Abstract
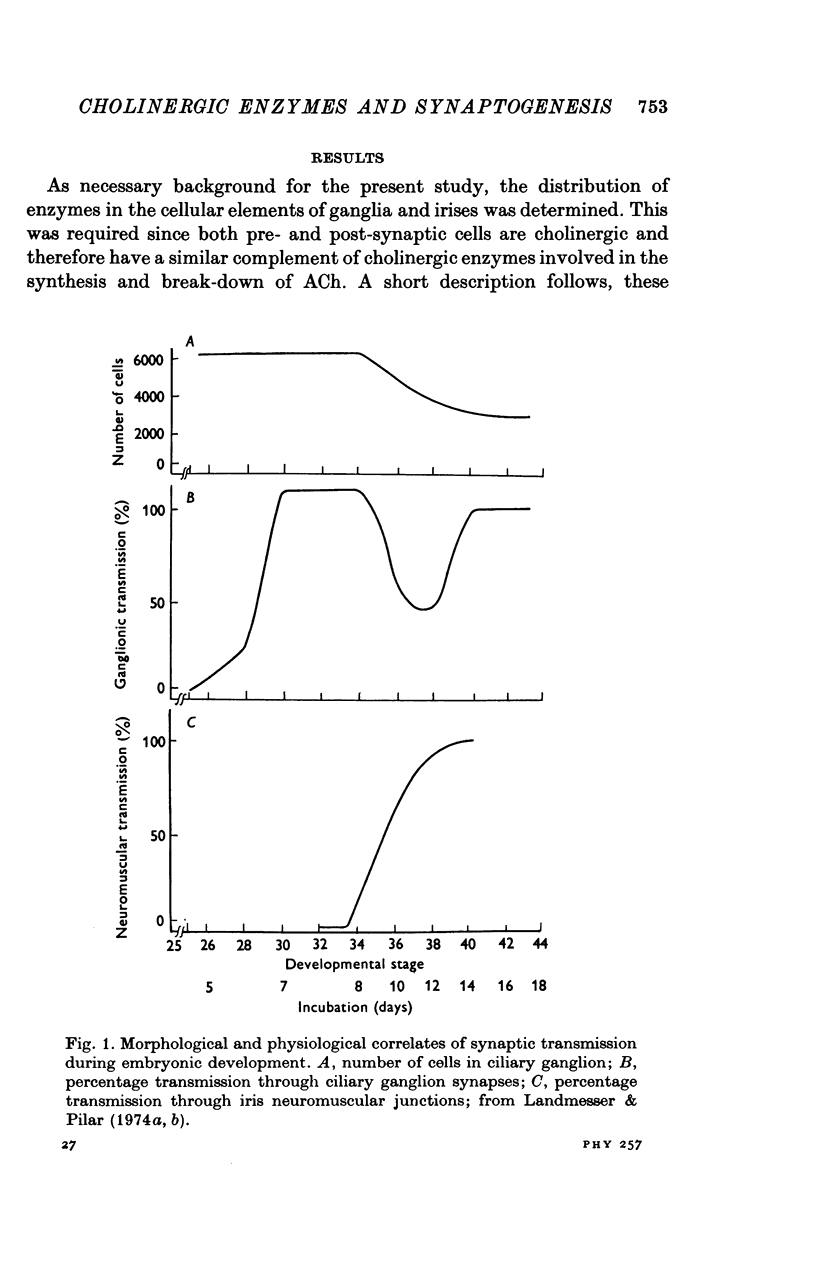
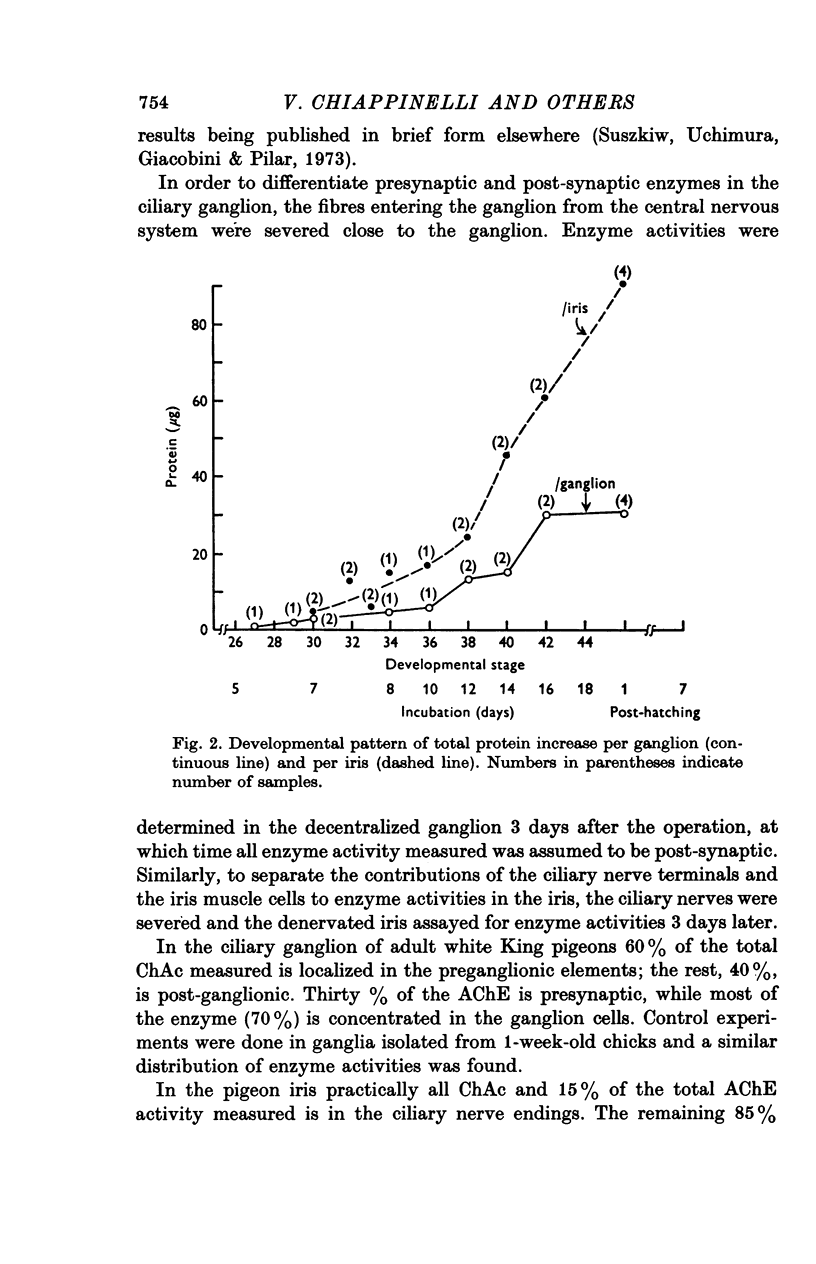
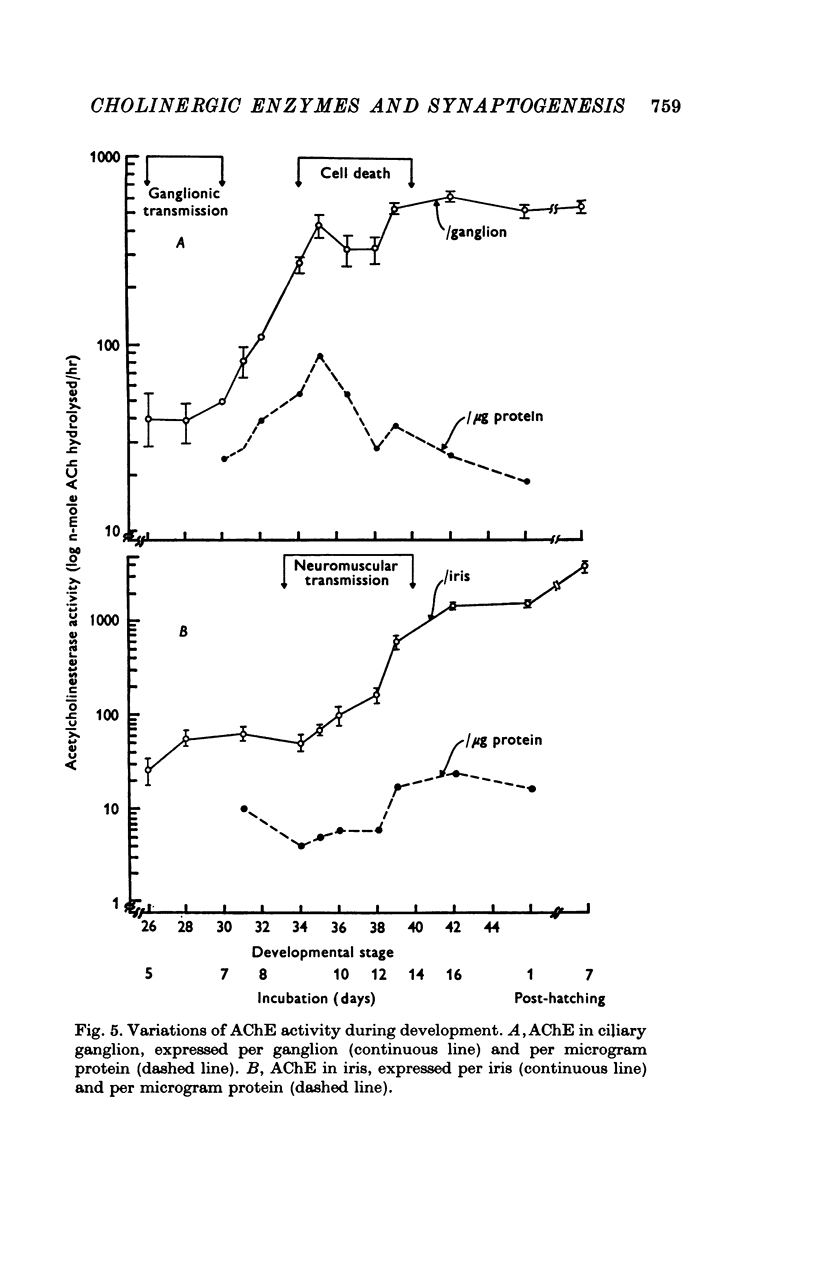
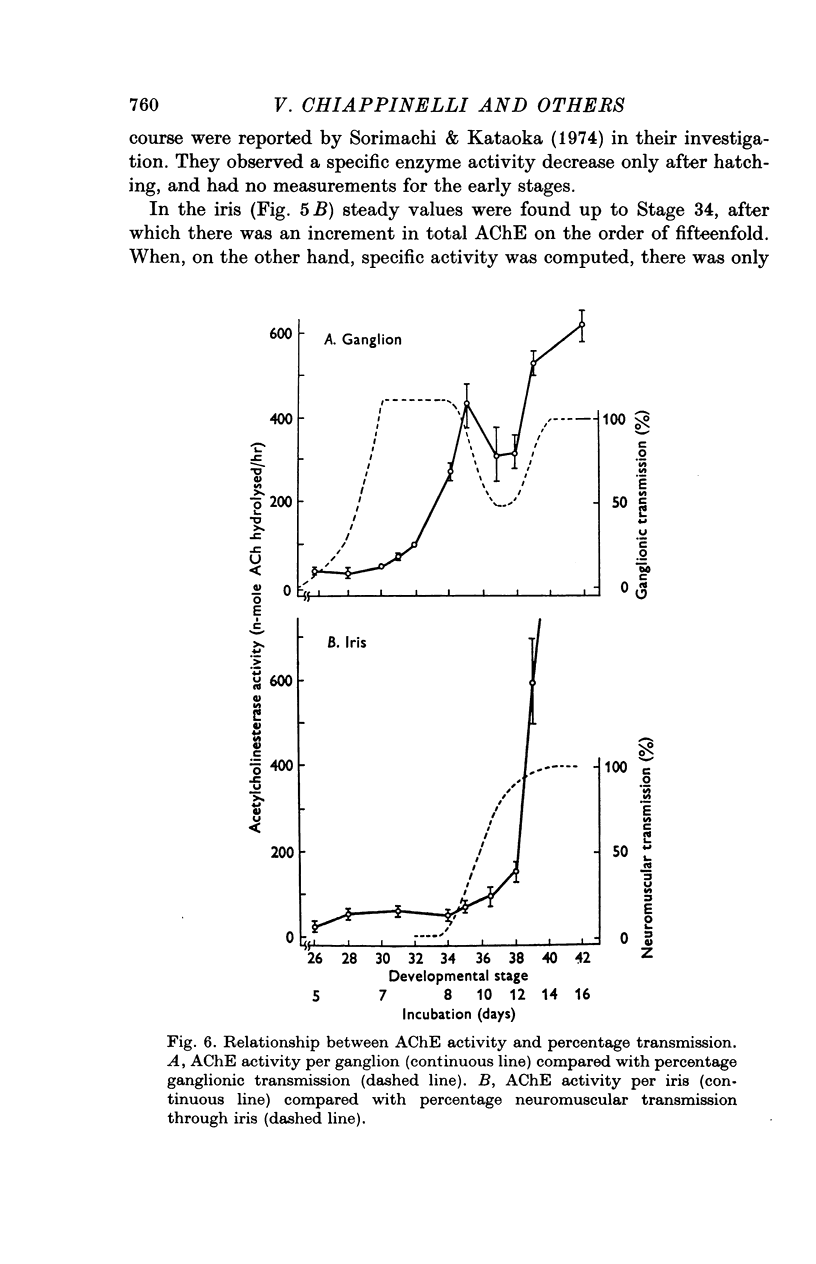
1. In chick ciliary ganglia and irises, cholineacetyltransferase (ChAc) and acetylcholinesterase (AChE) activities were measured from the fifth day of incubation until 1 week after hatching. The changes in enzyme activity were correlated in time with previous electrophysiological and morphological findings of synapse formation in these tissues. 2. At Stage 26 (Hamburger & Hamilton, 1951; before synapse formation in the ganglia) low activities of ChAc (12 +/- 4 [mean +/- S.E.] p-mole of ACh synthesized/hr) were measured in the iris nerve terminals, indicating that ganglion cells are biochemically differentiated, immediately after cell migration is completed. The specific acitivities of ChAc and AChE rose during development and these increases were closely related to the onset and maturation of ganglionic and iris synaptic transmission. These increases in enzyme activities can be used in cholinergic synapses as an index of synapse formation. 3. The 200-fold specific increase of ChAc in iris nerve terminals which occurs at Stage 34 probably reflects an increase in synthesis of the enzyme in ganglion cells and suggests that the formation of the iris neuromuscular junction triggers the enzyme induction. It is implied that the cell responds to a signal ascending the axon from the terminal. 4. The initial increase of AChE specific activity in the ganglion occurs after transmission is established in all cells between Stage 30 and 34 and is mainly due to enzyme synthesis by the ganglion cells. In the iris there is a twofold increase in specific activity after the formation of neuromuscular junctions which probably reflects enzyme induction in the muscle subneural region. It is concluded that the specific induction of AChE in post-junctional cells is due to an influence of the prejunctional element. 5. During synaptic formation in the ciliary ganglion, reciprocal interactions between the neurones and their targets result in the induction of ChAc in the prejunctional elements and AChE in the post-junctional cells.
Full text
PDF




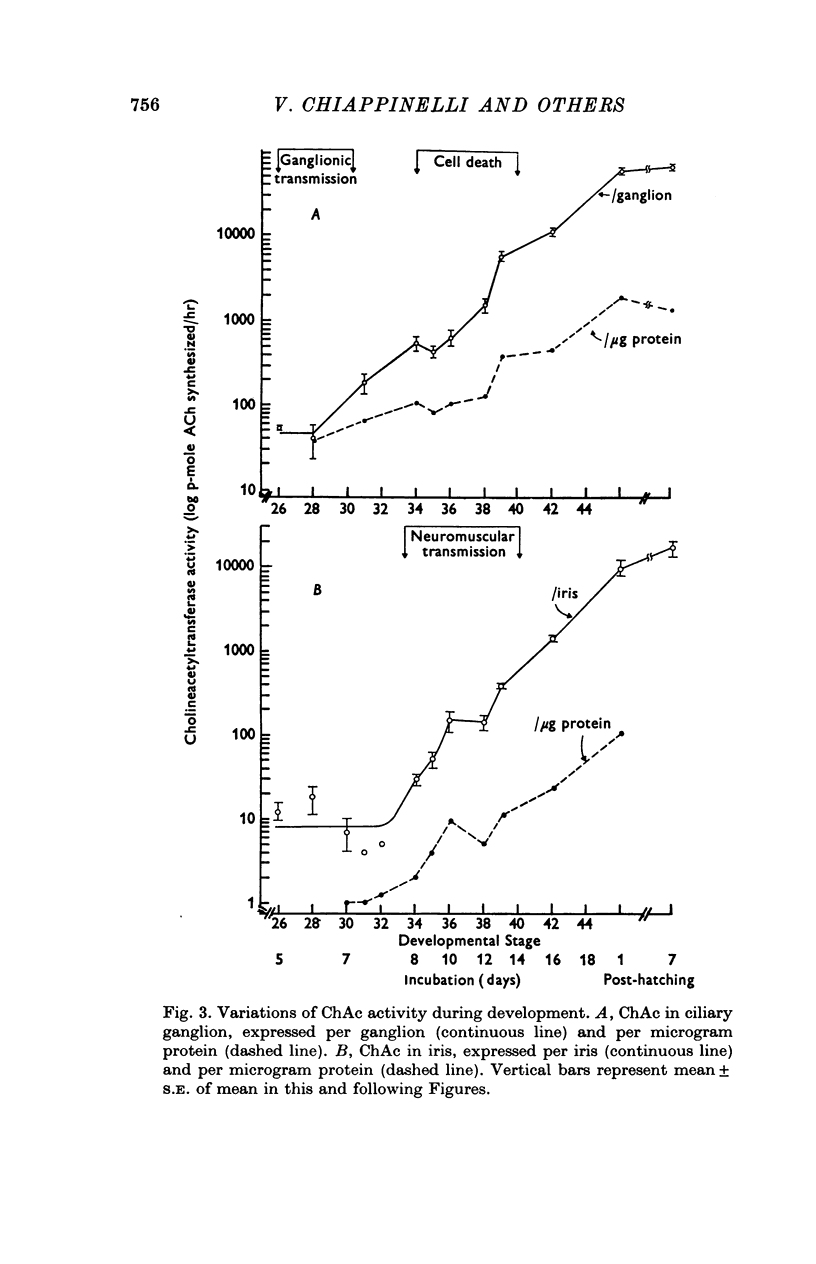

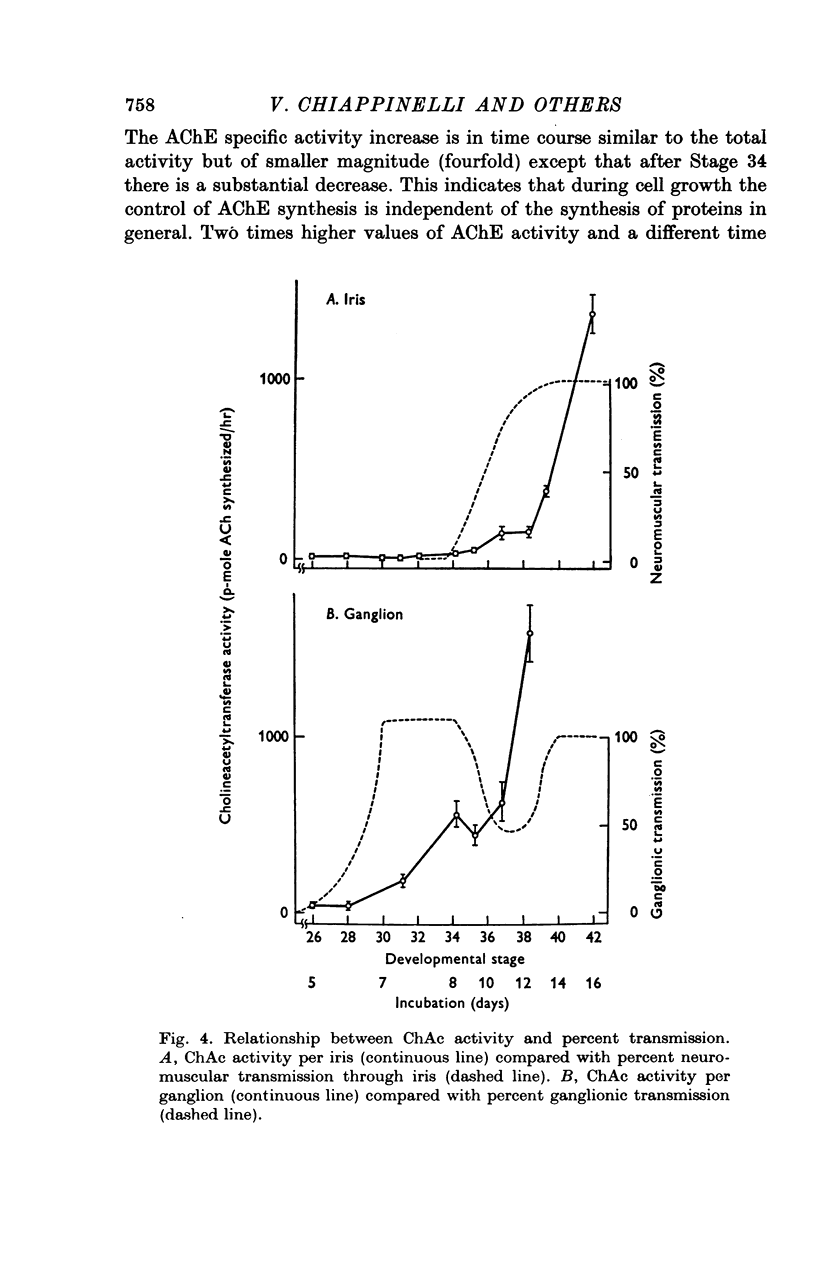






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Teitelman G. Aggregates formed by mixtures of embryonic neural cells: activity of enzymes of the cholinergic system. Dev Biol. 1974 Aug;39(2):317–321. doi: 10.1016/0012-1606(74)90243-7. [DOI] [PubMed] [Google Scholar]
- Black I. B., Geen S. C. Inhibition of the biochemical and morphological maturation of adrenergic neurons by nicotinic receptor blockade. J Neurochem. 1974 Feb;22(2):301–306. doi: 10.1111/j.1471-4159.1974.tb11594.x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. The role of post-synaptic neurones in the biochemical maturation of presynaptic cholinergic nerve terminals in a mouse sympathetic ganglion. J Physiol. 1972 Feb;221(1):149–159. doi: 10.1113/jphysiol.1972.sp009745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Black I. B., Joh T. H., Reis D. J. Accumulation of tyrosine hydroxylase molecules during growth and development of the superior cervical ganglion. Brain Res. 1974 Jul 19;75(1):133–144. doi: 10.1016/0006-8993(74)90775-6. [DOI] [PubMed] [Google Scholar]
- Burt A. M. Acetylcholinesterase and choline acetyltransferase activity in the developing chick spinal cord. J Exp Zool. 1968 Sep;169(1):107–112. doi: 10.1002/jez.1401690112. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A., Hanbauer I. Do cyclic nucleotides promote the trans-synaptic induction of tyrosine hydroxylase? Life Sci. 1974 Apr 1;14(7):1169–1188. doi: 10.1016/0024-3205(74)90425-1. [DOI] [PubMed] [Google Scholar]
- Coughlin M. D. Target organ stimulation of parasympathetic nerve growth in the developing mouse submandibular gland. Dev Biol. 1975 Mar;43(1):140–158. doi: 10.1016/0012-1606(75)90137-2. [DOI] [PubMed] [Google Scholar]
- Dolezalova H., Giacobini E., Giacobini G., Rossi A., Toschi G. Developmental variations of choline acetyl-transferase, dopamine-beta-hydroxylase and monoamineoxidase in chicken embryo and chicken sympathetic ganglia. Brain Res. 1974 Jun 20;73(2):309–320. doi: 10.1016/0006-8993(74)91051-8. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969 Nov;115(3):465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIACOBINI E., HOLMSTEDT B. Cholinesterase in muscles: a histochemical and microgasometric study. Acta Pharmacol Toxicol (Copenh) 1960;17:94–105. doi: 10.1111/j.1600-0773.1960.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Giller E. L., Jr, Schrier B. K., Shainberg A., Fisk H. R., Nelson P. G. Choline acetyltransferase activity is increased in combined cultures of spinal cord and muscle cells from mice. Science. 1973 Nov 9;182(4112):588–589. doi: 10.1126/science.182.4112.588. [DOI] [PubMed] [Google Scholar]
- HAMMOND W. S., YNTEMA C. L. Origin of ciliary ganglia in the chick. J Comp Neurol. 1958 Dec;110(3):367–389. doi: 10.1002/cne.901100304. [DOI] [PubMed] [Google Scholar]
- Hendry I. A. The retrograde trans-synaptic control of the development of cholinergic terminals in sympathetic ganglia. Brain Res. 1975 Mar 28;86(3):483–487. doi: 10.1016/0006-8993(75)90900-2. [DOI] [PubMed] [Google Scholar]
- Hess A. Developmental changes in the structure of the synapse on the myelinated cell bodies of the chicken ciliary ganglion. J Cell Biol. 1965 Jun;25(3 Suppl):1–19. doi: 10.1083/jcb.25.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACS T., KOVER A., BALOGH G. Studies on the localization of cholinesterase in various types of muscle. J Cell Comp Physiol. 1961 Apr;57:63–71. doi: 10.1002/jcp.1030570202. [DOI] [PubMed] [Google Scholar]
- Koenig H. L. Relations entre la distribution de l'activité acétylcholinestérasique et celle de l'ergastoplasme dans les neurones du ganglion ciliaire du poulet. Arch Anat Microsc Morphol Exp. 1965 Oct-Dec;54(4):937–963. [PubMed] [Google Scholar]
- Koslow S. H., Giacobini E. An isotopic micromethod for the measurement of cholinesterase activity in individual cells. J Neurochem. 1969 Nov;16(11):1523–1528. doi: 10.1111/j.1471-4159.1969.tb09907.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Fate of ganglionic synapses and ganglion cell axons during normal and induced cell death. J Cell Biol. 1976 Feb;68(2):357–374. doi: 10.1083/jcb.68.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synapse formation during embryogenesis on ganglion cells lacking a periphery. J Physiol. 1974 Sep;241(3):715–736. doi: 10.1113/jphysiol.1974.sp010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974 Sep;241(3):737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol. 1972 May;222(3):691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974 Nov;41(1):162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- Oesch F., Thoenen H. Increased activity of the peripheral sympathetic nervous system: induction of choline acetyltransferase in the preganglionic cholinergic neurone. Nature. 1973 Apr 20;242(5399):536–537. doi: 10.1038/242536a0. [DOI] [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C. STUDIES ON THE INDUCTION AND REPRESSION OF ENZYMES IN RAT LIVER. I. INDUCTION OF THREONINE DEHYDRASE AND ORNITHINE-DELTA-TRANSAMINASE BY ORAL INTUBATION OF CASEIN HYDROLYSATE. J Biol Chem. 1964 Jun;239:1783–1788. [PubMed] [Google Scholar]
- Pilar G., Jenden D. J., Campbell B. Distribution of acetylcholine in the normal and denervated pigeon ciliary ganglion. Brain Res. 1973 Jan 30;49(2):245–256. doi: 10.1016/0006-8993(73)90421-6. [DOI] [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976 Feb;68(2):339–356. doi: 10.1083/jcb.68.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Sorimachi M., Kataoka K. Developmental change of choline acetyltransferase and acetylcholinesterase in the ciliary and the superior cervical ganglion of the chick. Brain Res. 1974 Apr 12;70(1):123–130. doi: 10.1016/0006-8993(74)90217-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Saner A., Kettler R., Angeletti P. U. Nerve growth factor and preganglionic cholinergic nerves; their relative importance to the development of the terminal adrenergic neuron. Brain Res. 1972 Sep 29;44(2):593–602. doi: 10.1016/0006-8993(72)90321-6. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Trans-synaptic enzyme induction. Life Sci. 1974 Jan 16;14(2):223–235. doi: 10.1016/0024-3205(74)90052-6. [DOI] [PubMed] [Google Scholar]


