Abstract
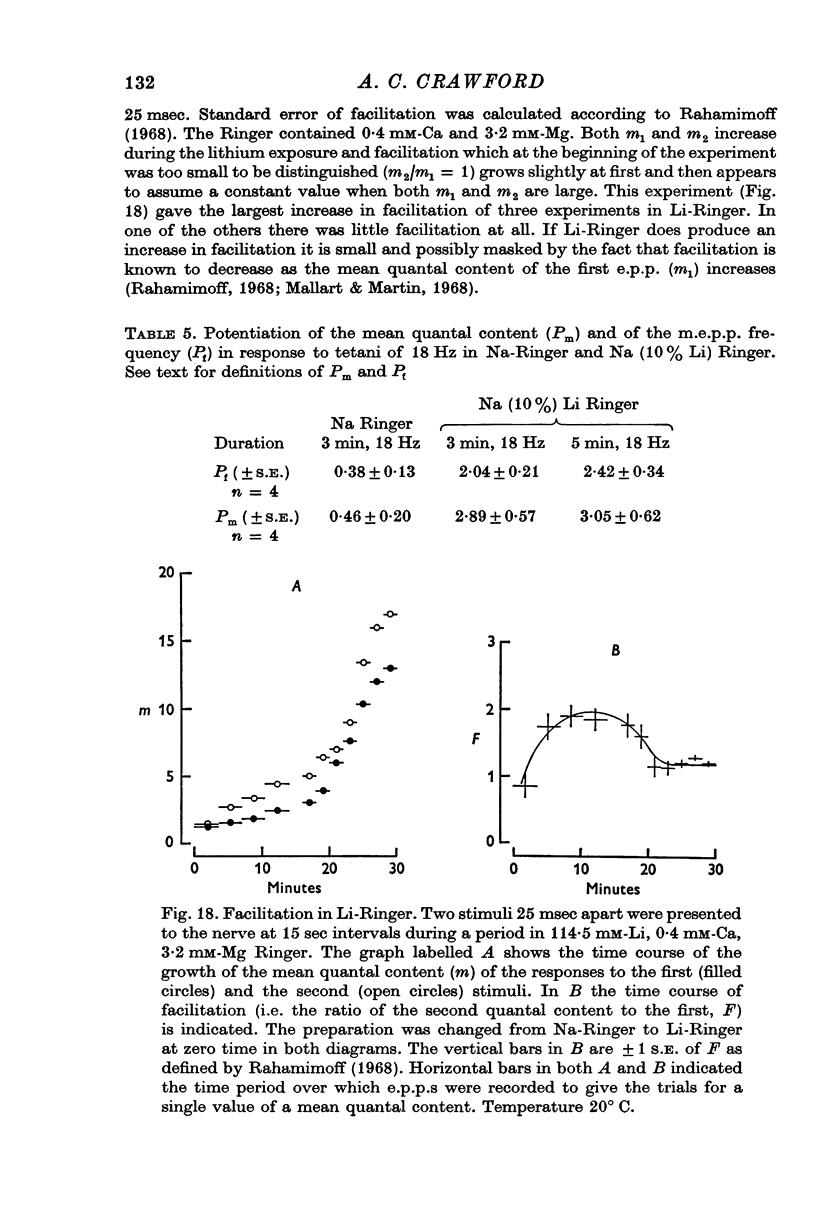
1. Transmitter release has been examined at the frog neuromuscular junction when all or part of the Na of the Ringer is replaced by Li ions. 2. No immediate change occurs in either the mean quantal content of the end-plate potential or the miniature end-plate potential frequency on changing to Li Ringer but over the following hour both these quantities increase by more than two orders of magnitude. 3. During thefirst 30-40 min of an exposure to Li-Ringer the m.e.p.p. frequency rises exponentially with a time constant of 10 min, and the mean quantal content of the e.p.p. grows by addition of extra evoked quanta, the increment rising exponentially with a time constant the same as that of the m.e.p.p. frequency. 4. Following this initial period in Li-Ringer the m. e.p.p. frequency accelerates to a peak of several hundred quanta per second and then declines slowly over the next few hours. Just before the m.e.p.p.frequency peak the conduction velocity of the presynaptic action potential declines and shortly afterwards synaptic transmission fails as the action potential no longer conducts into the terminals. 5. The rise in the m.e.p.p. frequency during the first 30-40 min is independent of the [Ca-2+]o. At subsequent times before the peak external Cabecomes progressively more effective in accelerating the m.e.p.p. frequency and in the presence of 1mM-EGTA spontaneous release stabilizes at 60-80 quanta/sec. 6. The [Li-+]o strongly influences the rate of increases in both evoked and spontaneous release but not their extent; replacing only half the Na of the Ringer by Li increases the time constants of the increases to about 30 min. 7. Rises in the m.e.p.p. frequency can be irreversibly accelerated by tetanizing the nerve in a Li-Ringer in which the Ca has been chelated by EGTA. The extent of the increases in the m.e.p.p. frequency is dependent on the number of pulses in the tetanus and is little affected by the frequency of stimulation. Accumulation of Li ions inside the presynaptic terminals probably underlies the changes in spontaneous release. 8. When only 10 percent of the Na of the Ringer is replaced by Li-+ ions the magnitude of post-tetanic potentiation of the e.p.p. and of the post-tetanic rise in the m.e.p.p. frequency is increased. Under these conditions changes in facilitation of the e.p.p. are small. 9. Various mechanisms by which Li could alter transmitter release are discussed and it is suggested that intracellular Ca sequestering mechanisms of the presynaptic terminals are affected when an end-plate is exposed to Li-Ringer.
Full text
PDF


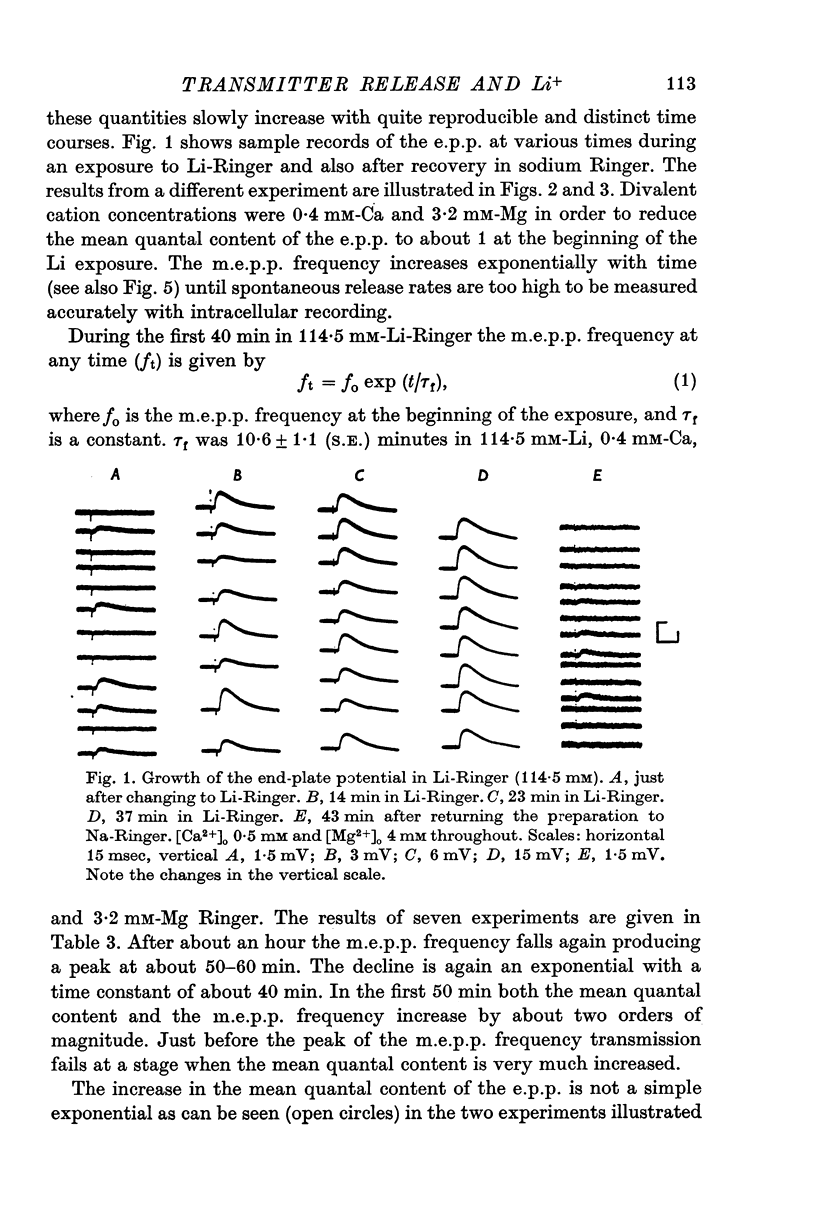
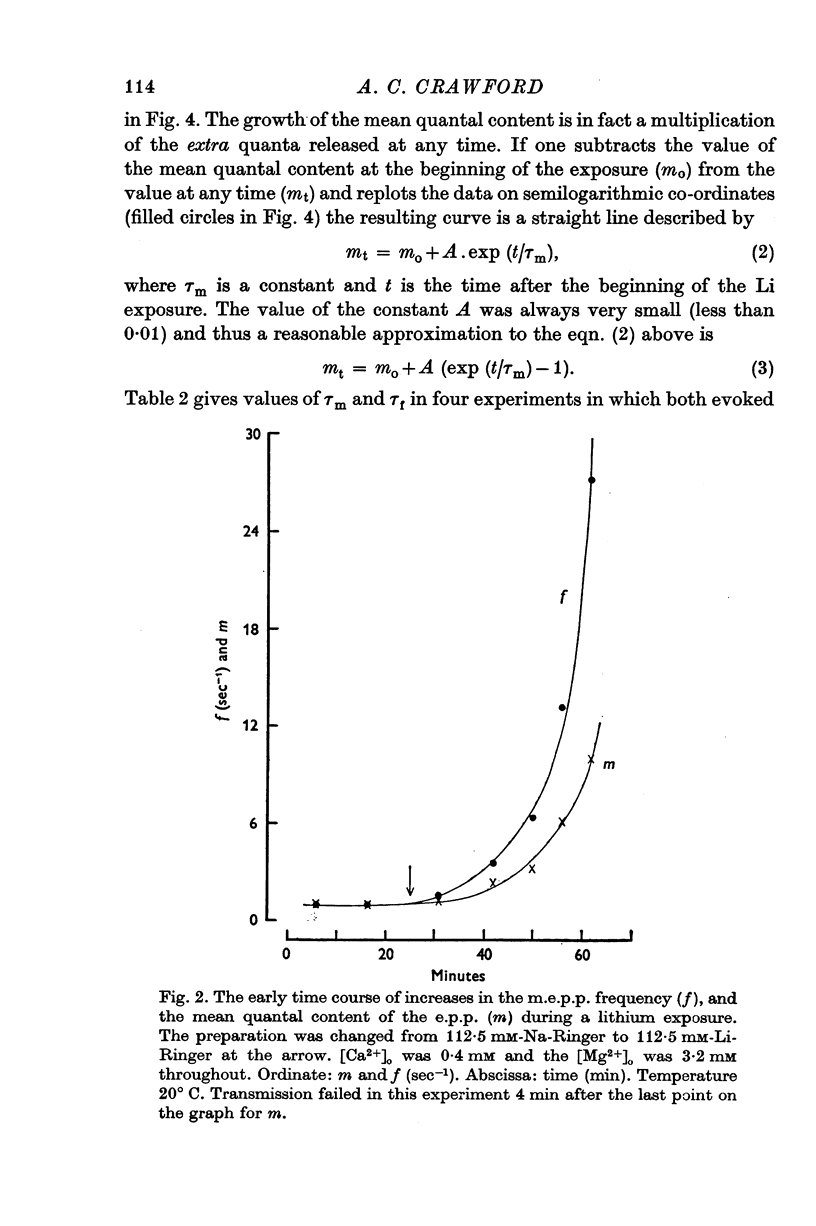
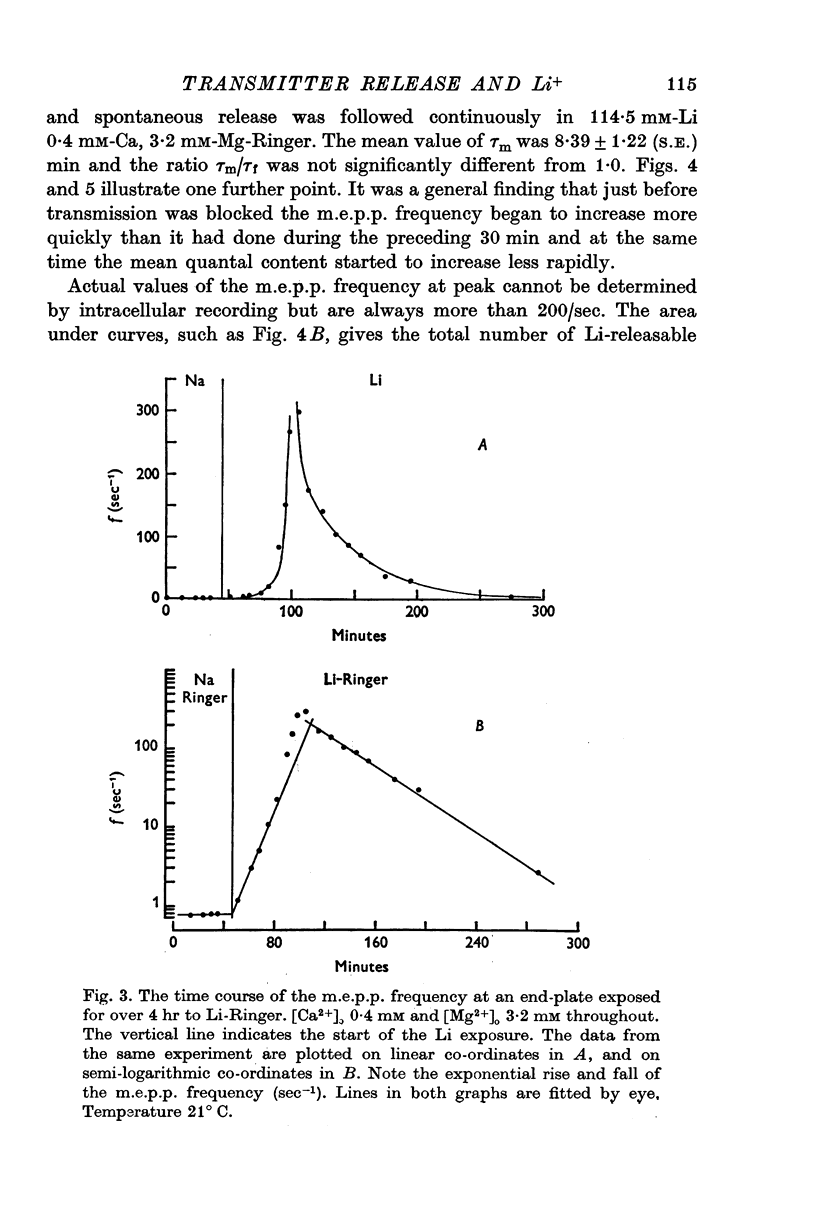
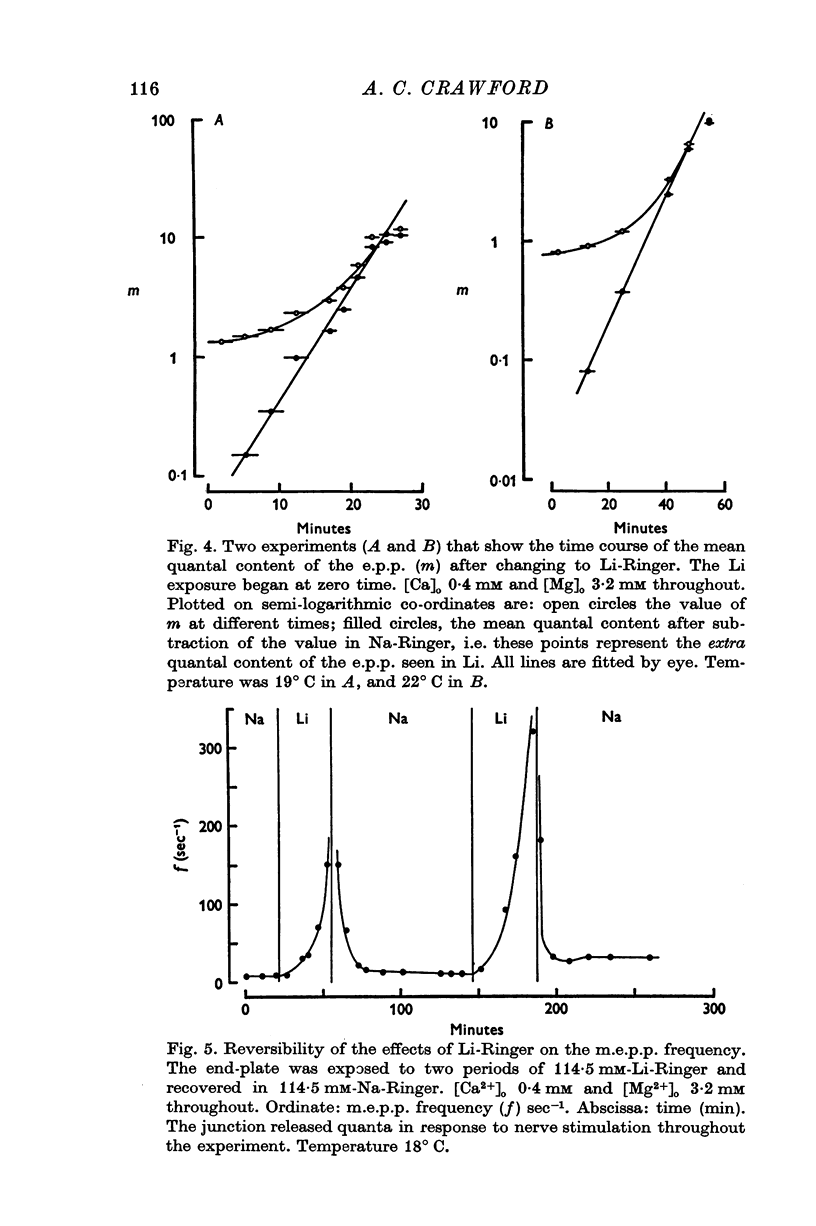

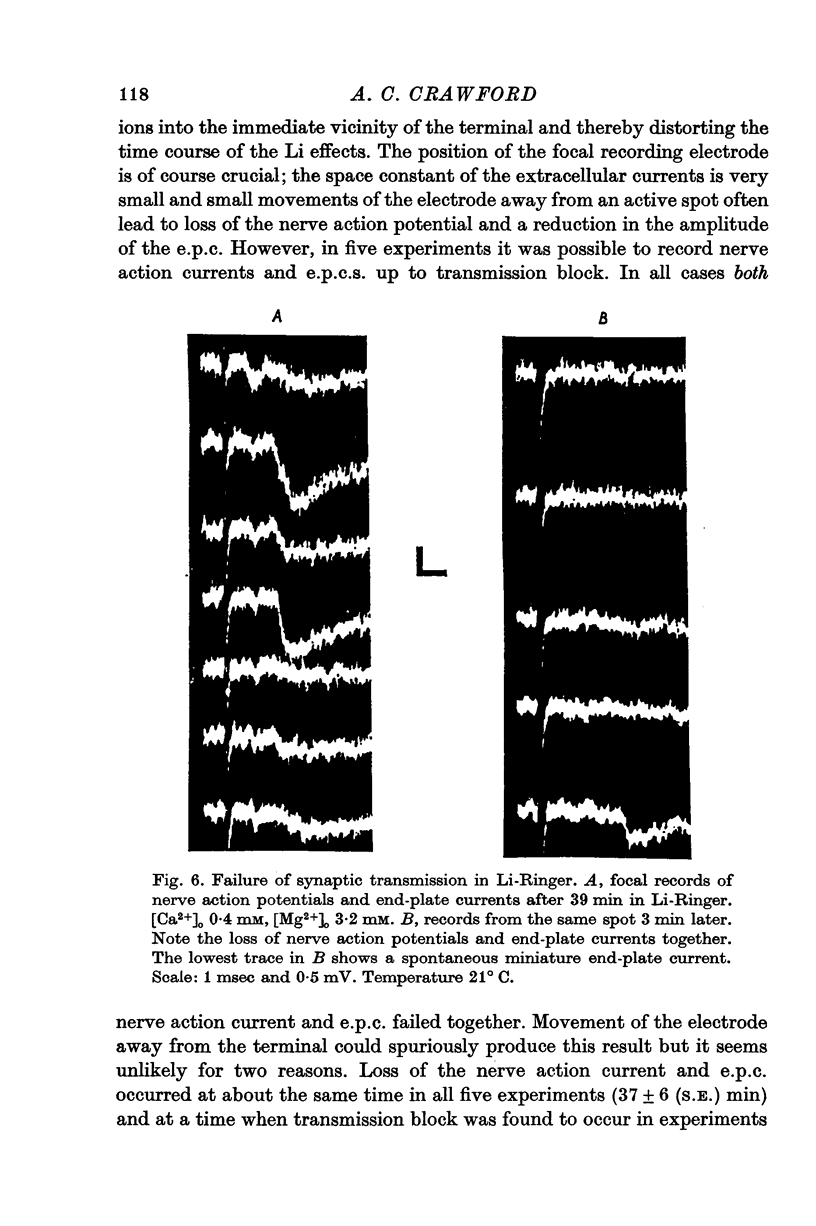
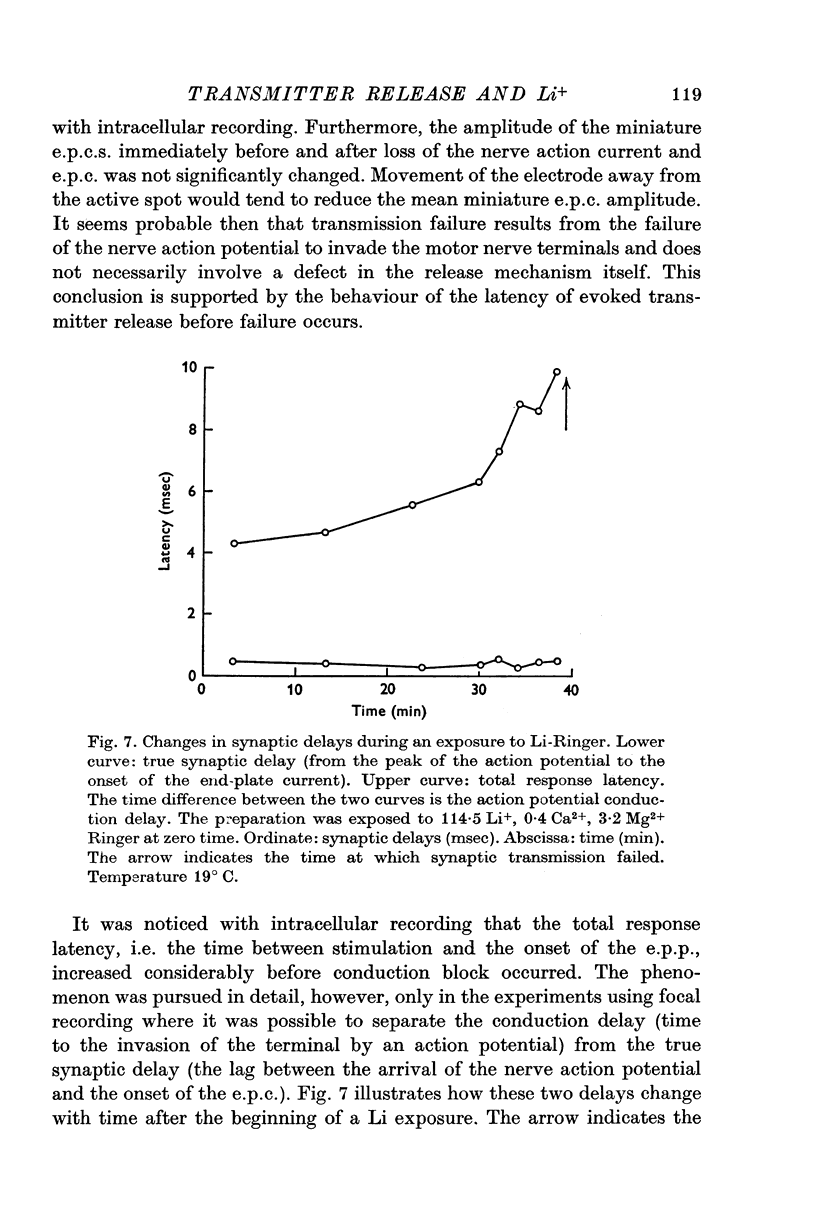

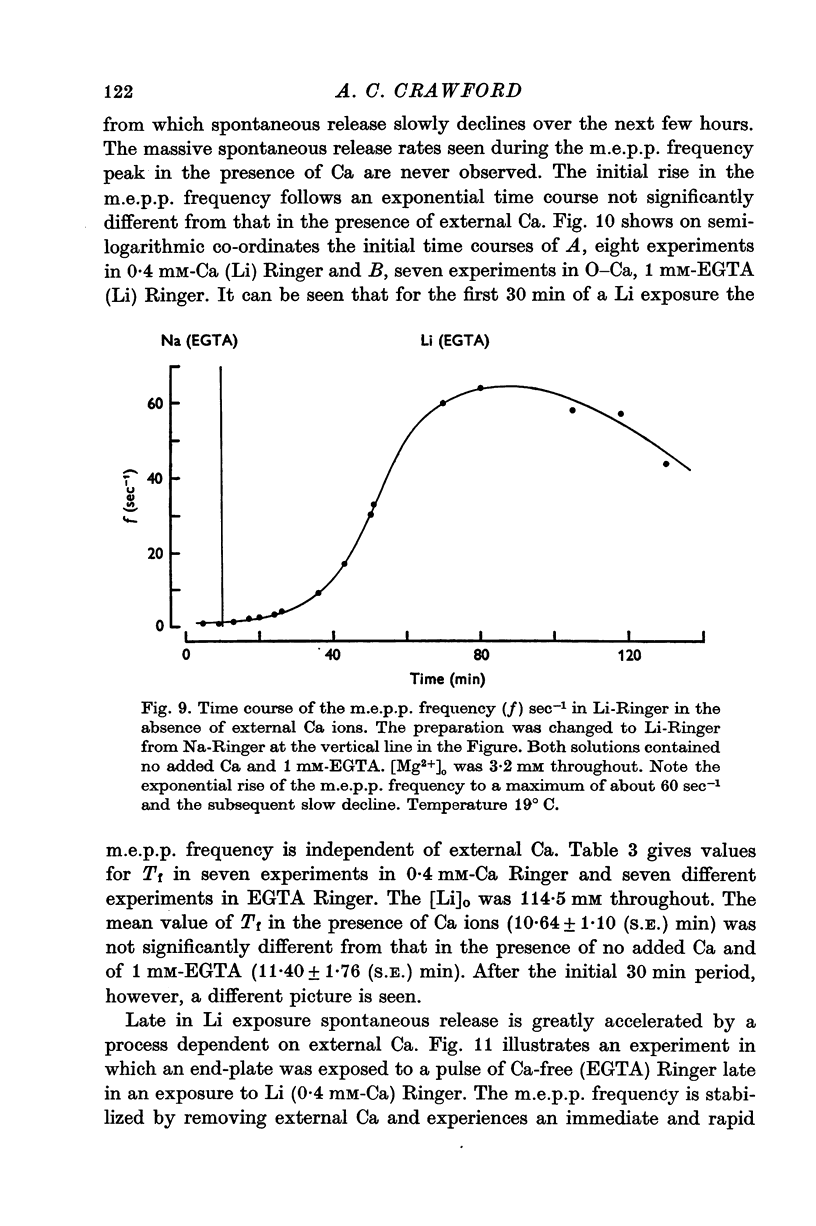
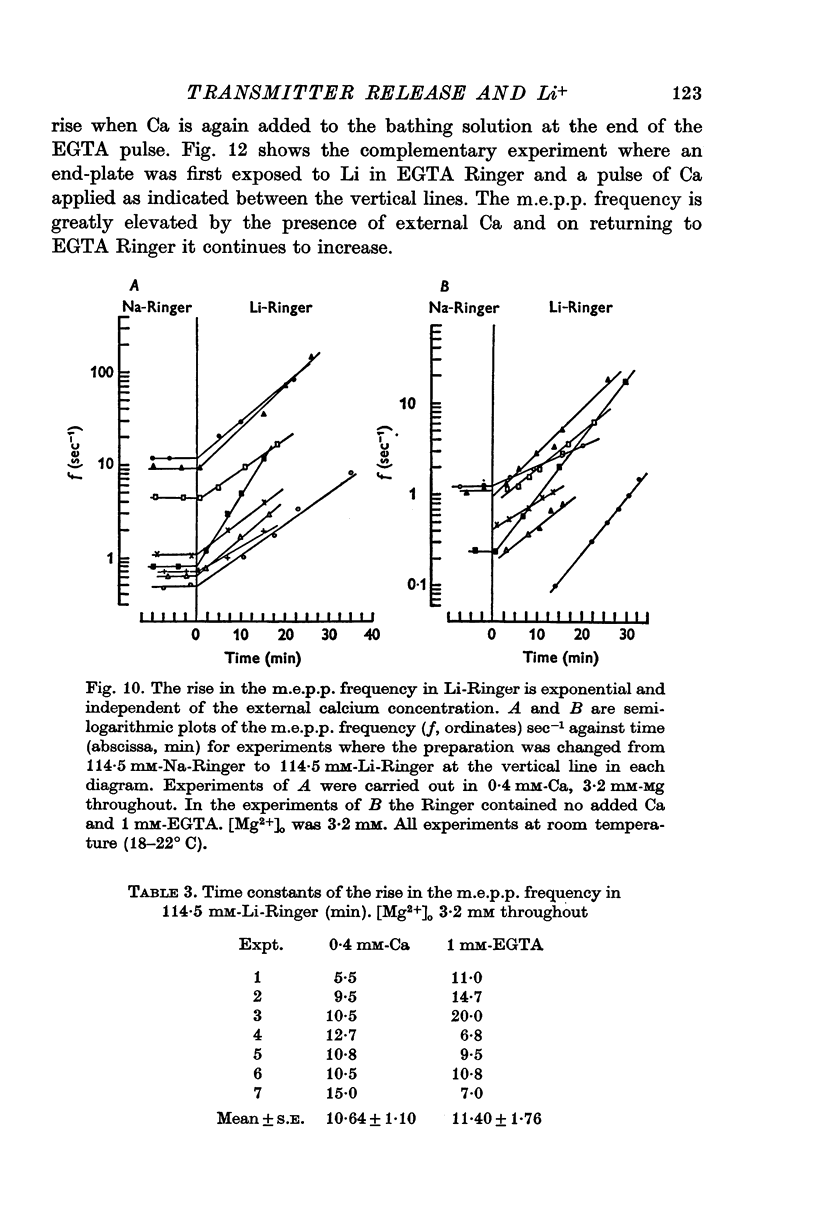
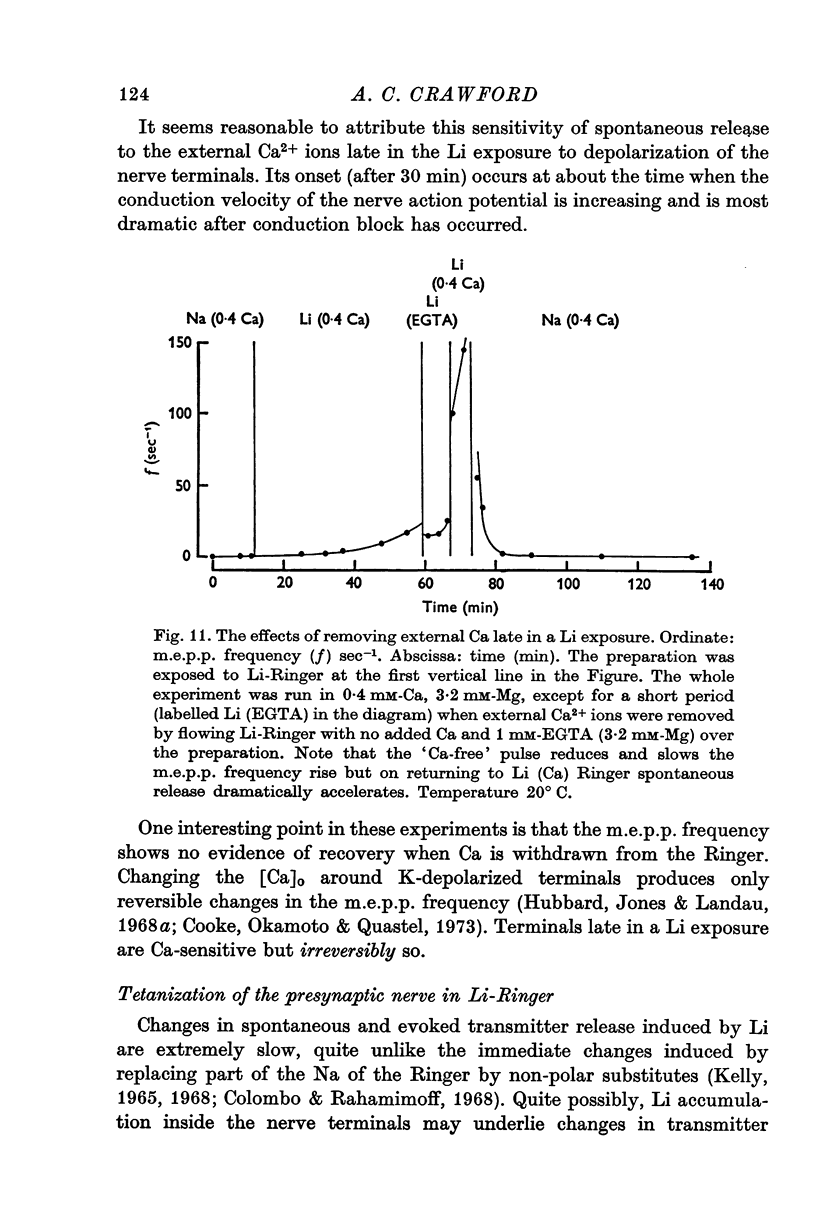
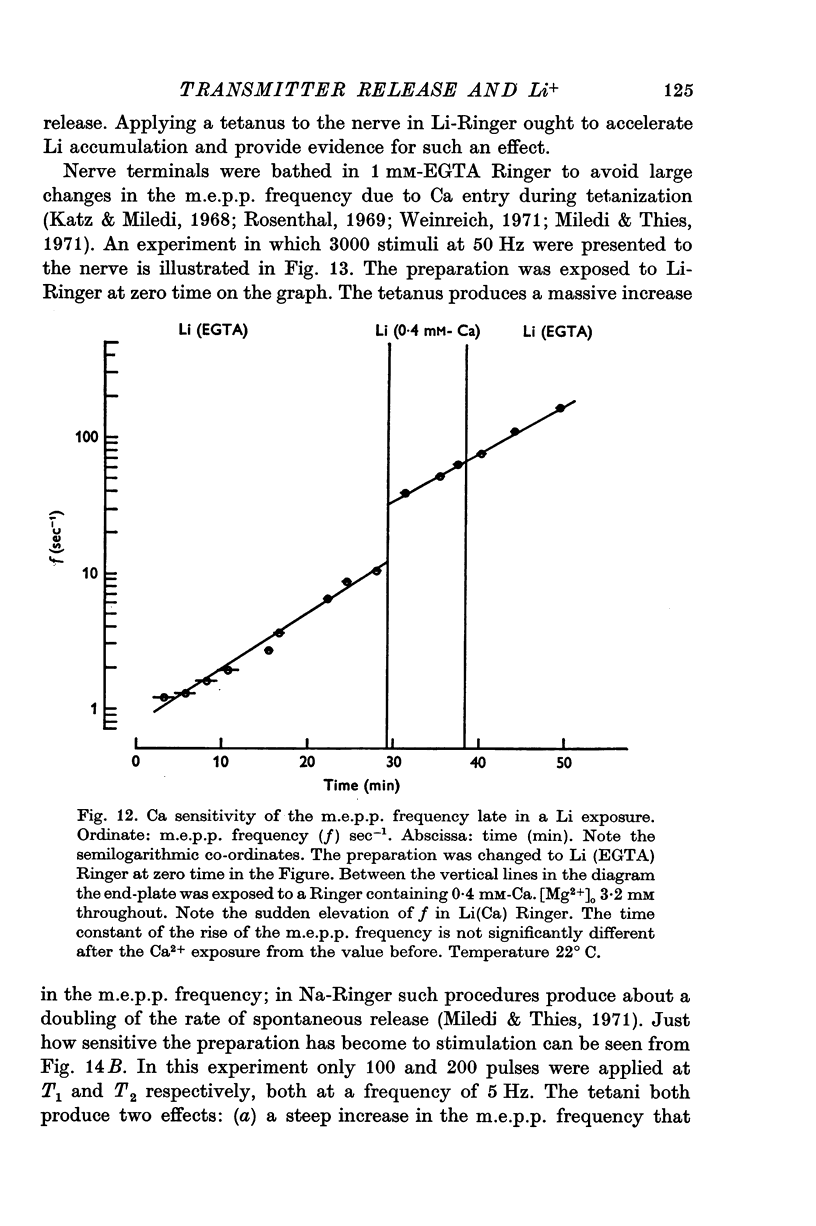



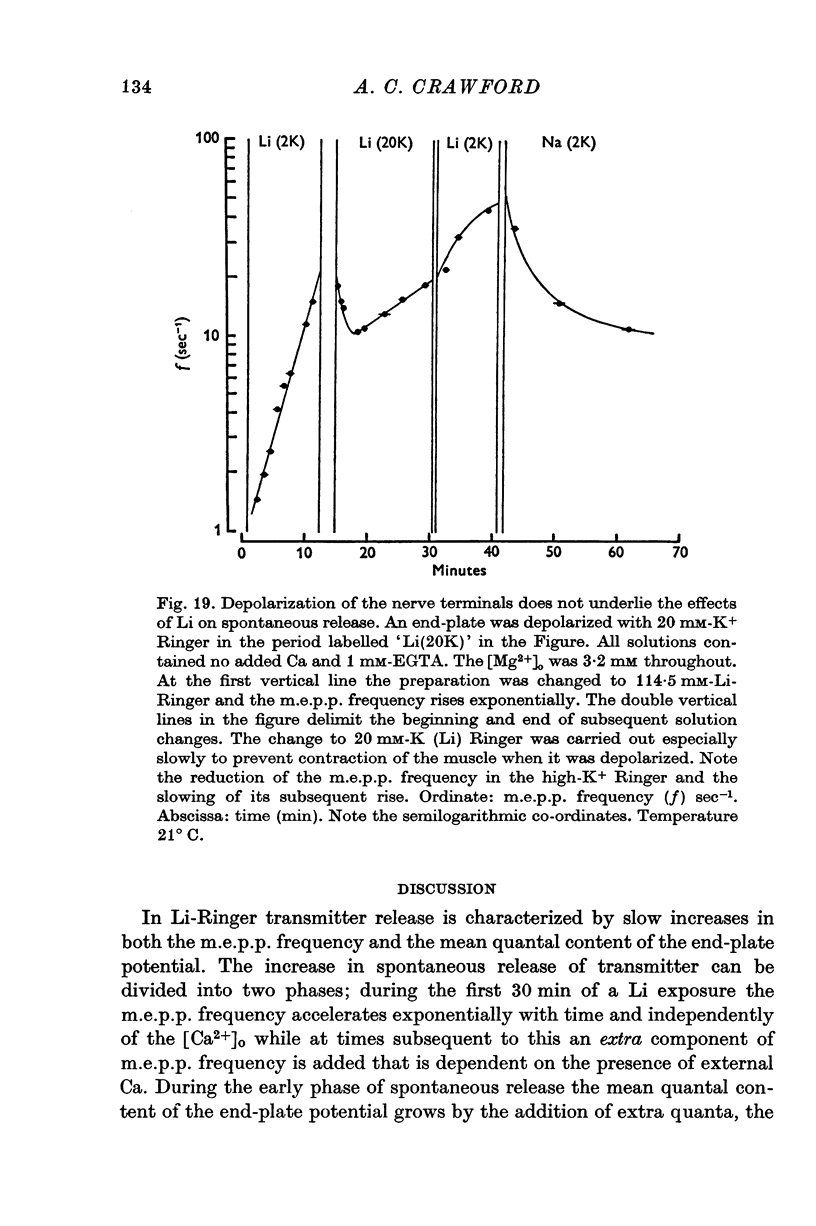







Selected References
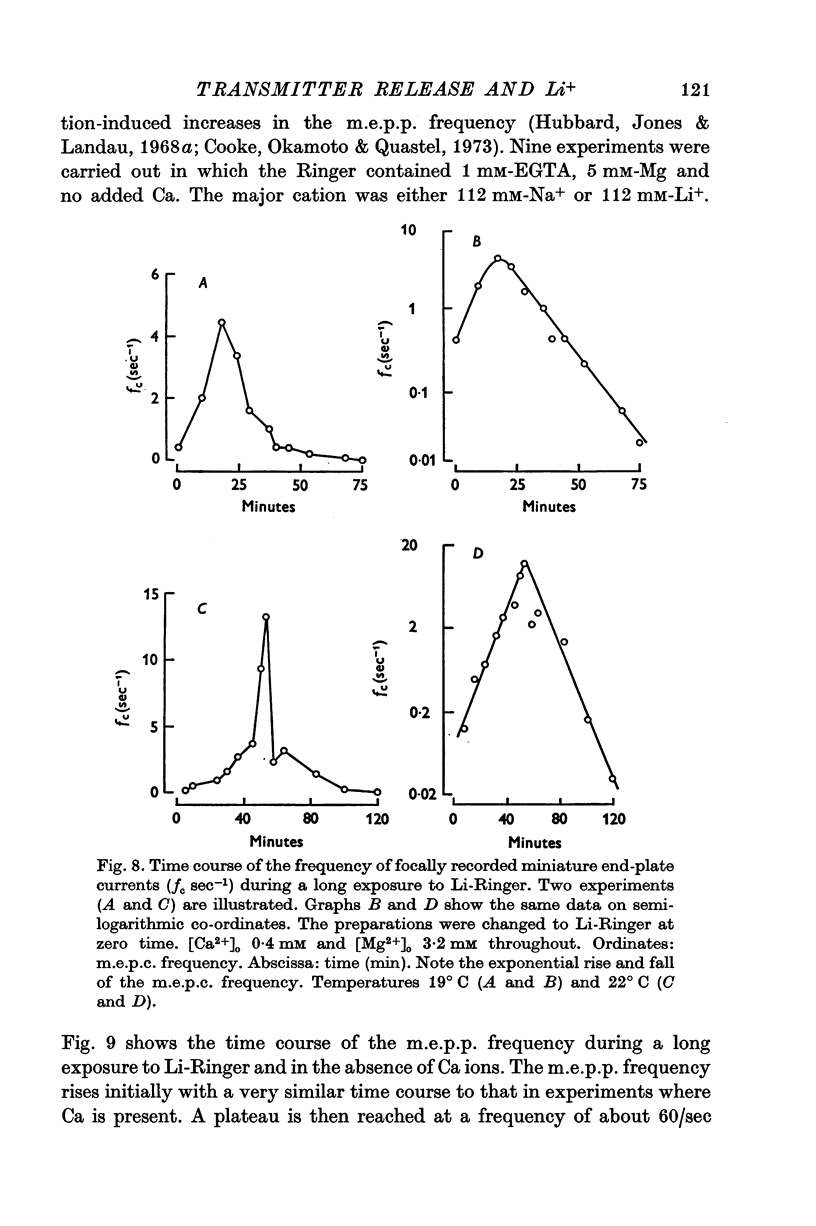
These references are in PubMed. This may not be the complete list of references from this article.
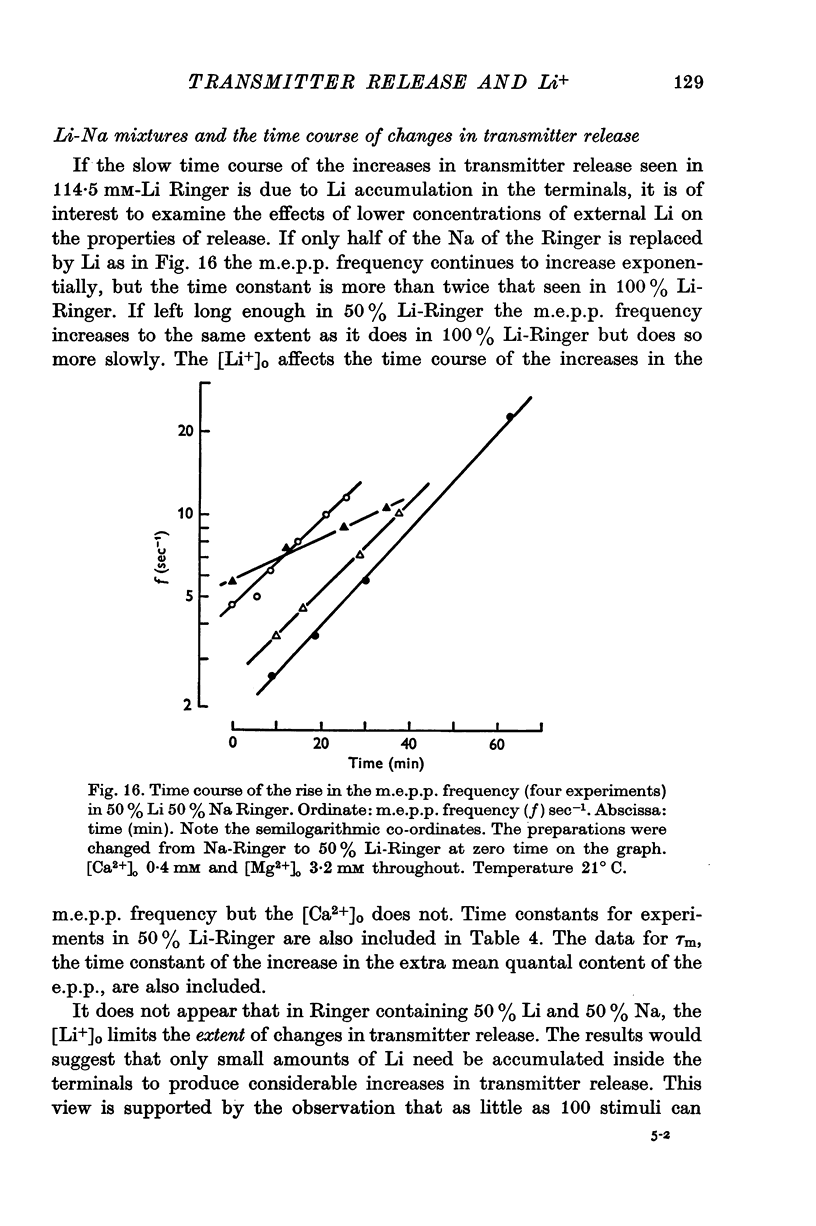
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
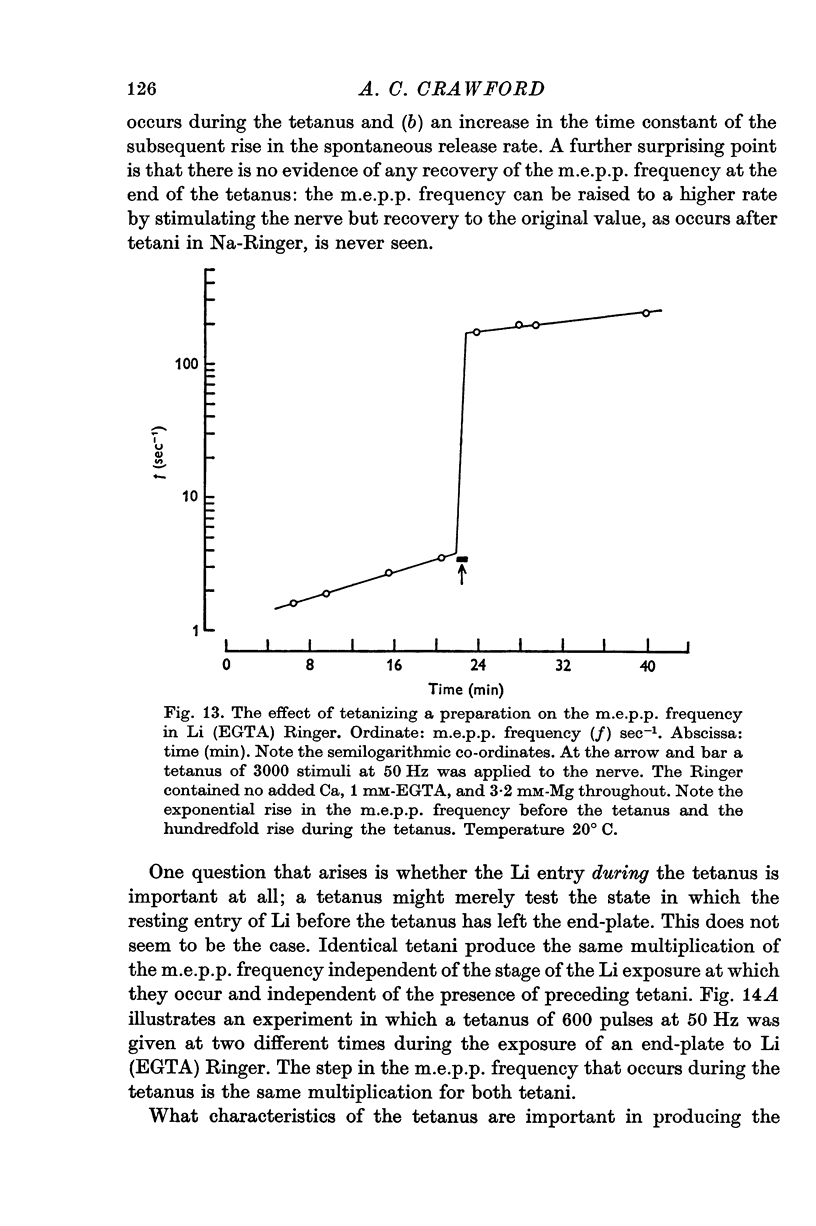
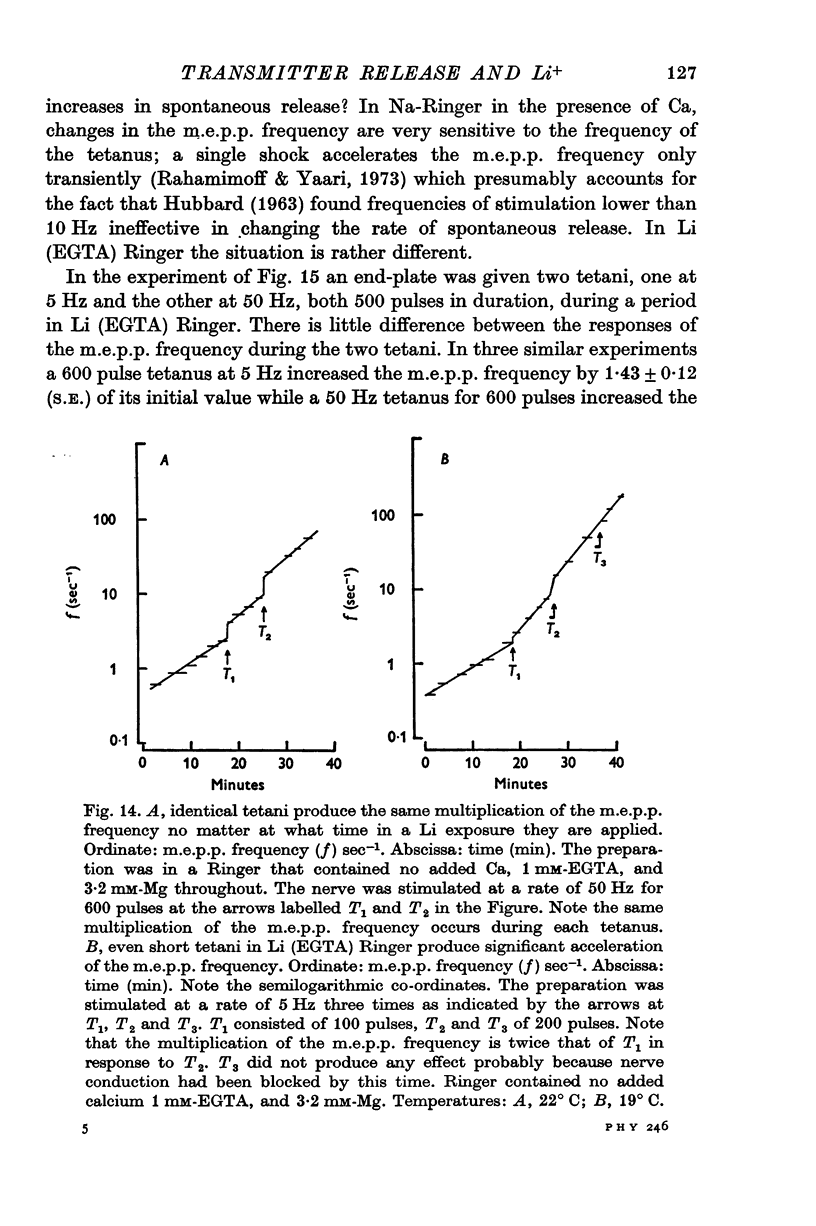
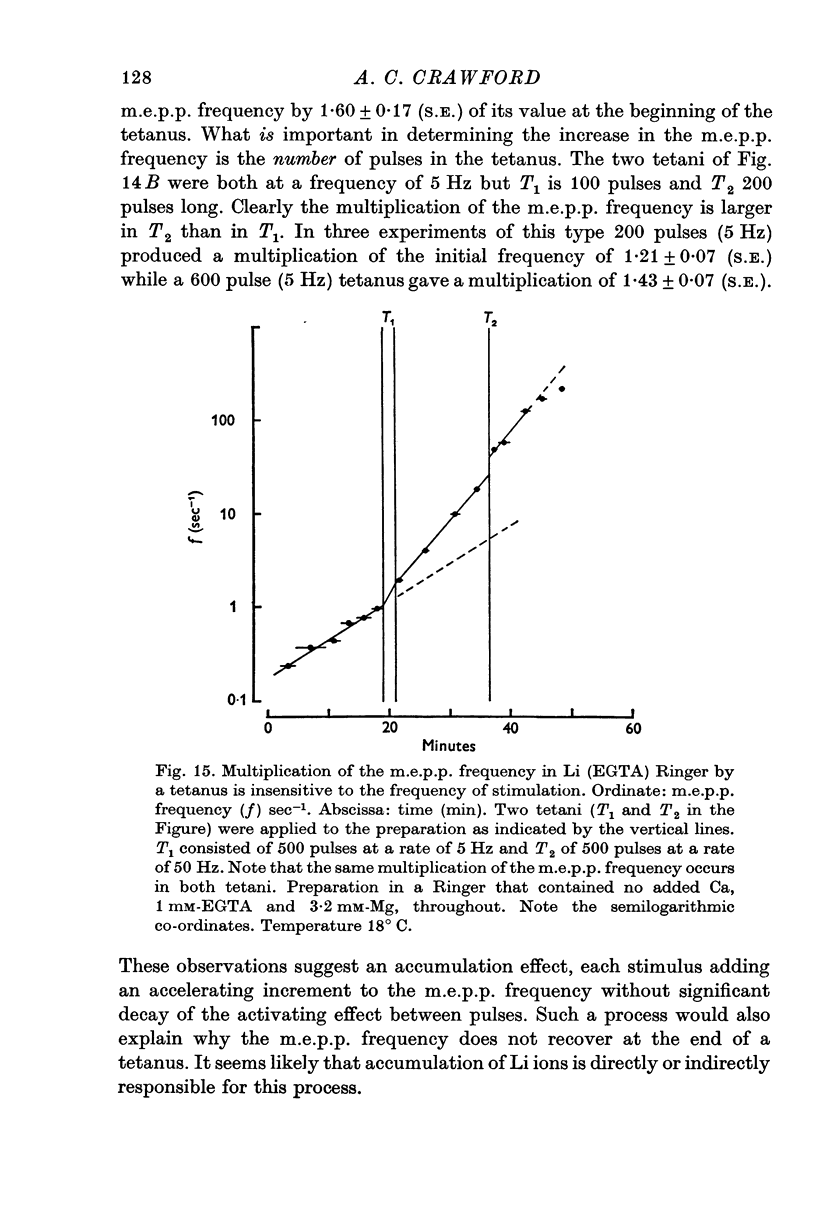
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
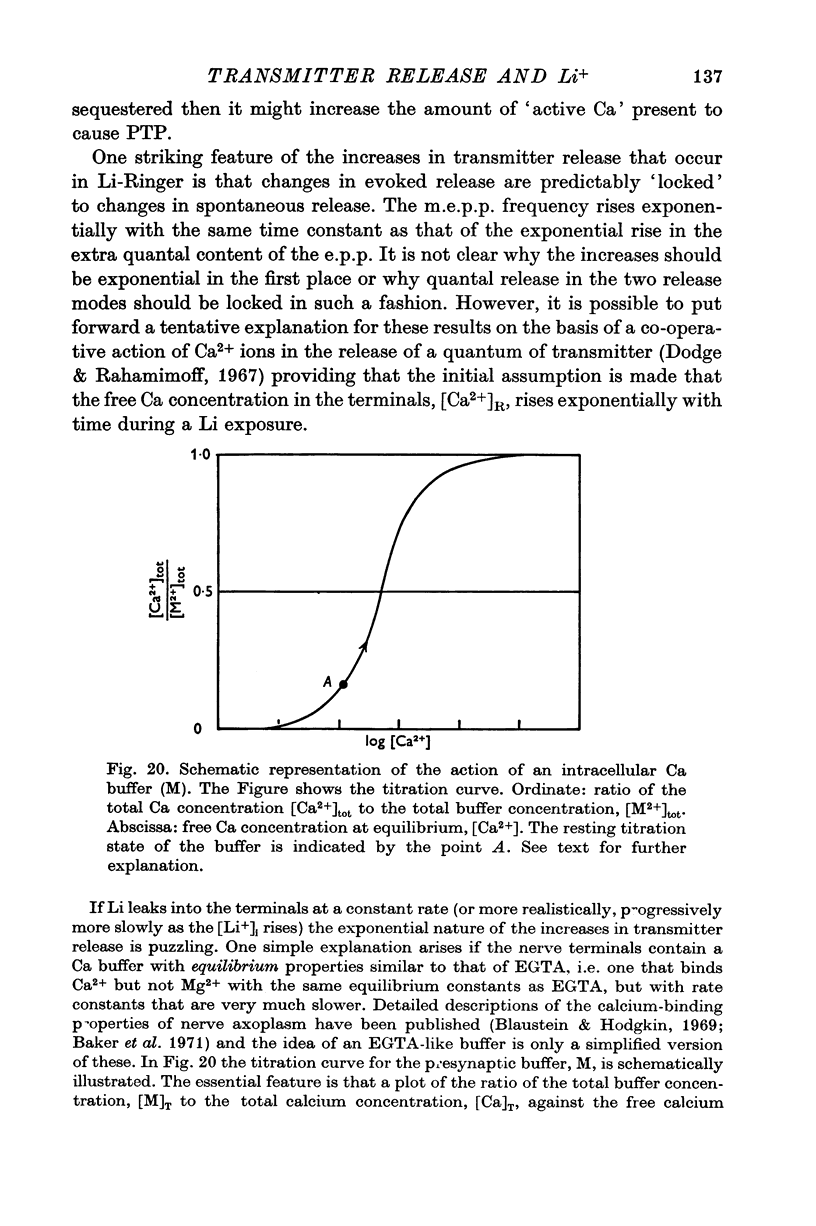
- Balnave R. J., Gage P. W. On facilitation of transmitter release at the toad neuromuscular junction. J Physiol. 1974 Jun;239(3):657–675. doi: 10.1113/jphysiol.1974.sp010588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARMELIET E. E. INFLUENCE OF LITHIUM IONS ON THE TRANSMEMBRANE POTENTIAL AND CATION CONTENT OF CARDIAC CELLS. J Gen Physiol. 1964 Jan;47:501–530. doi: 10.1085/jgp.47.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J. J., Gage P. W. Lithium stimulates secretion of acetylcholine in the absence of extracellular calcium. Brain Res. 1973 Feb 28;50(2):476–479. doi: 10.1016/0006-8993(73)90755-5. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Hurlbut W. P., Mauro A. Changes in the fine structure of the neuromuscular junction of the frog caused by black widow spider venom. J Cell Biol. 1972 Jan;52(1):1–14. doi: 10.1083/jcb.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomo F., Rahamimoff R. Interaction between sodium and calcium ions in the process of transmitter release at the neuromuscular junction. J Physiol. 1968 Sep;198(1):203–218. doi: 10.1113/jphysiol.1968.sp008602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. The dependence of evoked transmitter release on external calcium ions at very low mean quantal contents. J Physiol. 1974 Jul;240(2):255–278. doi: 10.1113/jphysiol.1974.sp010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Miledi R., Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969 Jan;200(1):267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FATT P. The electromotive action of acetylcholine at the motor end-plate. J Physiol. 1950 Oct 16;111(3-4):408–422. doi: 10.1113/jphysiol.1950.sp004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLYNN F., MAIZELS M. Cation control in human erythrocytes. J Physiol. 1949 Dec;110(3-4):301–318. doi: 10.1113/jphysiol.1949.sp004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., STAMPFLI R. Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibers. J Physiol. 1951 Feb;112(3-4):496–508. doi: 10.1113/jphysiol.1951.sp004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J., Katz B., Miledi R. Structural and functional changes of frog neuromuscular junctions in high calcium solutions. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):407–415. doi: 10.1098/rspb.1971.0072. [DOI] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Microphysiology of vertebrate neuromuscular transmission. Physiol Rev. 1973 Jul;53(3):674–723. doi: 10.1152/physrev.1973.53.3.674. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KELLY J. S. ANTAGONISM BETWEEN NA+ AND CA2+ AT THE NEUROMUSCULAR JUNCTION. Nature. 1965 Jan 16;205:296–297. doi: 10.1038/205296a0. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The intracellular calcium contents of some invertebrate nerves. J Physiol. 1956 Nov 28;134(2):399–407. doi: 10.1113/jphysiol.1956.sp005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto N., Kirpekar S. M. Effect of manganese and lanthanum on spontaneous release of acetylcholine at frog motor nerve terminals. Nat New Biol. 1972 Jan 5;235(53):29–30. doi: 10.1038/newbio235029a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S. The antagonism of Ca2+ by Na+ and other monovalent ions at the frog neuromuscular junction. Q J Exp Physiol Cogn Med Sci. 1968 Jul;53(3):239–249. doi: 10.1113/expphysiol.1968.sp001967. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of tetanic and post-tetanic potentiation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):353–371. doi: 10.1113/jphysiol.1973.sp010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Activation of transmitter release by strontium and calcium ions at the neuromuscular junction. J Physiol. 1971 Jul;215(3):709–726. doi: 10.1113/jphysiol.1971.sp009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Neuromuscular transmission: inhibition by manganese ions. Science. 1972 Apr 21;176(4032):308–309. doi: 10.1126/science.176.4032.308. [DOI] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dependence of the action potential of the frog's heart on the external and intracellular sodium concentration. J Physiol. 1966 May;184(2):312–334. doi: 10.1113/jphysiol.1966.sp007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Yamakawa K. The effects of lithium on the neuromuscular junction of the frog. Jpn J Physiol. 1966 Oct 15;16(5):541–550. doi: 10.2170/jjphysiol.16.541. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D. Ionic mechanism of post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1971 Jan;212(2):431–446. doi: 10.1113/jphysiol.1971.sp009333. [DOI] [PMC free article] [PubMed] [Google Scholar]


