L. G., Baer P. G., Wallace S. L., McDaniel J. K. Reduced intrarenal resistance and autoregulatory capacity after hyperoncotic dextran. Am J Physiol. 1971 Jul;221(1):329–334. doi: 10.1152/ajplegacy.1971.221.1.329. [DOI] [PubMed] [Google Scholar]
- Nizet A. Influence of serumalbumin and dextran on sodium and water excretion by the isolated dog kidney. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(1):7–15. doi: 10.1007/BF00412414. [DOI] [PubMed] [Google Scholar]
- Patek A. J., Mankin H., Colcher H., Lowell A., Earle D. P. THE EFFECTS OF INTRAVENOUS INJECTION OF CONCENTRATED HUMAN SERUM ALBUMIN UPON BLOOD PLASMA, ASCITES AND RENAL FUNCTIONS IN THREE PATIENTS WITH CIRRHOSIS OF THE LIVER. J Clin Invest. 1948 Jan;27(1):135–144. doi: 10.1172/JCI101916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski J. Effects of renal artery infusion of various hypertonic solutions on the renal blood flow and renal handling of PAH in the dog. Pflugers Arch. 1972;334(1):85–102. doi: 10.1007/BF00586003. [DOI] [PubMed] [Google Scholar]
- Sadowski J. Glomerular filtration changes during renal artery infusion of various hypertonic solutions in the dog. Pflugers Arch. 1972;337(1):53–58. doi: 10.1007/BF00587872. [DOI] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J., Wright F. S., Davis J. M., von Stackelberg W., Grill G. Regulation of superficial nephron filtration rate by tubulo-glomerular feedback. Pflugers Arch. 1970;318(2):147–175. doi: 10.1007/BF00586493. [DOI] [PubMed] [Google Scholar]
- Windhager E. E., Lewy J. E., Spitzer A. Intrarenal control of proximal tubular reabsorption of sodium and water. Nephron. 1969;6(3):247–259. doi: 10.1159/000179732. [DOI] [PubMed] [Google Scholar]
- Young D. B., Rostorfer H. H. Blood flow and filtration rate responses to alterations in renal arterial osmolarity. Am J Physiol. 1973 Nov;225(5):1003–1008. doi: 10.1152/ajplegacy.1973.225.5.1003. [DOI] [PubMed] [Google Scholar]


