Abstract
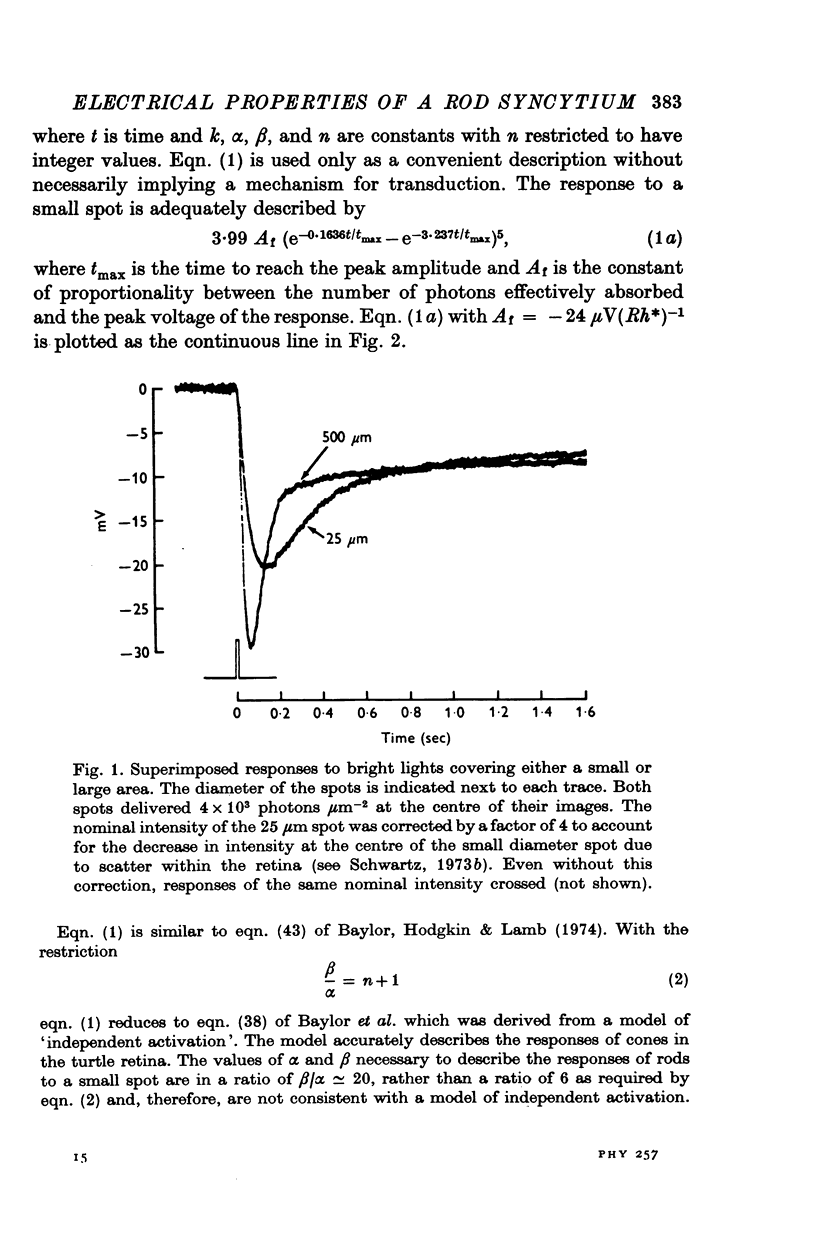
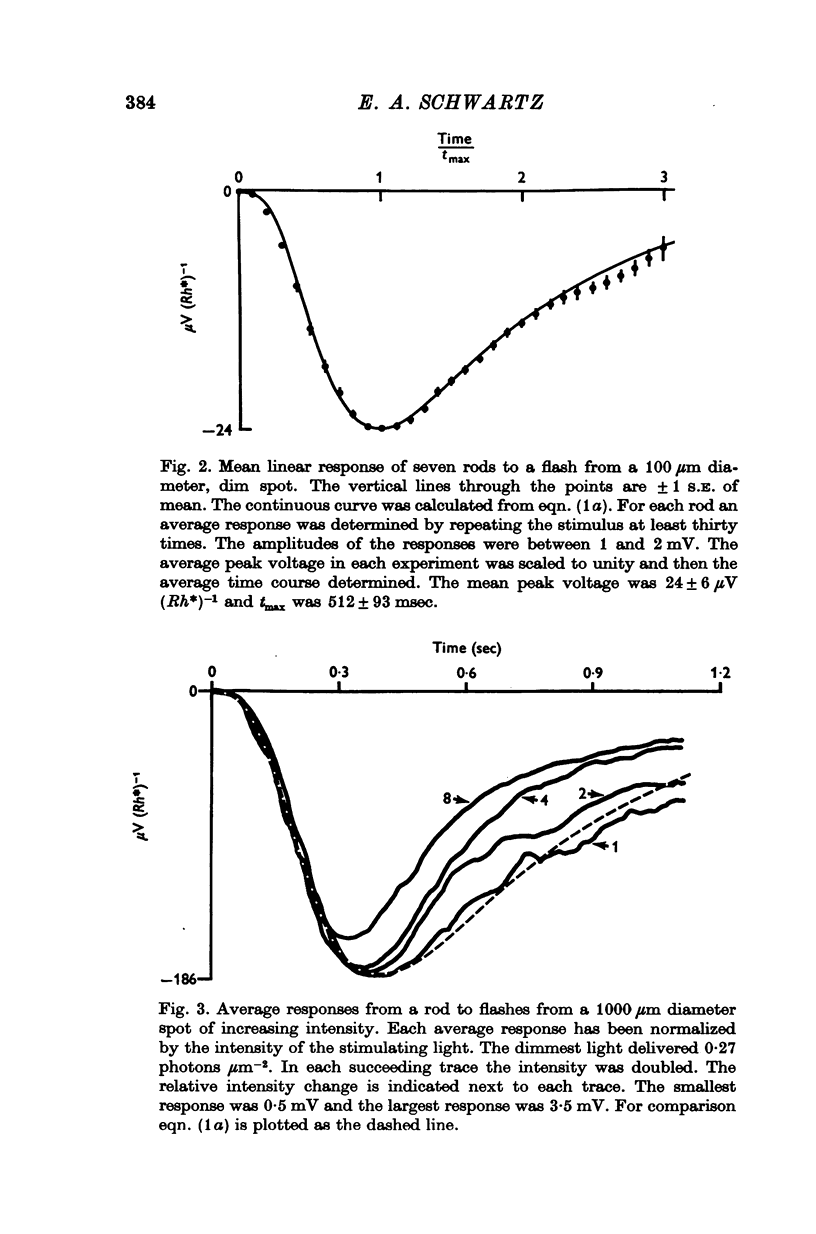
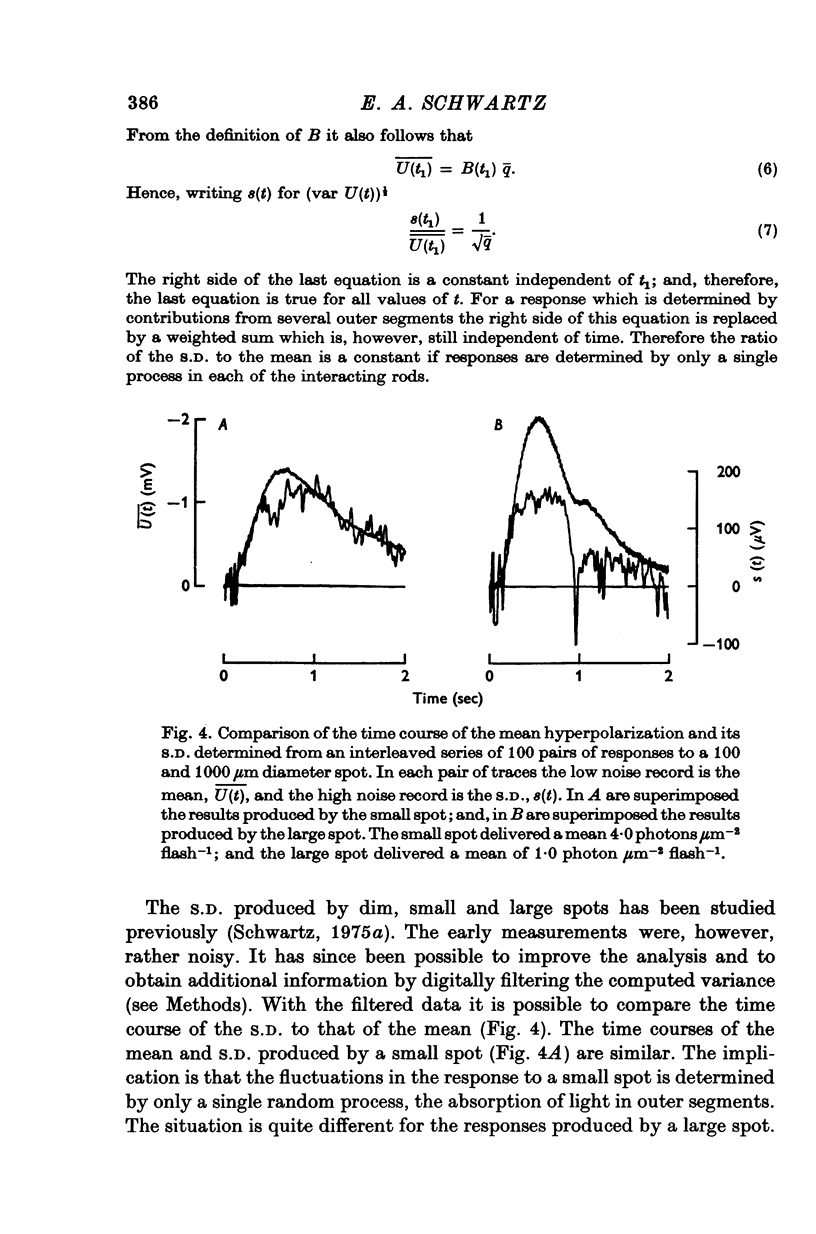
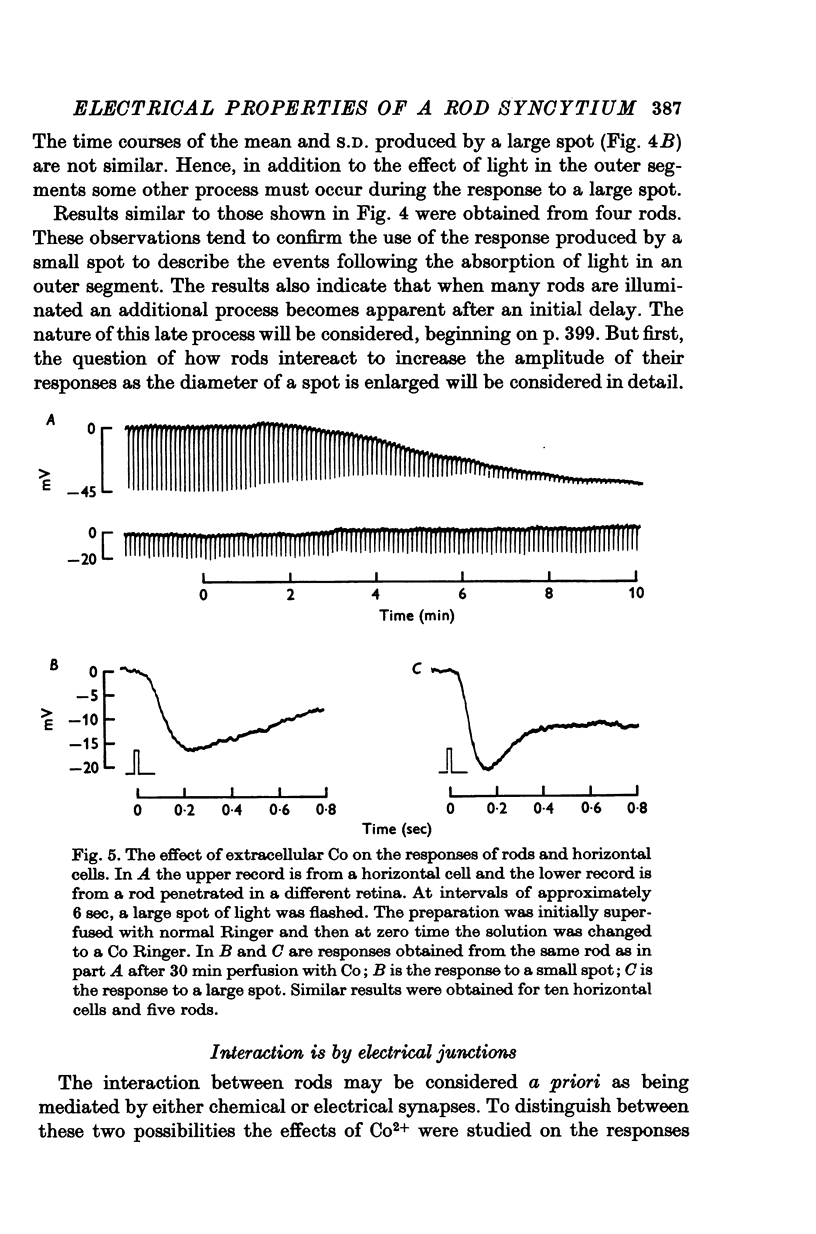
1. Intracellular responses were recorded from rods in isolated eye-cups of the snapping turtle. Chelydra serpentina. Responses to flashes of small (less than 100 mum diameter) and large (1000 mum diameter) spots of 500 nm light were studied. 2. Responses produced by small and large diameter spots which delivered less than 0-3 photons mum-2 had the same shape. The responses produced by large spots were, however, nearly ten times greater in amplitude. The difference in amplitude is termed enhancement. 3. Perfusing an eye-cup with a Co2+-containing medium blocked synaptic transmission from receptors to horizontal cells but did not affect the responses of rods. 4. The membrane conductance of a single rod, estimated by three independent methods, was approximately 1-2 X 10(-9) MHo. 5. Enhancement can be predicted by a mathematical model which treats rods as an electrical syncytium. The space coefficient describing the spread of current is approximately 65 mum indicating that the coupling conductance between rods was relatively high. 6. When the intensity of a small spot was increased from 0-3 photons mum-2 up to 6 photons mum-2, the shape of the response was unchanged. When the intensity of a large spot was increased to more than 0-3 photons mum-2, the voltage during the recovery phase was decreased. This decrease is termed disenhancement. 7. The voltages produced by bright, large and small diameter spots which delivered the same quantity of light to the impaled rod were compared. The voltage produced by a large diameter spot became for a short period during the recovery phase less than the voltage produced by a small diameter spot. This observation indicates that the response to a large spot included during recovery an active process which is not apparent in the response to a small spot.
Full text
PDF





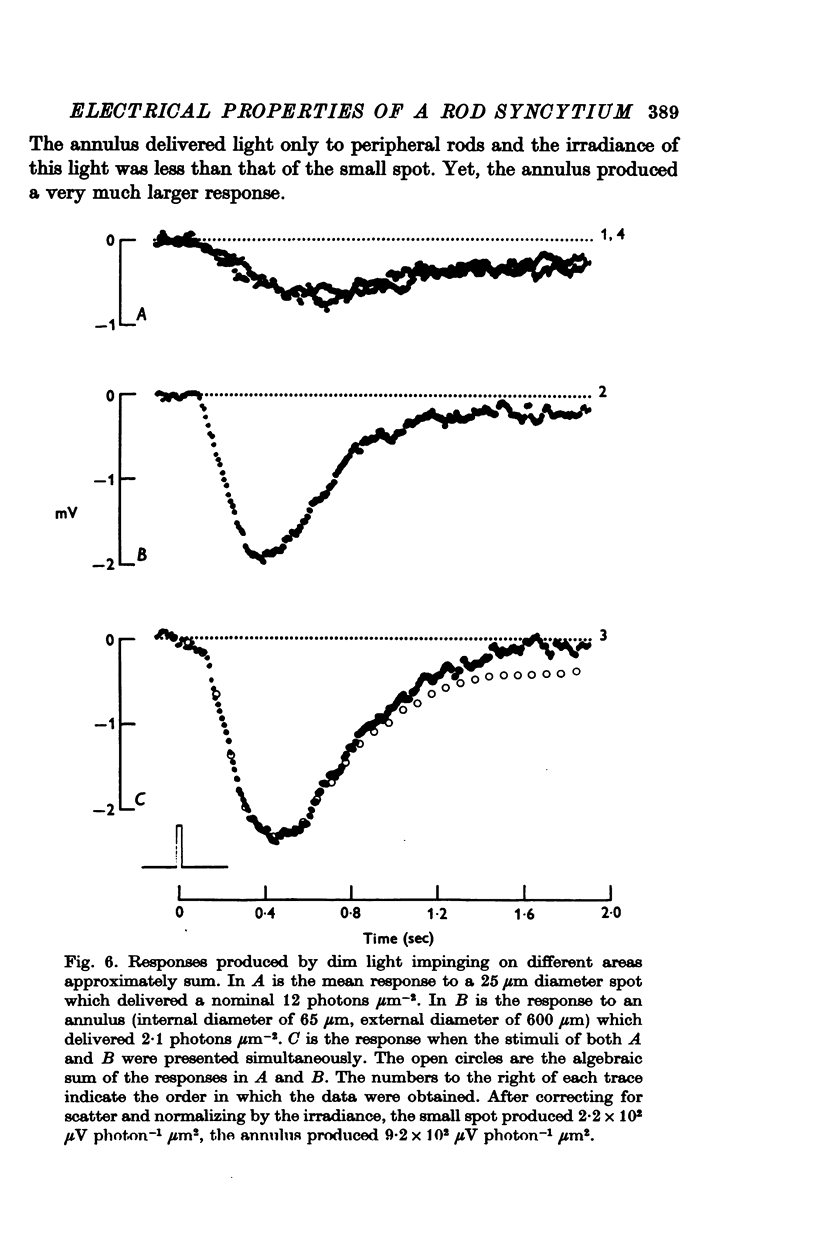

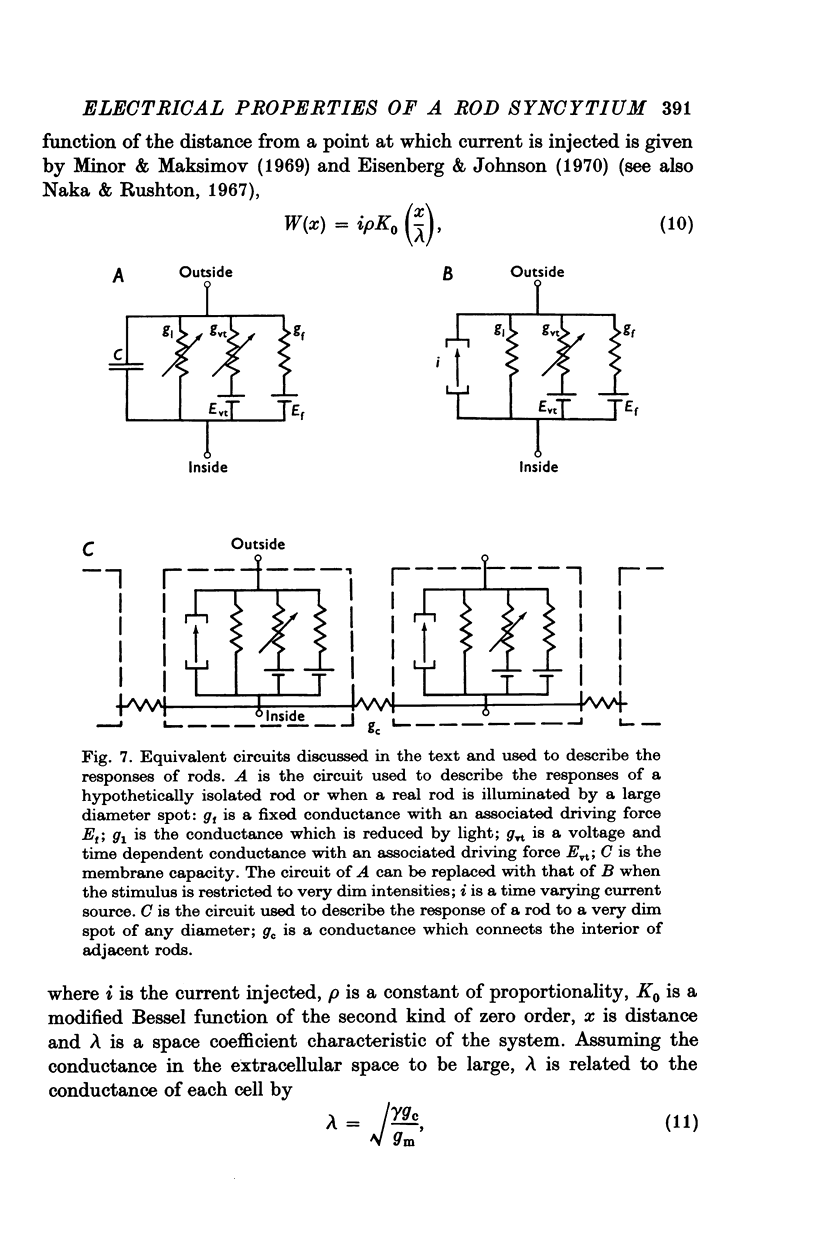


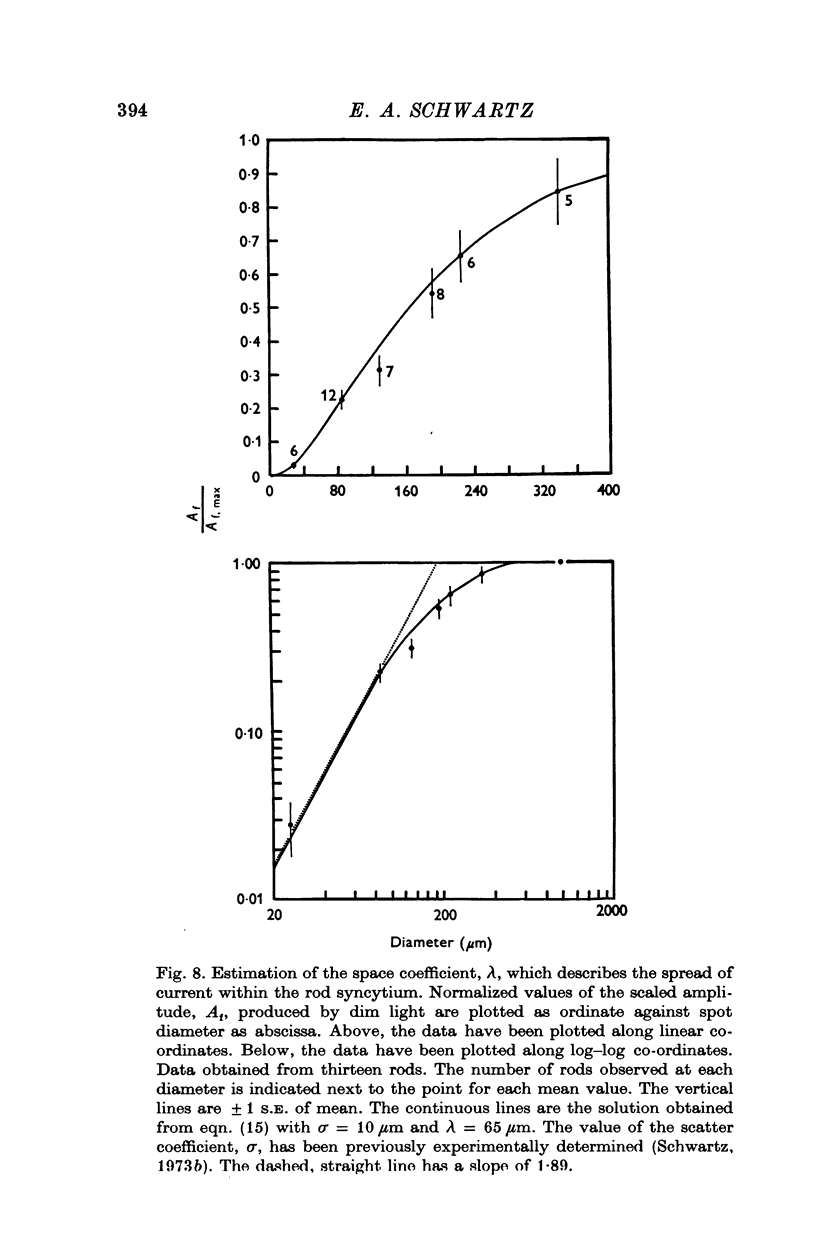
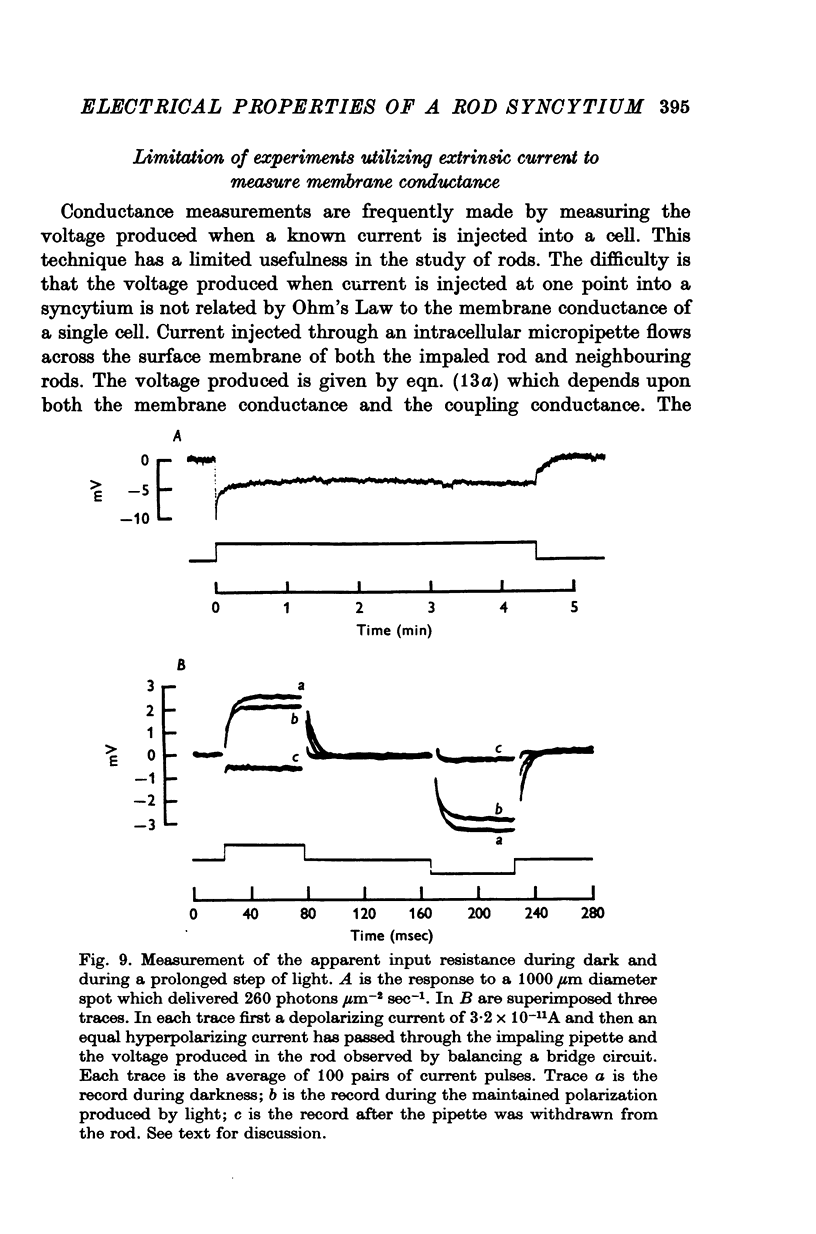








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Colburn T. R., Schwartz E. A. Linear voltage control of current passed through a micropipette with variable resistance. Med Biol Eng. 1972 Jul;10(4):504–509. doi: 10.1007/BF02474198. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Cones excite rods in the retina of the turtle. J Physiol. 1975 Apr;246(3):639–651. doi: 10.1113/jphysiol.1975.sp010908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]


