Abstract
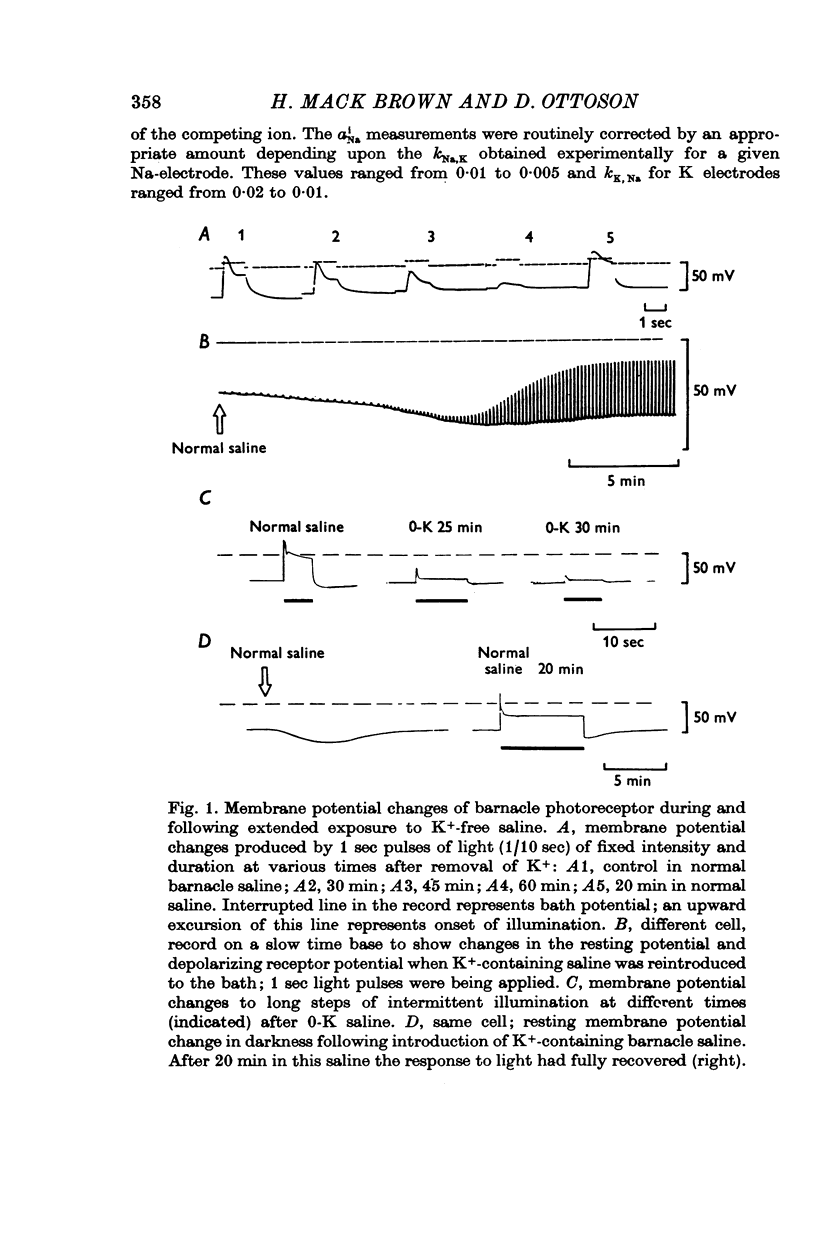
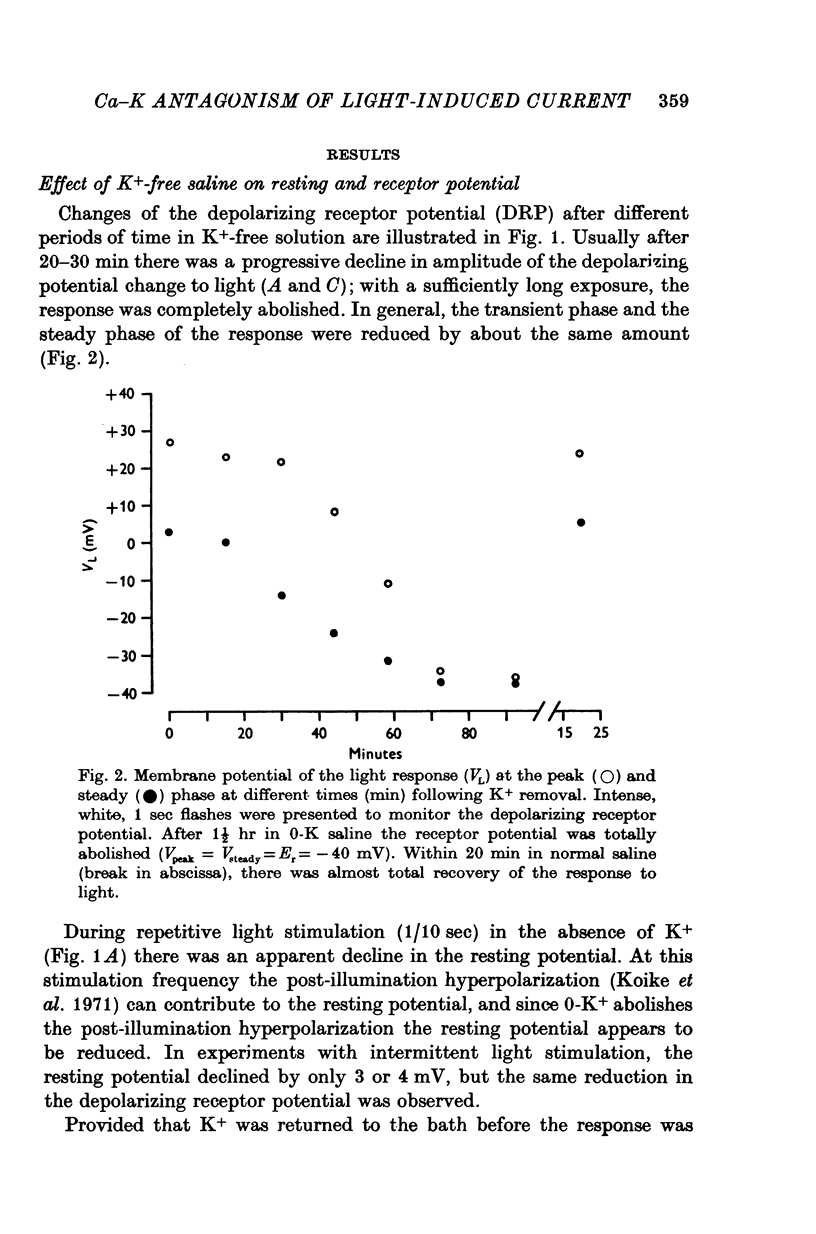
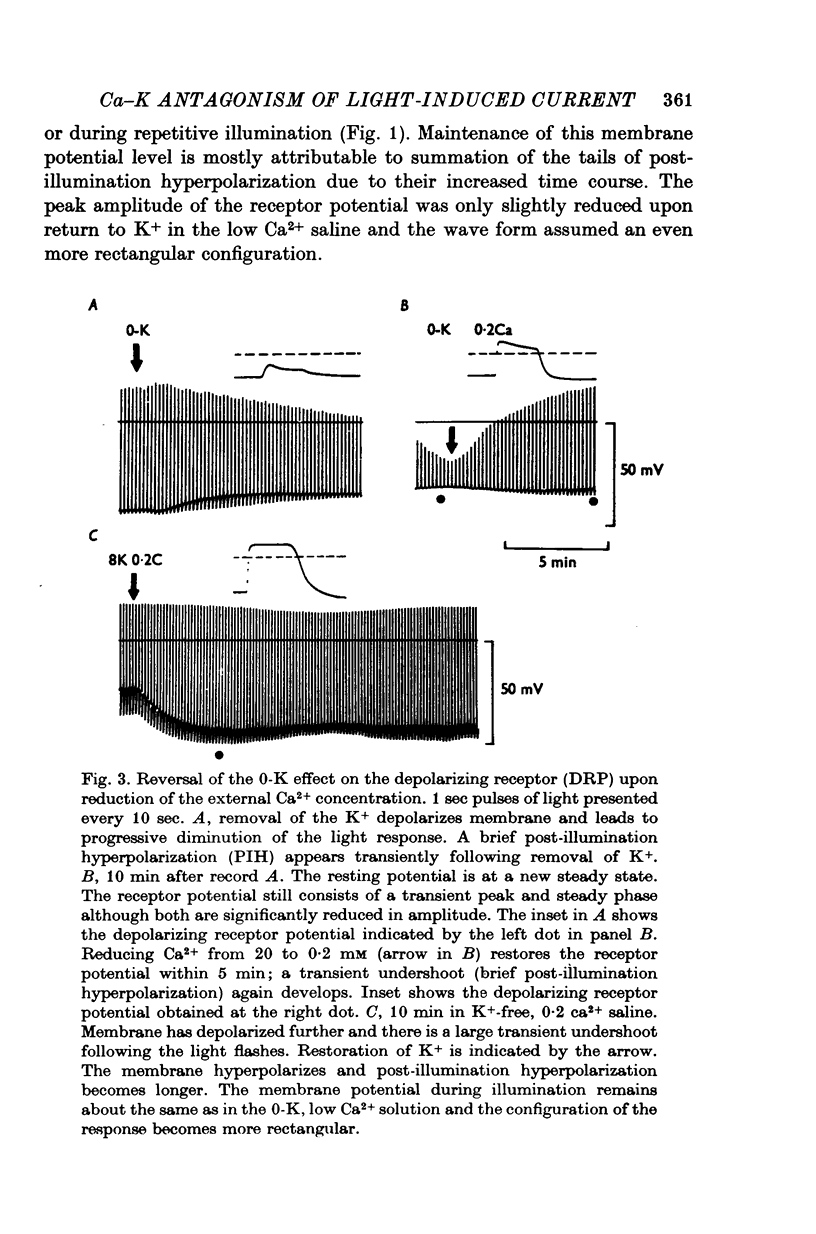
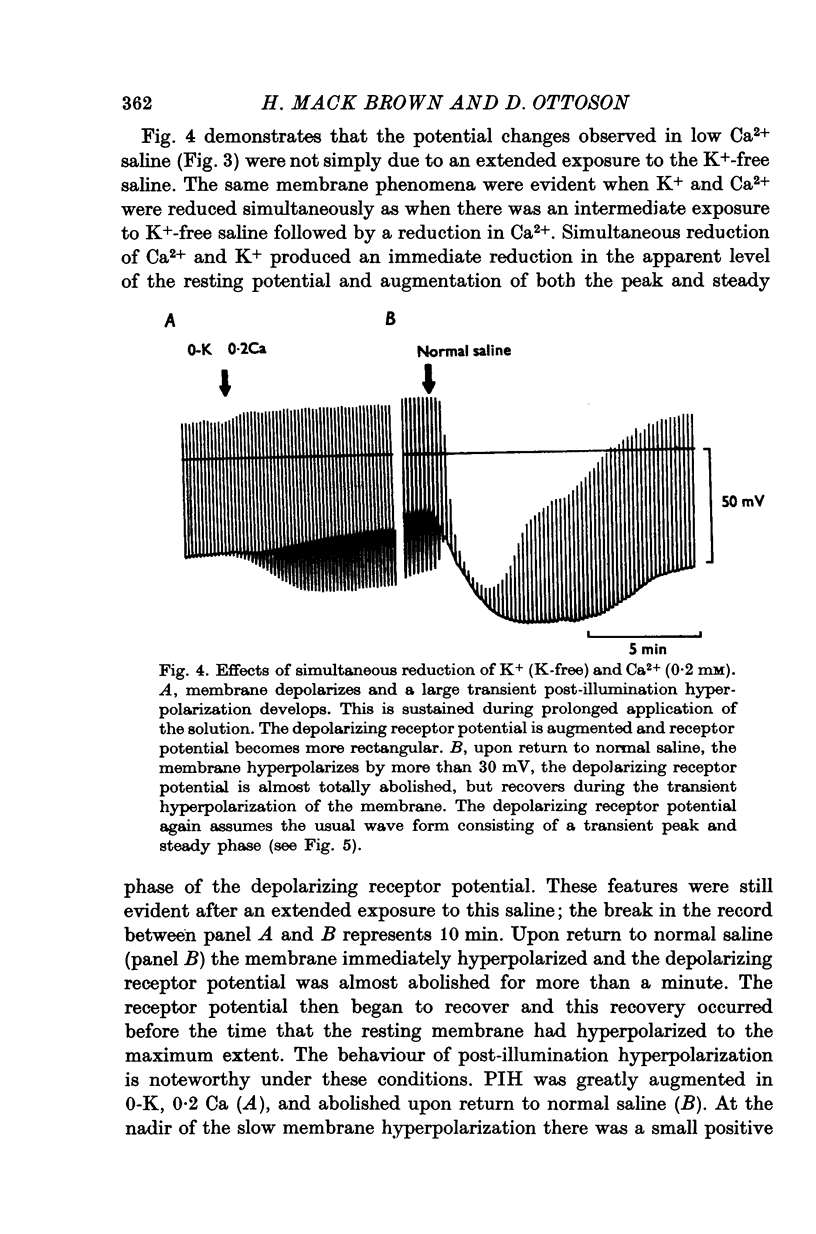
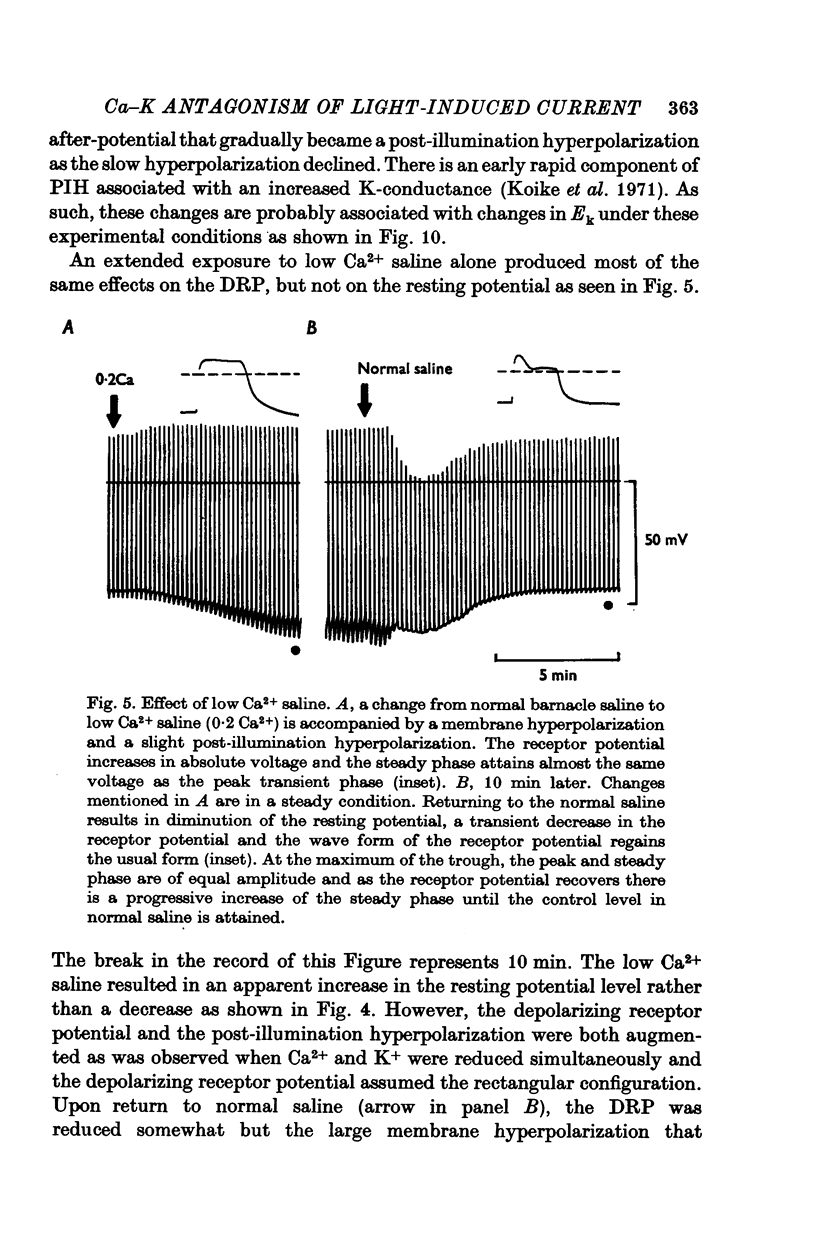
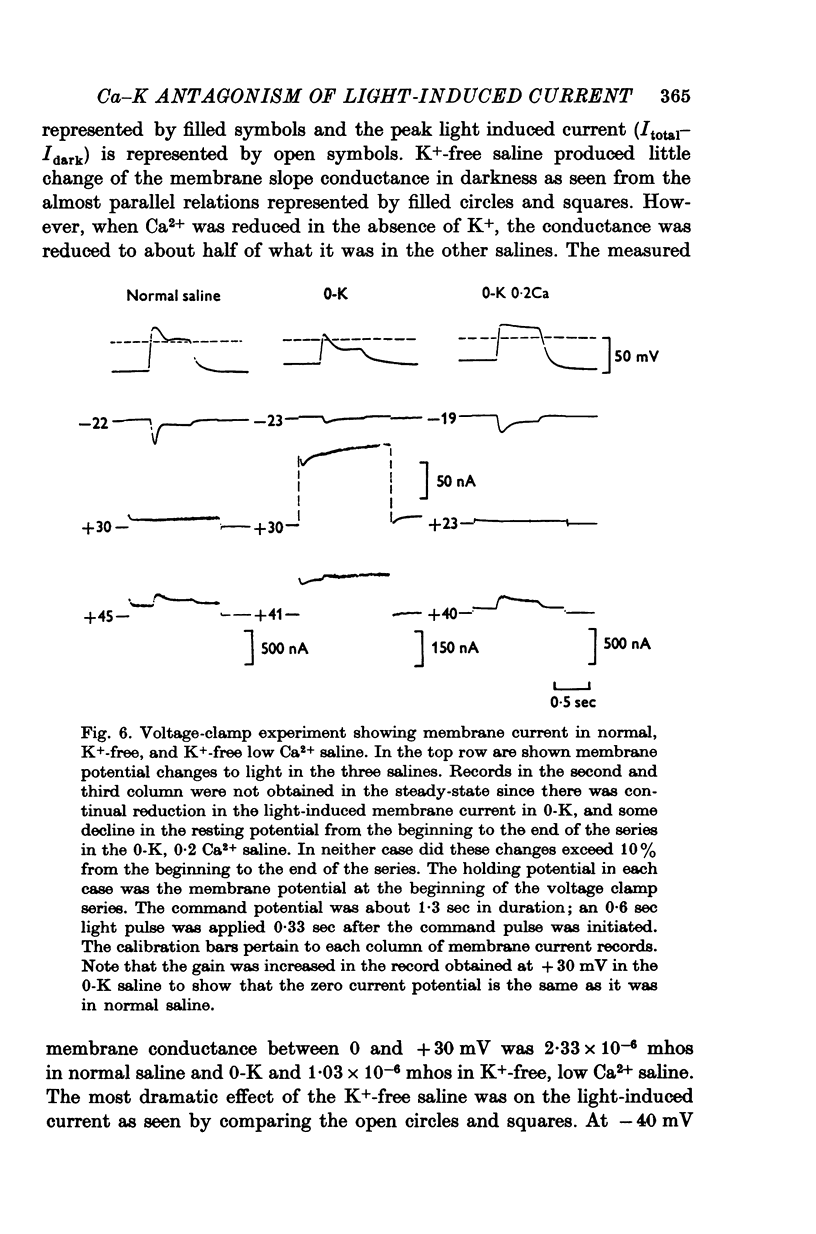
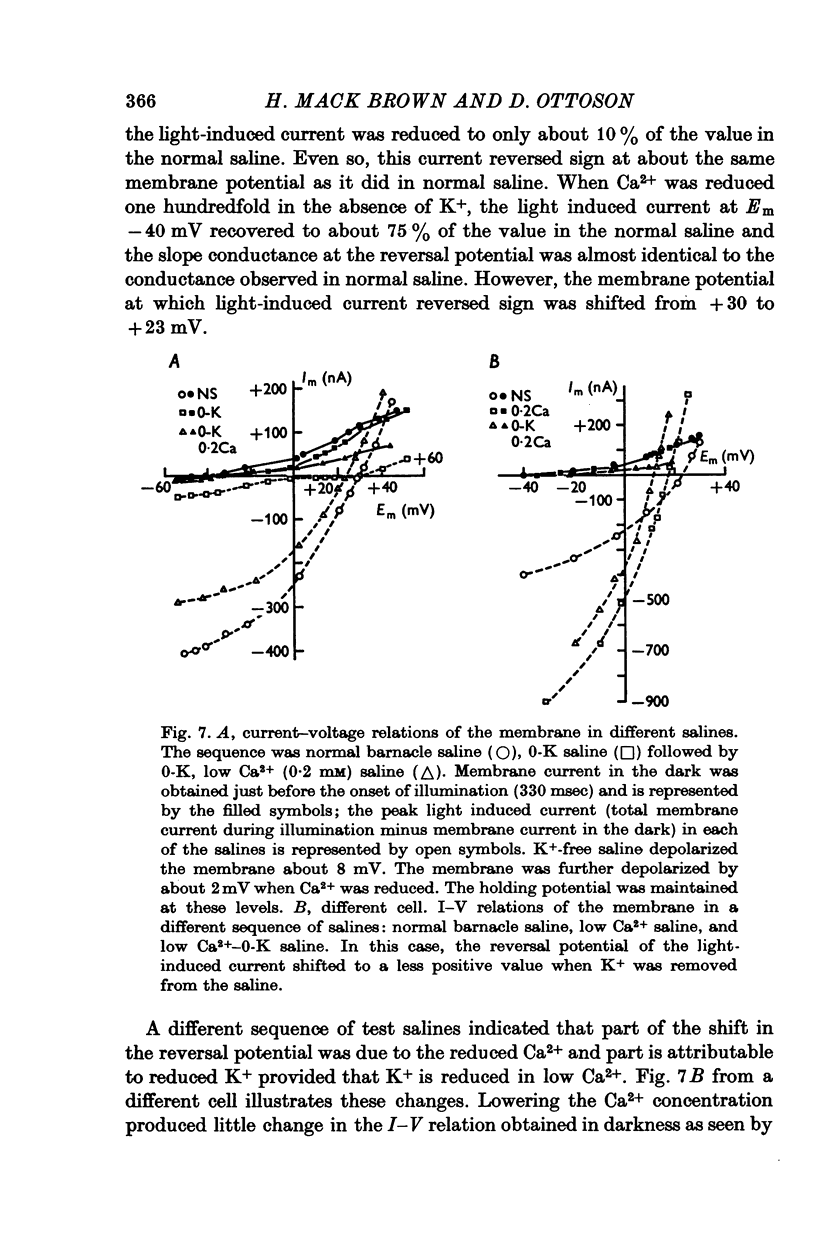
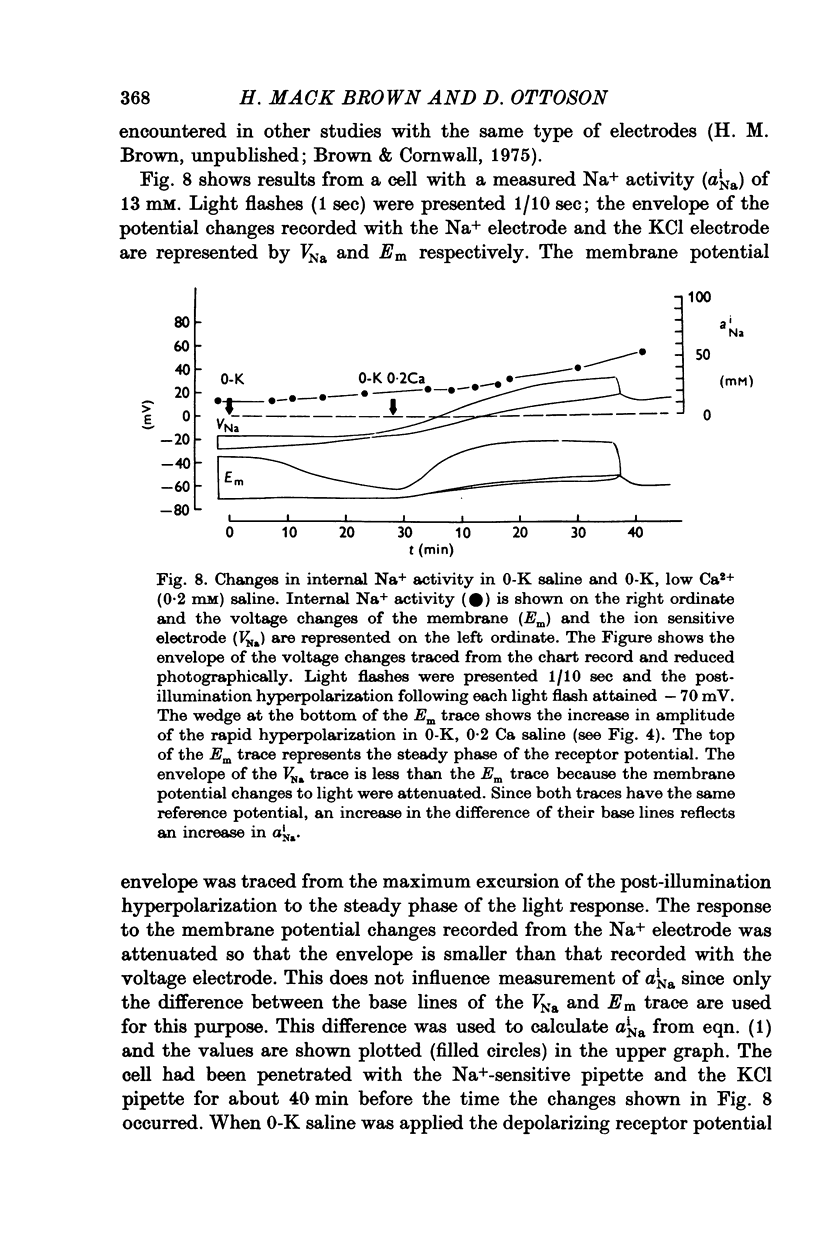
1. The mechanism of reduction and final abolition of the depolarizing receptor potential of Balanus eburneus photoreceptors in K+-free saline was examined with electro-physiological techniques including voltage-clamp and ion specific electrodes. 2. An extended exposure to K+-free saline reduces the transient peak and the steady phases of the depolarizing receptor potential by approximately equal amounts. The process can be reversed in normal saline although the wave form of the response is often more rectangular upon recovery. Restoration of K+ induces a transient hyperpolarization of the resting membrane for several minutes. 3. The depolarizing receptor potential can also be restored in K+-free solution by reducing the Ca2+ concentration. This saline depolarizes the resting membrane, and the wave form of the depolarizing receptor potential assumes a rectangular configuration. 4. Voltage-clamp experiments revealed that an extended exposure to K+-free saline produced an extreme reduction of the inward light-induced current (LIC), but no detectable change in the membrane potential at which the current reverses sign. Membrane conductance in darkness showed little change. Reduction of the Ca2+ concentration from 20 to 0-2 mM in K+-free restored the current and produced a negative 8-10 mV shift in the zero current potential. There was also a significant decrease in membrane conductance in darkness. 5. Current-voltage relations of the membrane in K+-free, low Ca2+, or K+-free low Ca2+ salines were somewhat dependent upon the order the salines were presented. 6. Low Ca2+ saline (0-2 mM) by itself produced a -5 mV shift in the zero-current potential. Removing K+ in low Ca2+ produced an additional shift (-5 mV) in the zero-current potential.
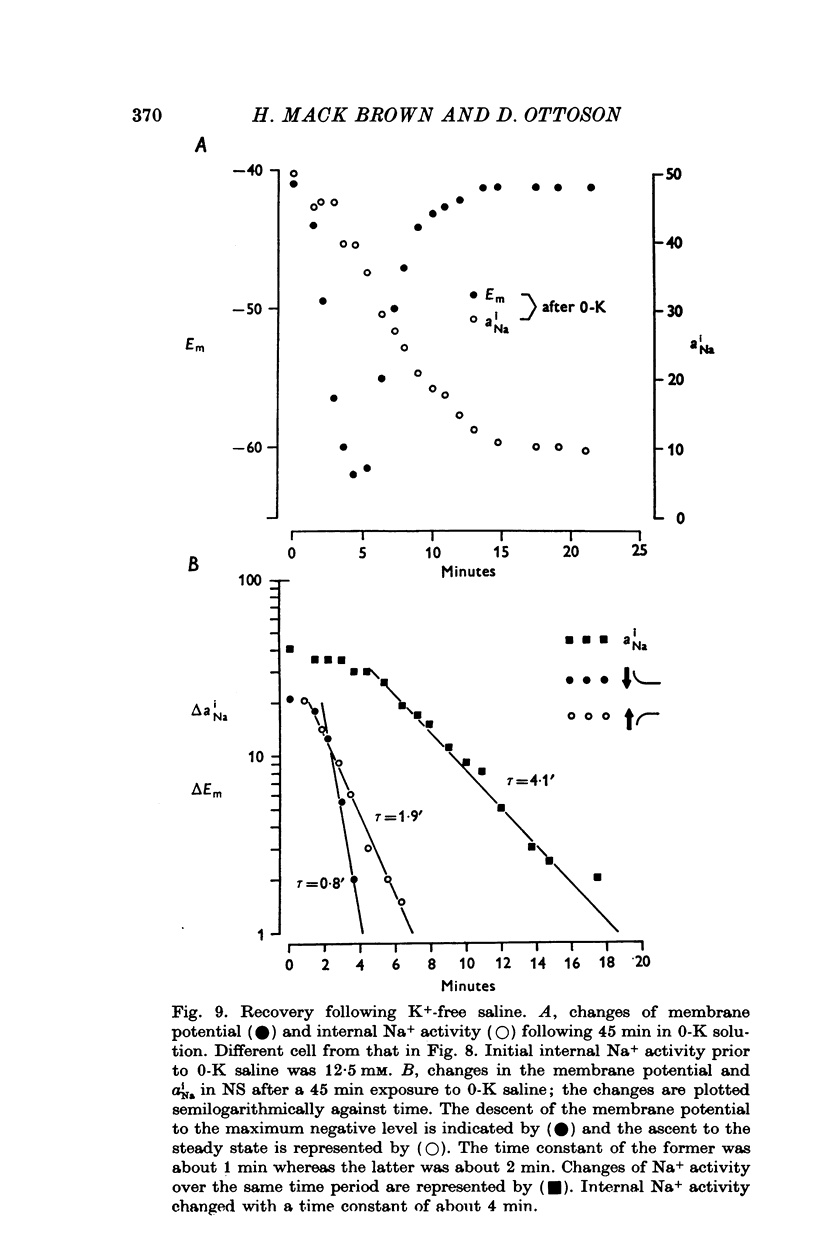
Full text
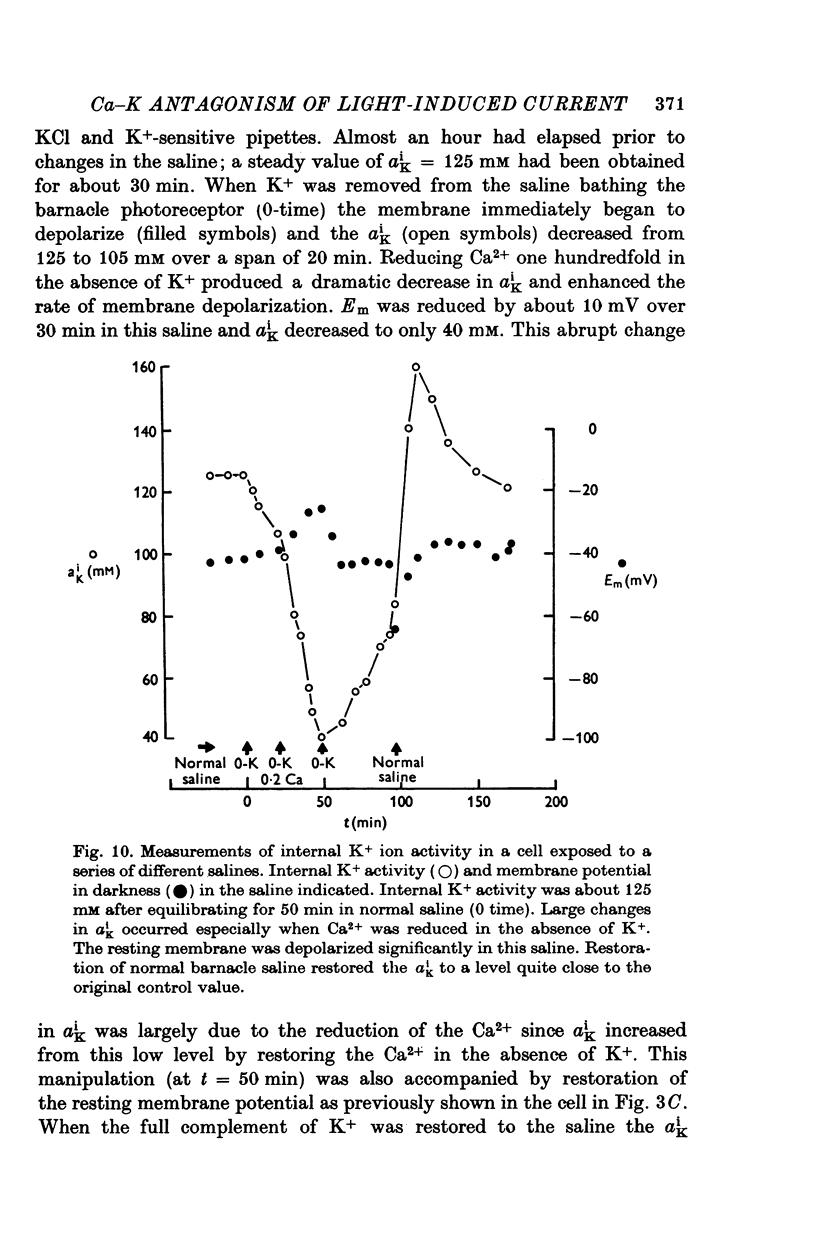
PDF







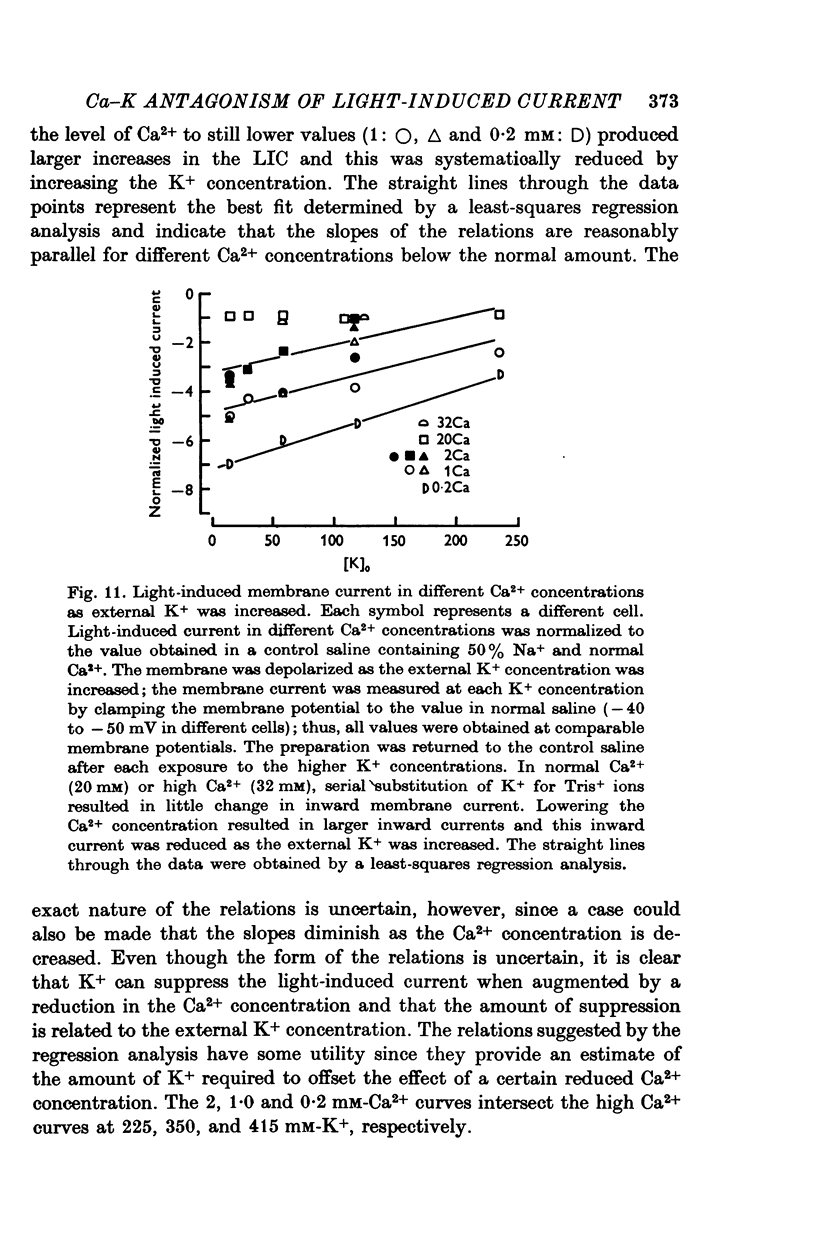





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeckh J., Priesner E., Schneider D., Jacobson M. Olfactory Receptor Response to the Cockroach Sexual Attractant. Science. 1963 Aug 23;141(3582):716–717. doi: 10.1126/science.141.3582.716. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Sutton R. B., Walker J. L., Jr Increased chloride conductance as the proximate cause of hydrogen ion concentration effects in Aplysia neurons. J Gen Physiol. 1970 Nov;56(5):559–582. doi: 10.1085/jgp.56.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Cornwall M. C. Ionic mechanism of a quasi-stable depolarization in barnacle photoreceptor following red light. J Physiol. 1975 Jul;248(3):579–593. doi: 10.1113/jphysiol.1975.sp010989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. W. Electrical characteristics of a barnacle photoreceptor. Fed Proc. 1971 Jan-Feb;30(1):69–78. [PubMed] [Google Scholar]
- Brown H. M., Meech R. W., Koike H., Hagiwara S. Current-voltage relations during illumination: photoreceptor membrane of a barnacle. Science. 1969 Oct 10;166(3902):240–243. doi: 10.1126/science.166.3902.240. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arrigo J. S. Possible screening of surface charges on crayfish axons by polyvalent metal ions. J Physiol. 1973 May;231(1):117–128. doi: 10.1113/jphysiol.1973.sp010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulpius B., Baumann F. Effects of sodium, potassium, and calcium ions on slow and spike potentials in single photoreceptor cells. J Gen Physiol. 1969 May;53(5):541–561. doi: 10.1085/jgp.53.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmark I., Ottoson D. The contribution of mechanical factors to the early adaptation of the spindle response. J Physiol. 1971 Feb;212(3):577–592. doi: 10.1113/jphysiol.1971.sp009343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., MENDELSON M. COMPONENTS OF RECEPTOR ADAPTATION IN A PACINIAN CORPUSCLE. J Physiol. 1965 Apr;177:377–397. doi: 10.1113/jphysiol.1965.sp007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoson D., Shepherd G. M. Transducer characteristics of the muscle spindle as revealed by its receptor potential. Acta Physiol Scand. 1971 Aug;82(4):545–554. doi: 10.1111/j.1748-1716.1971.tb05001.x. [DOI] [PubMed] [Google Scholar]
- Ozeki M., Sato M. Changes in the membrane potential and the membrane conductance associated with a sustained compression of the non-myelinated nerve terminal in Pacinian corpuscles. J Physiol. 1965 Sep;180(1):186–208. [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. G., Stell W. K., Brown J. E., Freeman J. A., Murray G. C. A role for the sodium pump in photoreception in Limulus. Science. 1968 Oct 25;162(3852):456–458. doi: 10.1126/science.162.3852.456. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]


