Abstract
1. A method for measuring bidirectional Cl fluxes has been used to estimate net Cl movements in short-circuited frog skin and to compare these with the short-circuit current (Isc) and Na fluxes. 2. In some experiments bidirectional fluxes of both Na and Cl were measured simultaneously. It was found that the algebraic sum of the net fluxes of these two ions did not differ significantly from the values of Isc, either in untreated or catecholamine-treated skins, except for the half-hour period immediately after catecholamine addition. 3. The net effluxes of Cl produced by noradrenaline (1-6 X 10(-5)M), isoprenaline (8 X 10(-7)M) and adrenaline (6 and 15 X 10(-6)M) were of similar magnitude for each catecholamine. The magnitude of the Cl response measured as a flux ratio was related to a certain extent to the precatecholamine Cl conductance. 4. The net Na influx was increased by isoprenaline and reduced by noradrenaline. 5. Addition of the beta-adrenergic blocking agent oxprenolol (4-5 X 10(-5)M) to skins stimulated by catecholamine resulted in the disappearance of the net Cl movement and fall in skin conductance and Isc. This fall was similar in magnitude to, and correlated with the mean rise in Isc produced by isoprenaline, but of significantly greater magnitude in the case of noradrenaline. 6. The changes in Na influx were strongly associated with the changes in Isc following catecholamine addition. Similarly, the changes in Na efflux and Cl efflux were correlated, suggesting the Na fluxes to be dissociated, influx and efflux changes perhaps taking place at different loci. 7. Acetazolamide (1-2 X 10(-4)M), added either before or during the noradrenaline stimulation, had no effect on the Cl efflux response. 8. The tissue exchange of Cl from the outside bathing medium after 4 hr was greater in catecholamine-stimulated skins than in those in which the response had been blocked by oxprenolol. 9. These findings were taken to support a model entailing a neutral NaCl pump resident in the mucous glands and an epithelial Na pump enhanced by beta- and inhibited by alpha-adrenergic stimulation.
Full text
PDF


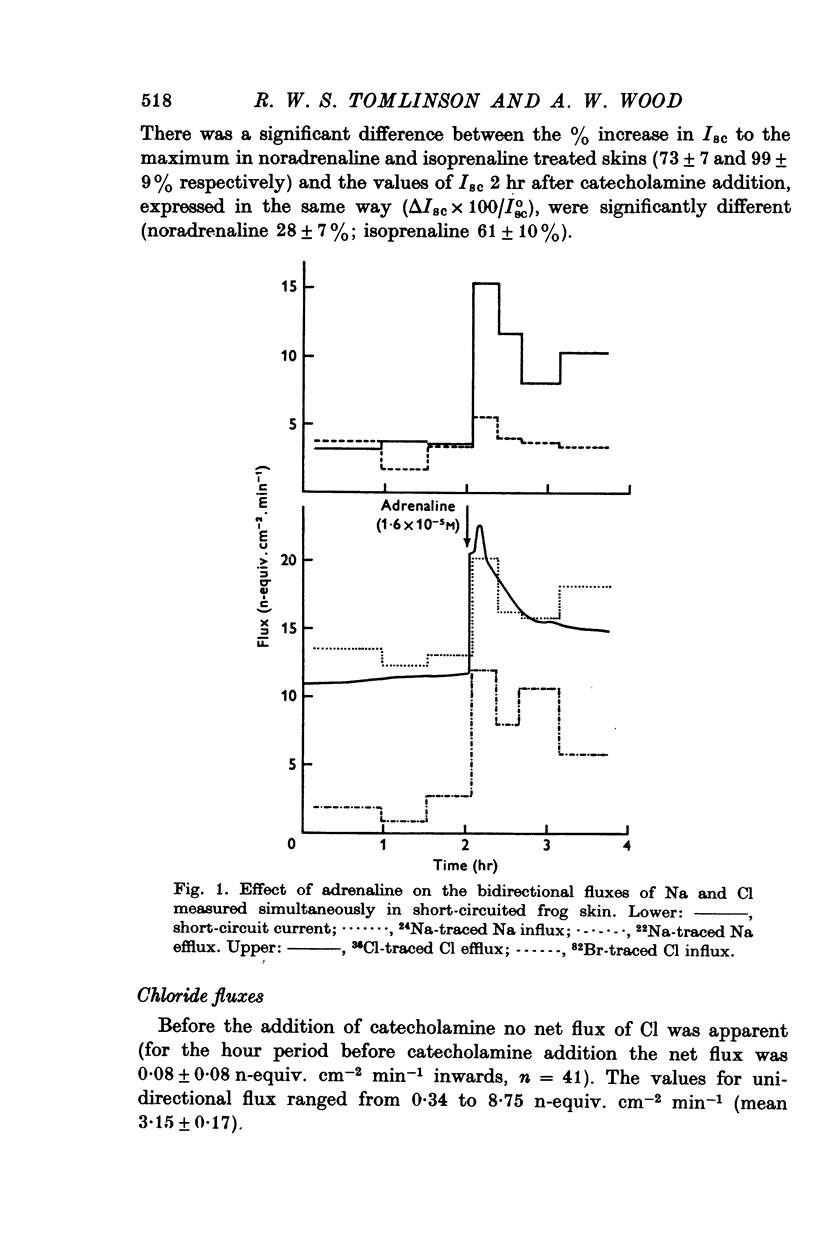
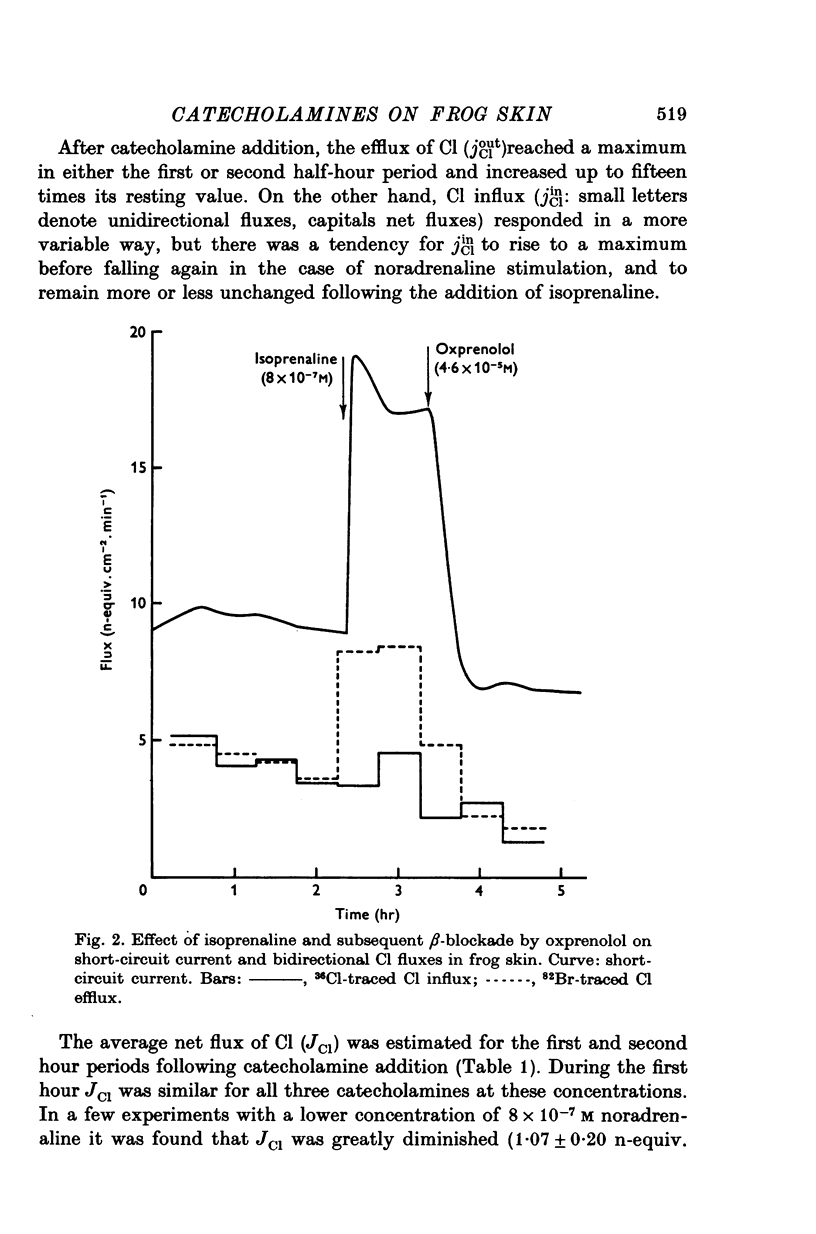
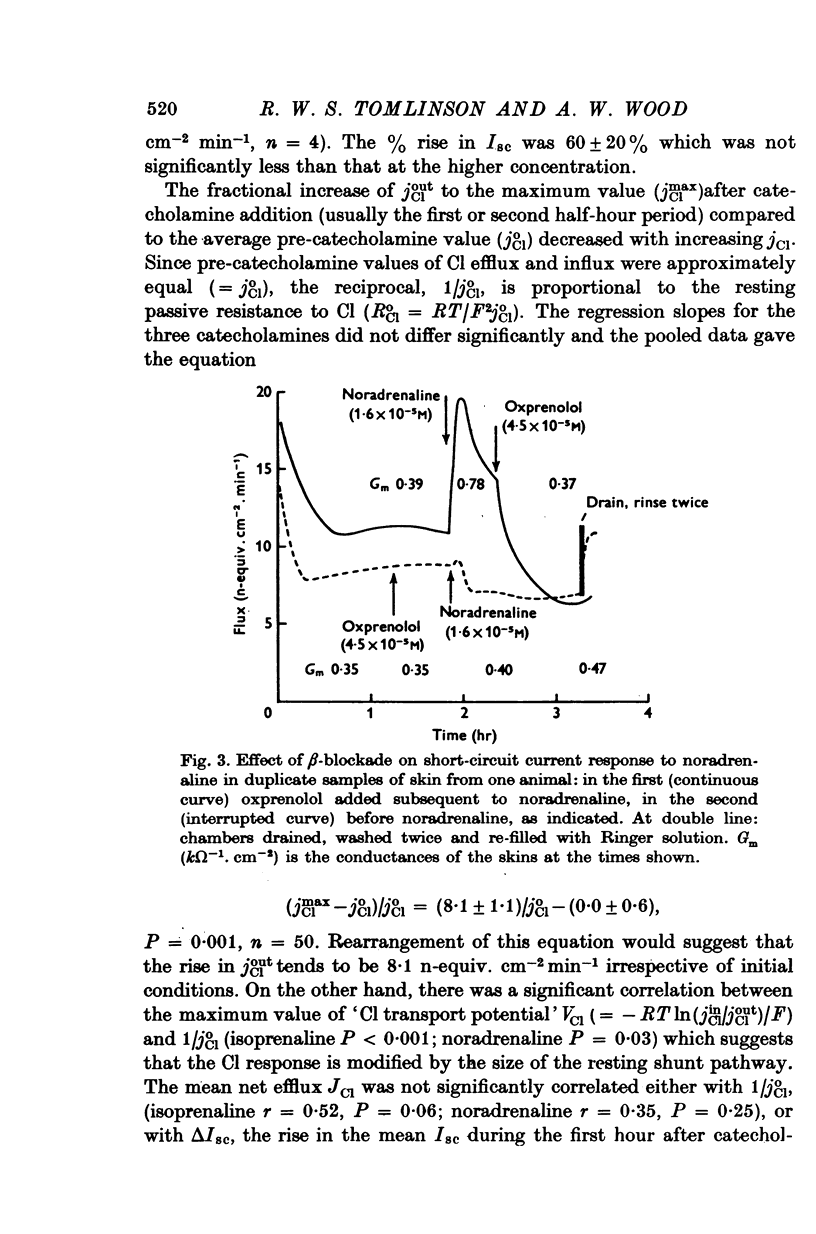
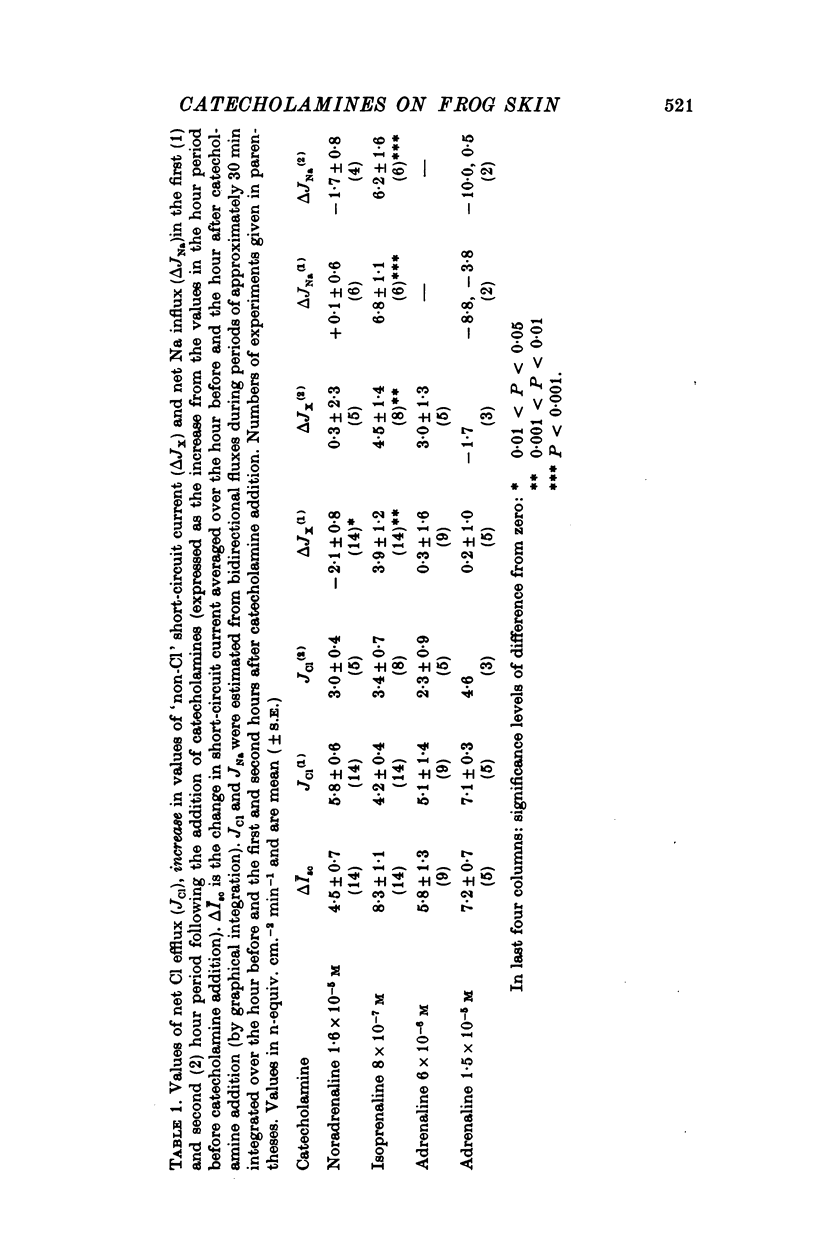






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambalavanar S., Foster R. W., Schnieden H. The adrenoceptors mediating catecholamine effects in frog isolated skin. J Pharm Pharmacol. 1973 Jan;25(1):55–59. doi: 10.1111/j.2042-7158.1973.tb09115.x. [DOI] [PubMed] [Google Scholar]
- Bastide F., Jard S. Actions de la noradrénaline et de l'oxytocine sur le transport actif de sodium et la permeabilité à l'eau de la peau de grenouille. Rôle du 3',5'-AMP cyclique. Biochim Biophys Acta. 1968 Jan 3;150(1):113–123. doi: 10.1016/0005-2736(68)90014-x. [DOI] [PubMed] [Google Scholar]
- Benson B. J., Hadley M. E. In vitro characterization of adrenergic receptors controlling skin gland secretion in two anurans Rana pipiens and Xenopus laevis. Comp Biochem Physiol. 1969 Sep 1;30(5):857–864. doi: 10.1016/0010-406x(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Fassina G., Capenedo F., Fiandini G. The effect of catecholamines and beta-anti-adrenergic drugs on isolated short-circuited skin of the frog. J Pharm Pharmacol. 1968 Mar;20(3):240–242. doi: 10.1111/j.2042-7158.1968.tb09732.x. [DOI] [PubMed] [Google Scholar]
- House C. R. The role of glandular activity in the electrical response of amphibian skin to noradrenaline. J Physiol. 1969 Jun;202(3):631–644. doi: 10.1113/jphysiol.1969.sp008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H., ZERAHN K. The origin of the short-circuit current in the adrenaline stimulated frog skin. Acta Physiol Scand. 1952;27(1):38–48. doi: 10.1111/j.1748-1716.1953.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Lang L., Sjöberg E., Skoglund C. R. Conductance recording of ionic outflow from frog skin glands during nerve stimulation. Acta Physiol Scand. 1975 Jan;93(1):67–76. doi: 10.1111/j.1748-1716.1975.tb05791.x. [DOI] [PubMed] [Google Scholar]
- McAfee R. D. The action of beta adrenergic site stimulating catecholamines on isolated frog skin. Biochim Biophys Acta. 1970 Mar 17;203(1):104–110. doi: 10.1016/0005-2736(70)90040-4. [DOI] [PubMed] [Google Scholar]
- Pinschmidt M. W., Campbell A. D., Huf E. G. Role of Na+ and anions in the triple response of isolated frog skin to norepinephrine. Biochim Biophys Acta. 1973 Oct 11;323(2):309–325. doi: 10.1016/0005-2736(73)90154-5. [DOI] [PubMed] [Google Scholar]
- Rajerison R. M., Montegut M., Jard S., Morel F. The isolated frog skin epithelium: presence of alpha and beta adrenergic receptors regulating active sodium transport and water permeability. Pflugers Arch. 1972;332(4):313–331. doi: 10.1007/BF00588578. [DOI] [PubMed] [Google Scholar]
- SCHOFFENIELS E., SALEE M. L. THE EFFECTS OF THE ELECTRICAL STIMULATION OF THE BRACHIAL PLEXUS ON THE POTENTIAL DIFFERENCE OF FROG SKIN. Comp Biochem Physiol. 1965 Apr;14:587–602. doi: 10.1016/0010-406x(65)90248-3. [DOI] [PubMed] [Google Scholar]
- Seldin J. P., Hoshiko T. Ionic requirement for epinephrine stimulation of frog skin gland secretion. J Exp Zool. 1966 Oct;163(1):111–114. doi: 10.1002/jez.1401630112. [DOI] [PubMed] [Google Scholar]
- Taylor A. E., Wright E. M., Schultz S. G., Curran P. F. Effect of sugars on ion fluxes in intest-ine. Am J Physiol. 1968 Apr;214(4):836–842. doi: 10.1152/ajplegacy.1968.214.4.836. [DOI] [PubMed] [Google Scholar]
- Watlington C. O. Effect of catecholamines and adrenergic blockade on sodium transport of isolated frog skin. Am J Physiol. 1968 May;214(5):1001–1007. doi: 10.1152/ajplegacy.1968.214.5.1001. [DOI] [PubMed] [Google Scholar]


