Abstract
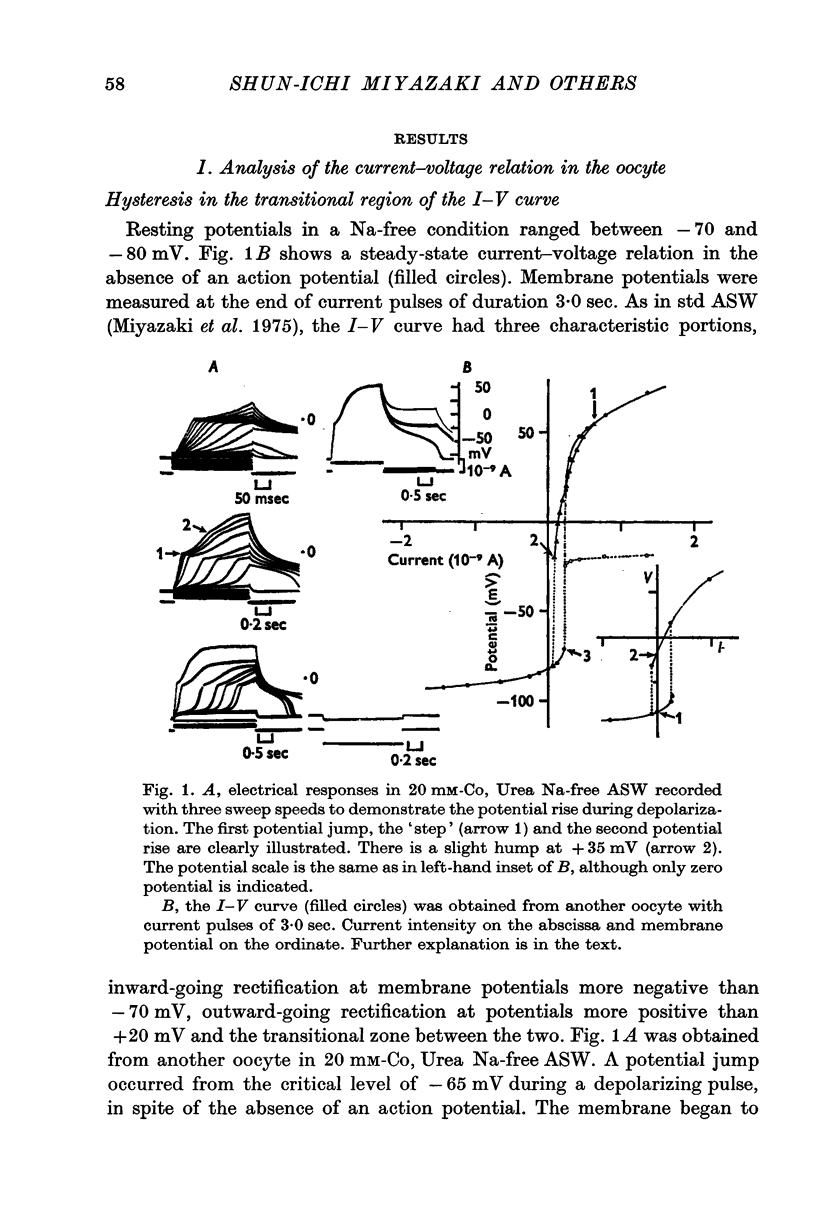
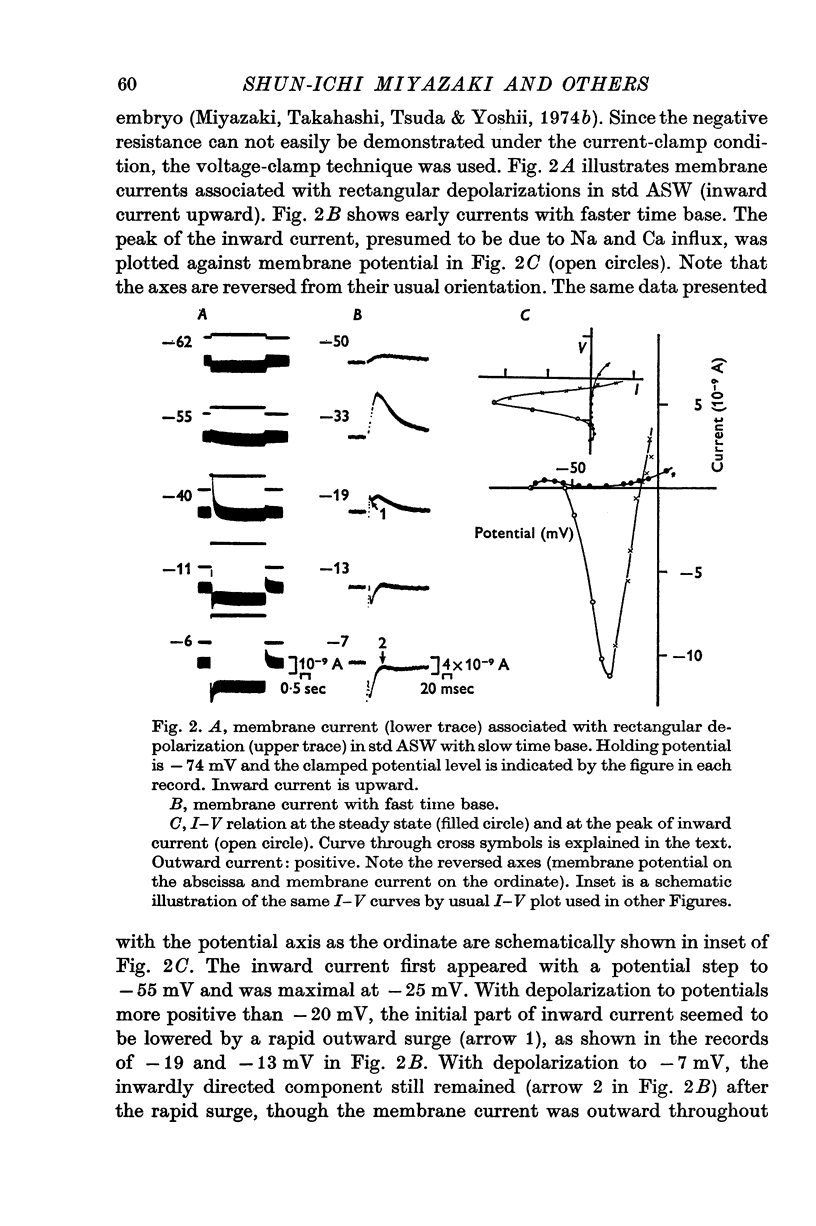
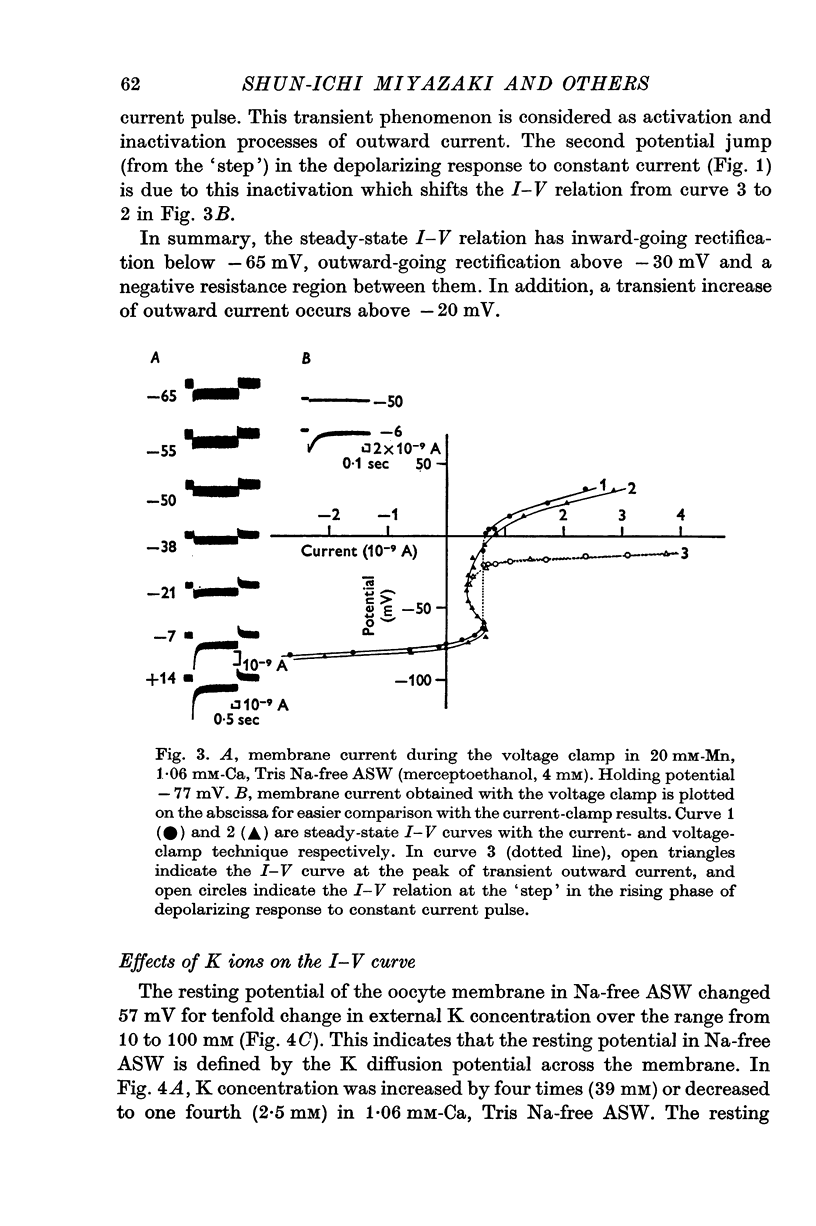
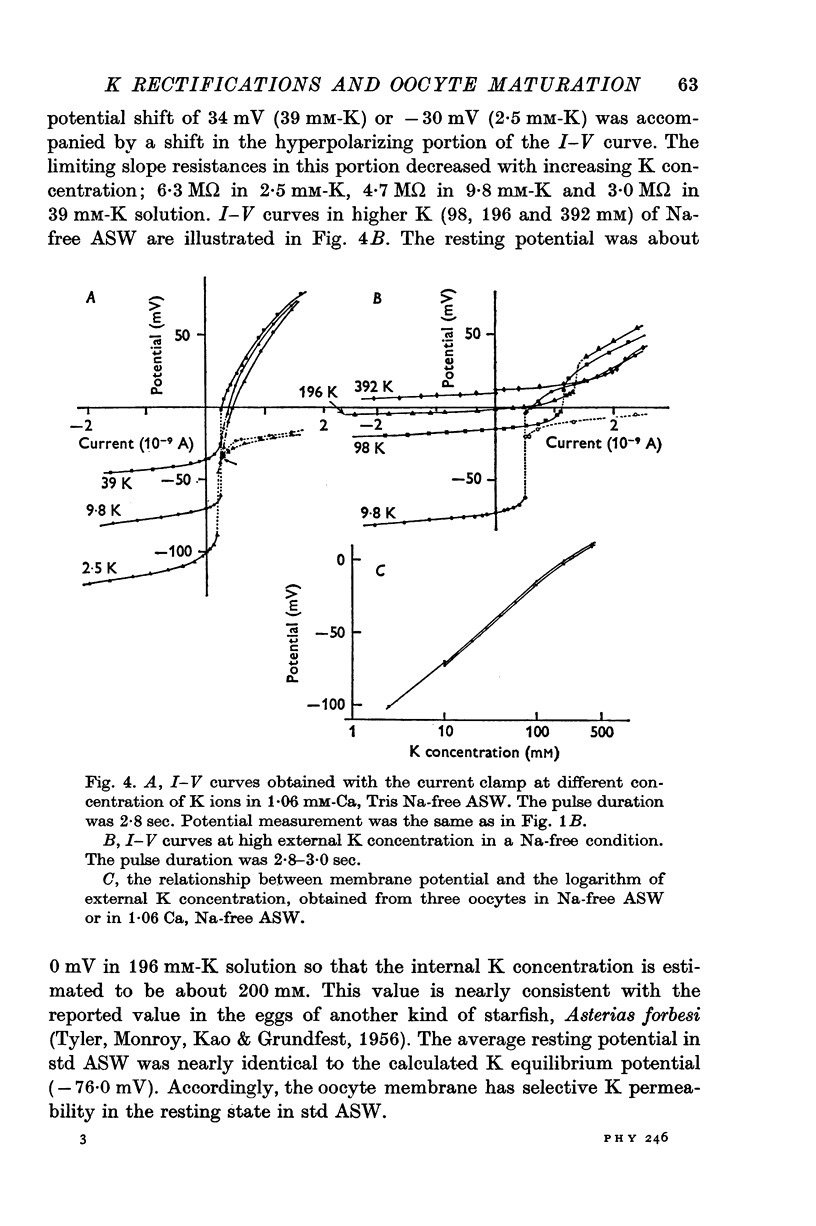
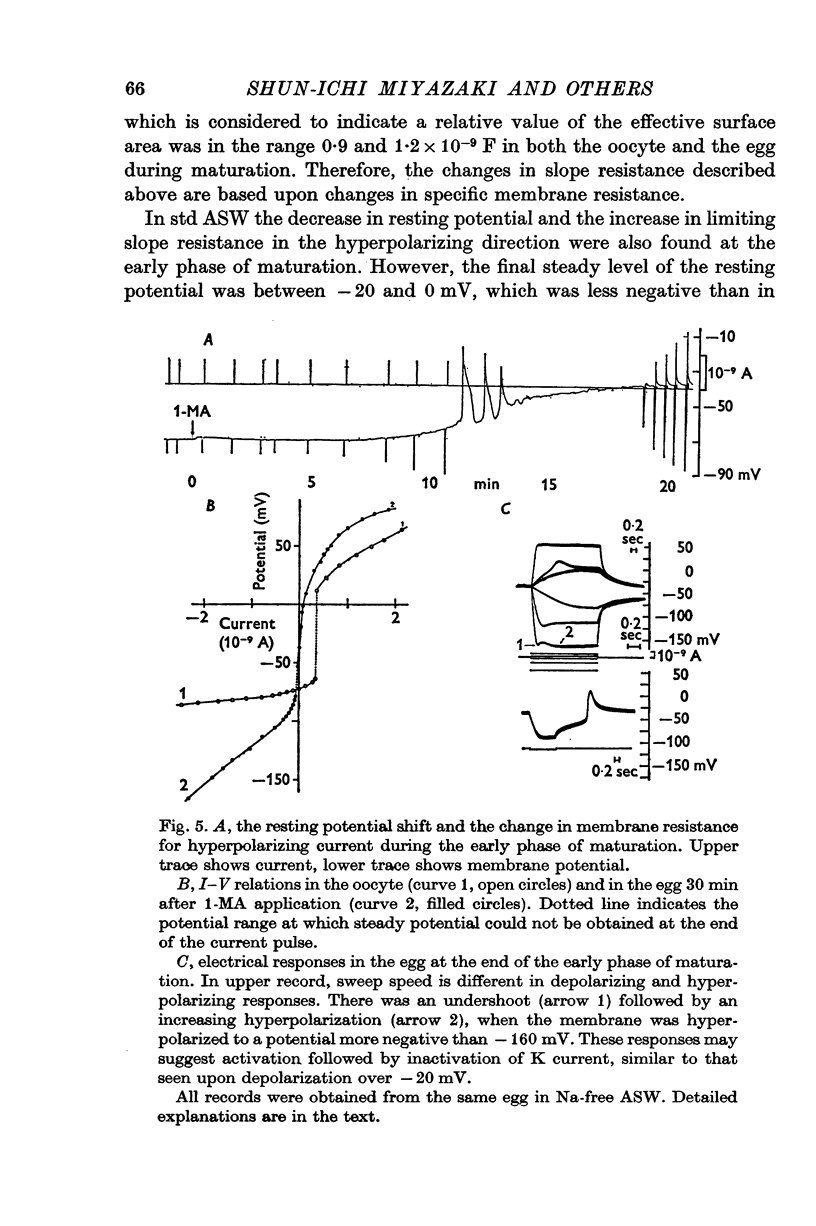
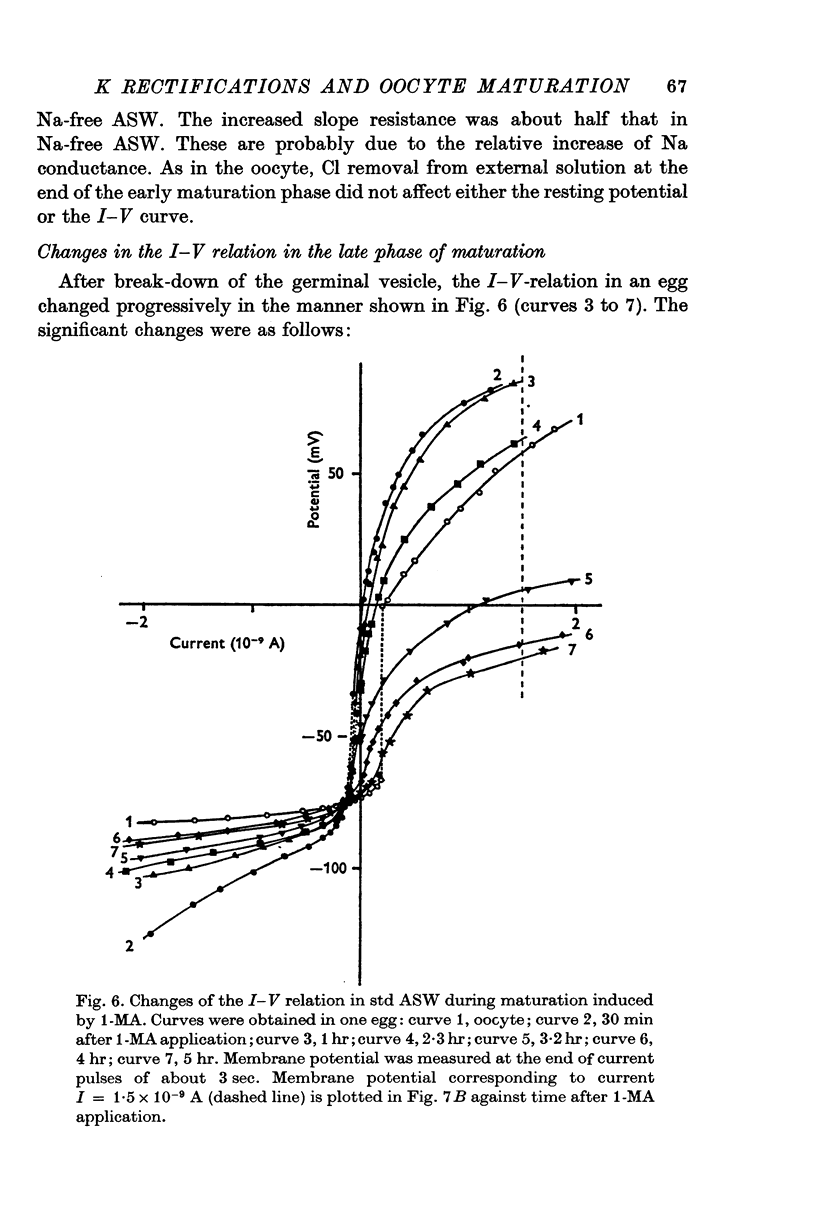
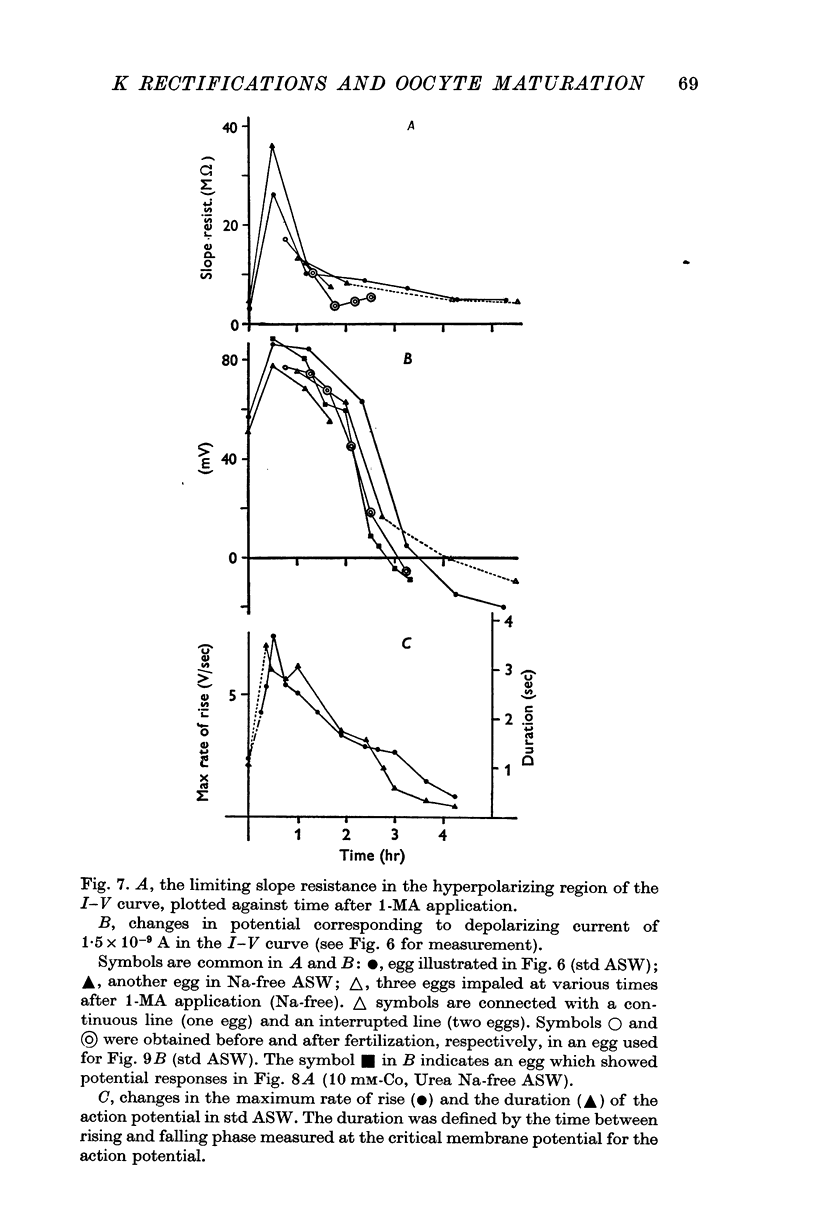
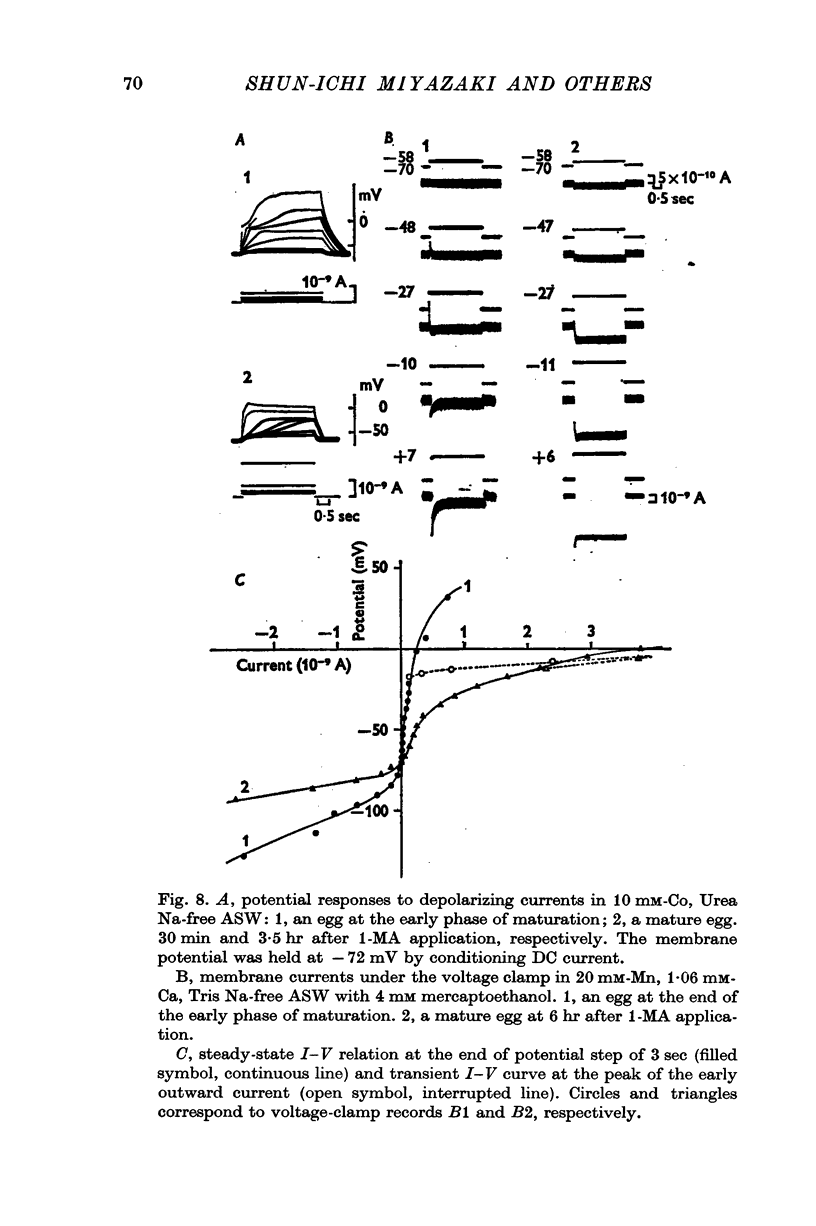
1. The current-voltage relations of the oocyte membrane of the starfish, Asterina pectinifera, and their changes during maturation were investigated using current-clamp techniques. 2. The resting potential of the oocyte membrane in sea water was found to be determined by the diffusion potential of K ions. 3. In the absence of Na and Ca inward currents the steady-state current-voltage relation of the oocyte membrane had inward-going K rectification at membrane potentials more negative than -65 mV and outward-going K rectification at potentials more positive than -30 mV, forming a S-shaped I-V curve. 4. A negative resistance region of the steady-state I-V curve was revealed with voltage-clamp technique in the potential range between -65 and -30 mV. 5.Transient K activation occurred when the membrane was brought from a resting potential of about -75 mV to potentials more positive than -20 mV, and this was immediately followed by K inactivation. Accordingly, the steady-state I-V relation showed only slight outward-going rectification. 6. At the beginning of meiosis, which is signalled by break-down of the nucleus, the limiting slope conductance in the inward rectifying region of the I-V curve decreased sevenfold. The cell membrane lost its selective permeability to K ions and was depolarized from -70 to between -20 and OmV in standard artificial sea-water. The depolarized resting potential was partly due to the relative increase in Na permeability. K conductance began to increase again within 30 min after breakdown of the nucleus. The resting potential became gradually larger and eventually attained -70 mV in the mature egg. 7. In the mature egg, K activation upon depolarization was no longer followed by inactivation. Accordingly, the slope conductance in the outward rectifying region of the I-V curve increased. 8. The action potential was augmented at the stage of nuclear breakdown. Thereafter the maximum rate of rise decreased and the duration of the action potential shortened. These changes were caused primarily by changes in K conductance during maturation. 9. Fertilization of the egg during the maturation process did not affect the changes in the I-V relation described above, except for a transient change of the membrane permeability upon fertilization.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHMAN R. F., KANNO Y., LOEWENSTEIN W. R. INTERCELLULAR ELECTRICAL COUPLING AT A FORMING MEMBRANE JUNCTION IN A DIVIDING CELL. Science. 1964 Aug 7;145(3632):604–605. doi: 10.1126/science.145.3632.604. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Grundfest H. Analysis of depolarizing and hyperpolarizing inactivation responses in gymnotid electroplaques. J Gen Physiol. 1966 Sep;50(1):141–169. doi: 10.1085/jgp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARMELIET E. E. Chloride ions and the membrane potential of Purkinje fibres. J Physiol. 1961 Apr;156:375–388. doi: 10.1113/jphysiol.1961.sp006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDFEST H. Ionic mechanisms in electrogenesis. Ann N Y Acad Sci. 1961 Sep 6;94:405–457. doi: 10.1111/j.1749-6632.1961.tb35554.x. [DOI] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundfest H. Heterogeneity of excitable membrane: electrophysiological and pharmacological evidence and some consequences. Ann N Y Acad Sci. 1966 Jul 14;137(2):901–949. doi: 10.1111/j.1749-6632.1966.tb50208.x. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Rectifying properties of heart muscle. Nature. 1960 Nov 5;188:495–495. doi: 10.1038/188495a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Yoshioka K. Effect of various ionic compositions upon the membrane potentials during activation of sea urchin eggs. Exp Cell Res. 1973 Mar 30;78(1):191–200. doi: 10.1016/0014-4827(73)90054-2. [DOI] [PubMed] [Google Scholar]
- Ito S., Yoshioka K. Renal activation potential observed in sea urchin egg during fertilization. Exp Cell Res. 1972 Jun;72(2):547–551. doi: 10.1016/0014-4827(72)90026-2. [DOI] [PubMed] [Google Scholar]
- Kanatani H. Induction of spawning and oocyte maturation by L-methyl-adenine in starfishes. Exp Cell Res. 1969 Oct;57(2):333–337. doi: 10.1016/0014-4827(69)90158-x. [DOI] [PubMed] [Google Scholar]
- Kanatani H., Shirai H., Nakanishi K., Kurokawa T. Isolation and indentification on meiosis inducing substance in starfish Asterias amurensis. Nature. 1969 Jan 18;221(5177):273–274. doi: 10.1038/221273a0. [DOI] [PubMed] [Google Scholar]
- MAENO T. Electrical characteristics and activation potential of Bufo eggs. J Gen Physiol. 1959 Sep;43:139–157. doi: 10.1085/jgp.43.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Action potential and non-linear current-voltage relation in starfish oocytes. J Physiol. 1975 Mar;246(1):37–54. doi: 10.1113/jphysiol.1975.sp010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K. Electrical excitability in the egg cell membrane of the tunicate. J Physiol. 1974 Apr;238(1):37–54. doi: 10.1113/jphysiol.1974.sp010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K., Yoshii M. Analysis of non-linearity observed in the current-voltage relation of the tunicate embryo. J Physiol. 1974 Apr;238(1):55–77. doi: 10.1113/jphysiol.1974.sp010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Takahashi K., Tsuda K. Calcium and sodium contributions to regenerative responses in the embryonic excitable cell membrane. Science. 1972 Jun 30;176(4042):1441–1443. doi: 10.1126/science.176.4042.1441. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Rosenthal J., Watson D. E. Membrane permeability changes in amphibian eggs at ovulation. J Cell Physiol. 1966 Jun;67(3):375–381. doi: 10.1002/jcp.1040670303. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA S., IWASAKI S., OBATA K. Delayed rectification and anomalous rectification in frog's skeletal muscle membrane. J Gen Physiol. 1962 Sep;46:97–115. doi: 10.1085/jgp.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S. Analysis of K inactivation and TEA action in the supramedullary cells of puffer. J Gen Physiol. 1966 Mar;49(4):629–640. doi: 10.1085/jgp.49.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kusano K. Behavior of delayed current under voltage clamp in the supramedullary neurons of puffer. J Gen Physiol. 1966 Mar;49(4):613–628. doi: 10.1085/jgp.49.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. Analysis of Spike Electrogenesis and Depolarizing K Inactivation in Electroplaques of Electrophorus electricus, L. J Gen Physiol. 1965 Nov 1;49(2):321–349. doi: 10.1085/jgp.49.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUBEN J. P., WERMAN R., GRUNDFEST H. The ionic mechanisms of hyperpolarizing responses in lobster muscle fibers. J Gen Physiol. 1961 Nov;45:243–265. doi: 10.1085/jgp.45.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Lundin L., Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Shen S., Mazia D. Membrane potential, membrane resistance and an energy requirement for the development of potassium conductance in the fertilization reaction of echinoderm eggs. Exp Cell Res. 1972 May;72(1):195–203. doi: 10.1016/0014-4827(72)90581-2. [DOI] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTWEIN W., KASSEBAUM D. G. On the mechanism of spontaneous impulse generation in the pacemaker of the heart. J Gen Physiol. 1961 Nov;45:317–330. doi: 10.1085/jgp.45.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Miyazaki S. I., Kidokoro Y. Development of excitability in embryonic muscle cell membranes in certain tunicates. Science. 1971 Jan 29;171(3969):415–418. doi: 10.1126/science.171.3969.415. [DOI] [PubMed] [Google Scholar]
- Tupper J., Saunders J. W., Jr, Edwards C. The onset of electrical communication between cells in the developing starfish embryo. J Cell Biol. 1970 Jul;46(1):187–191. doi: 10.1083/jcb.46.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


