Abstract
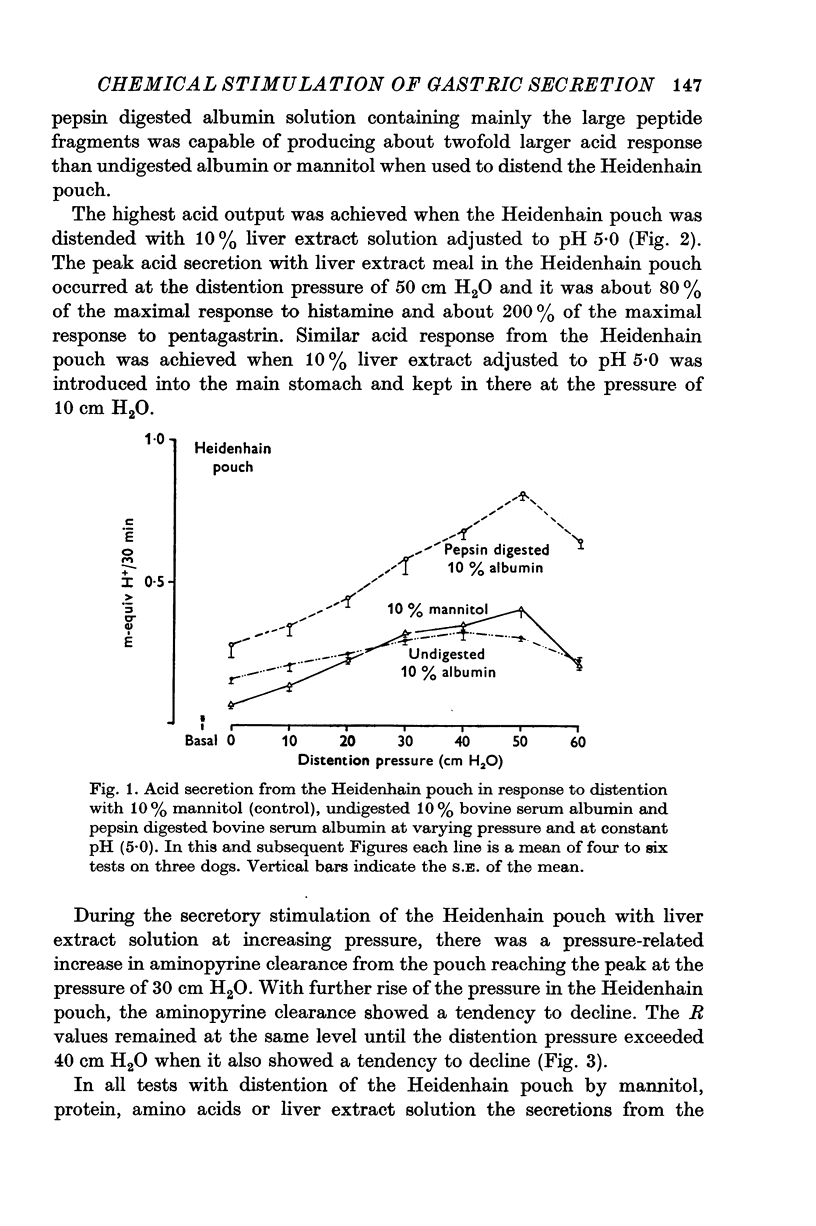
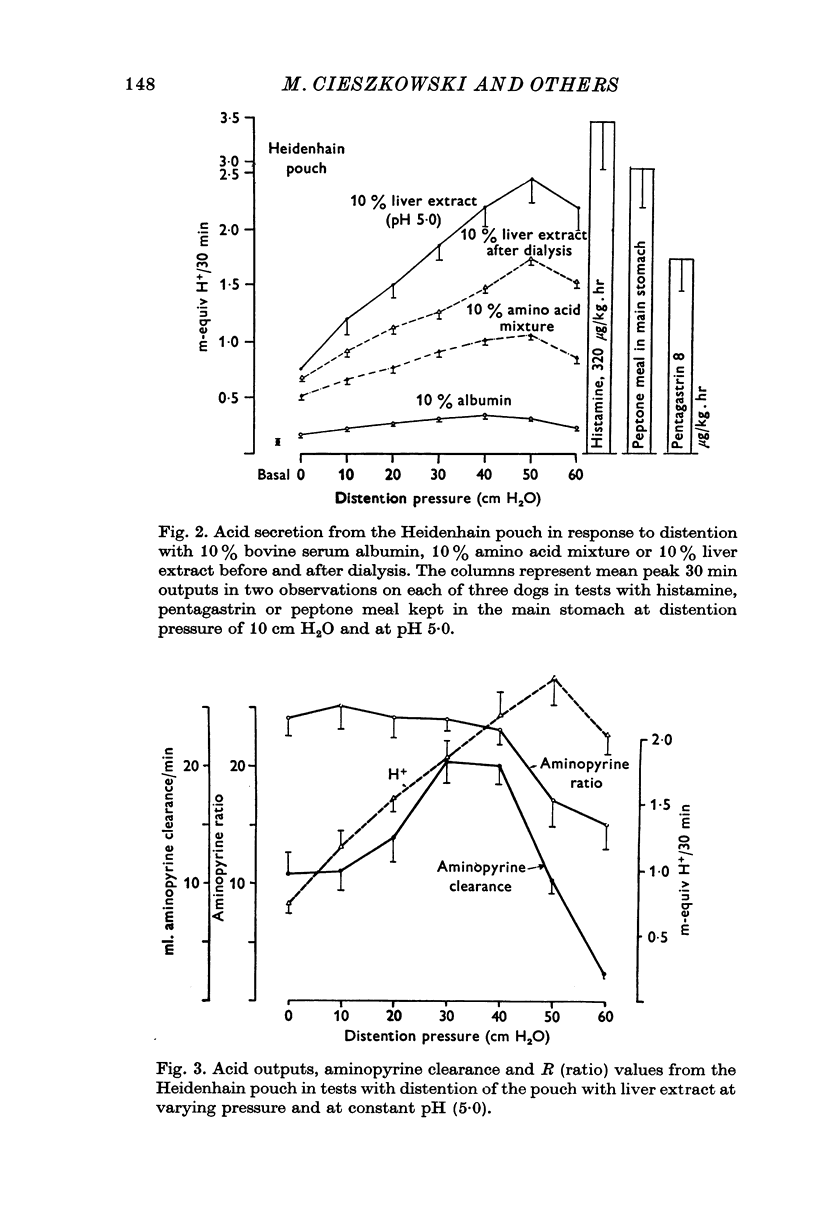
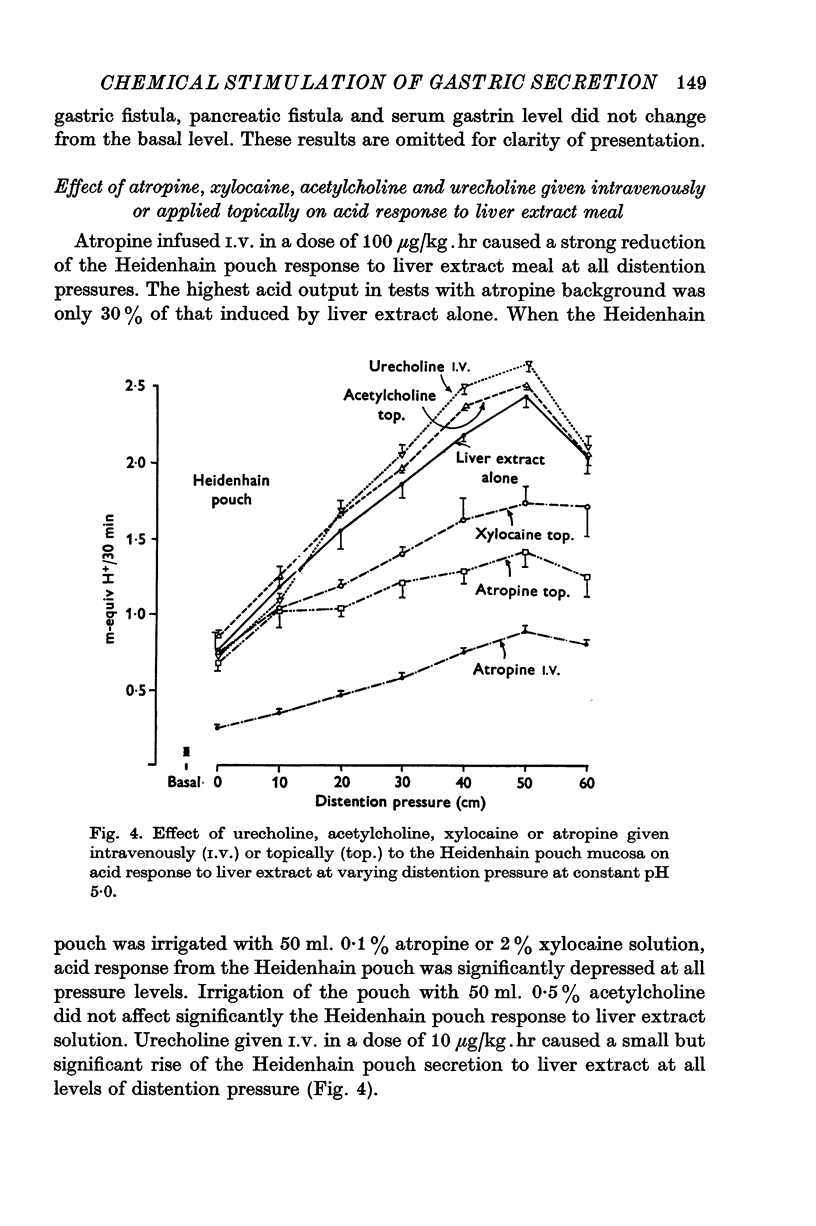
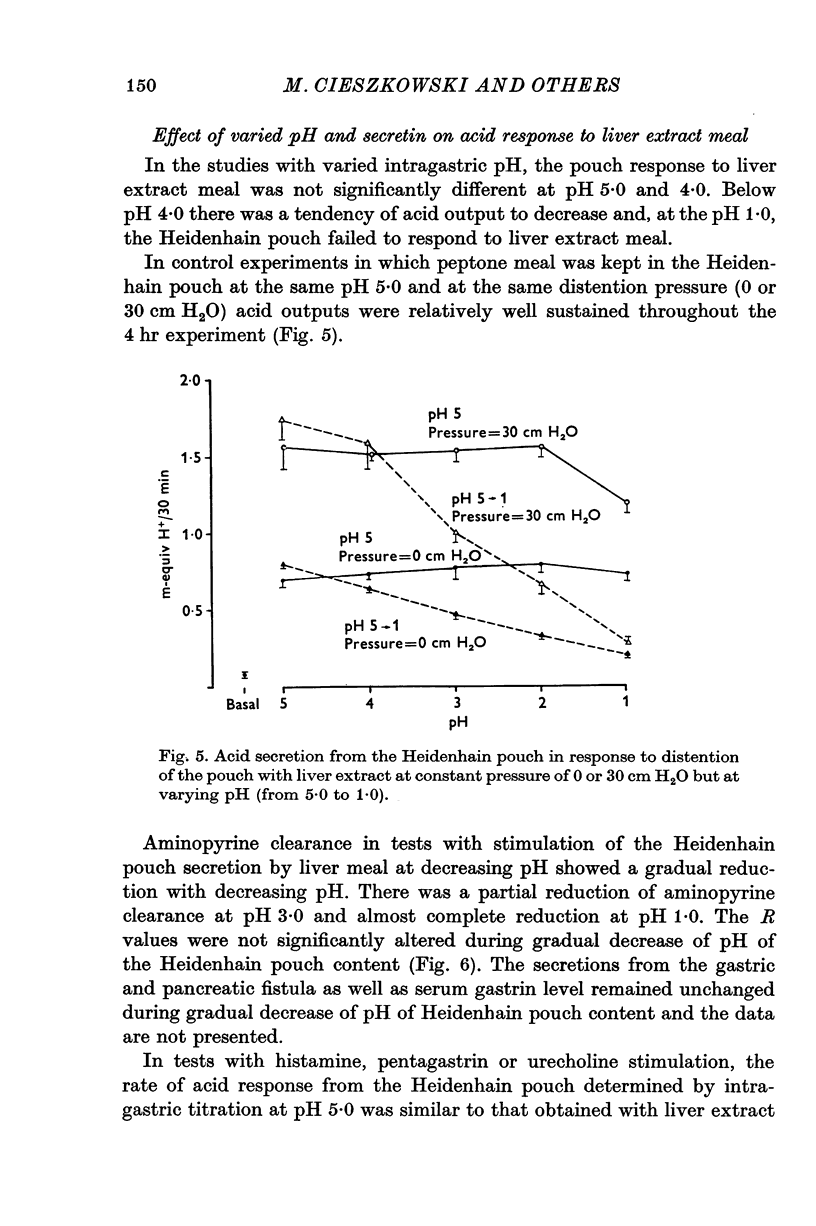
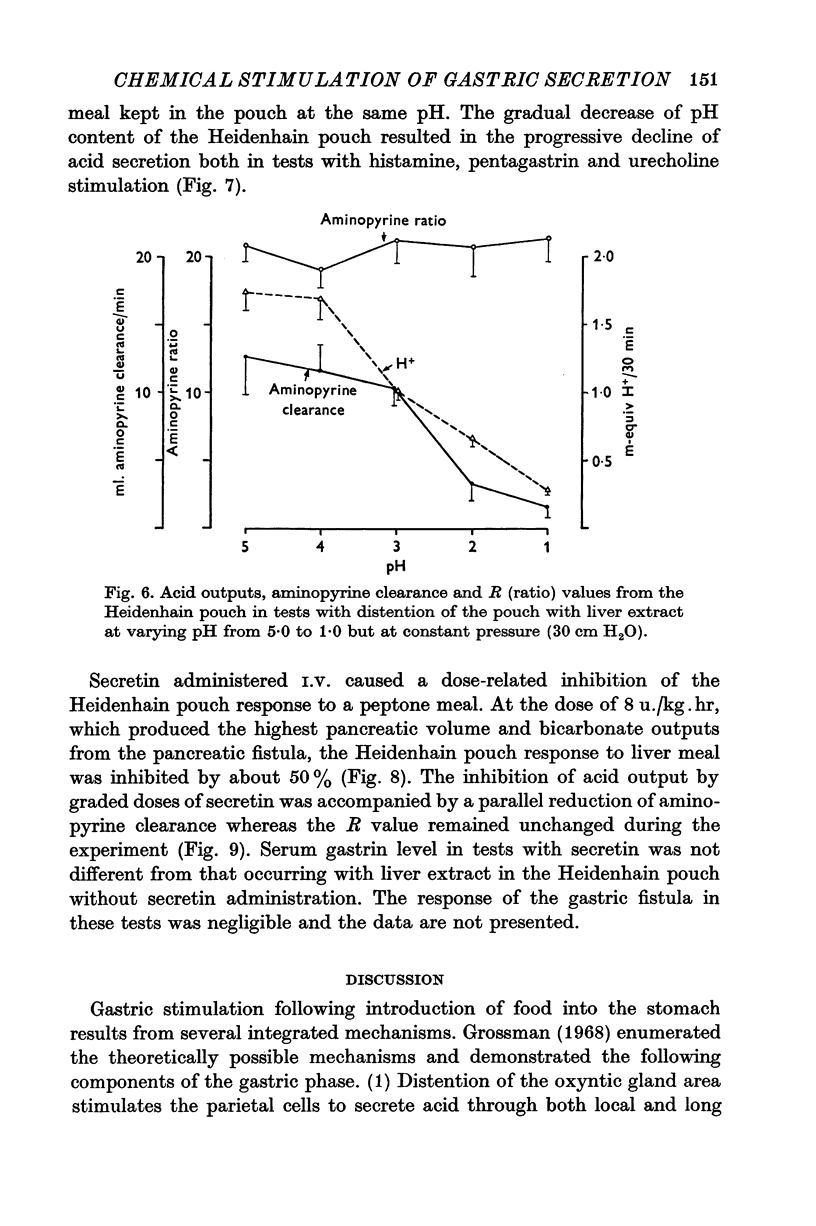
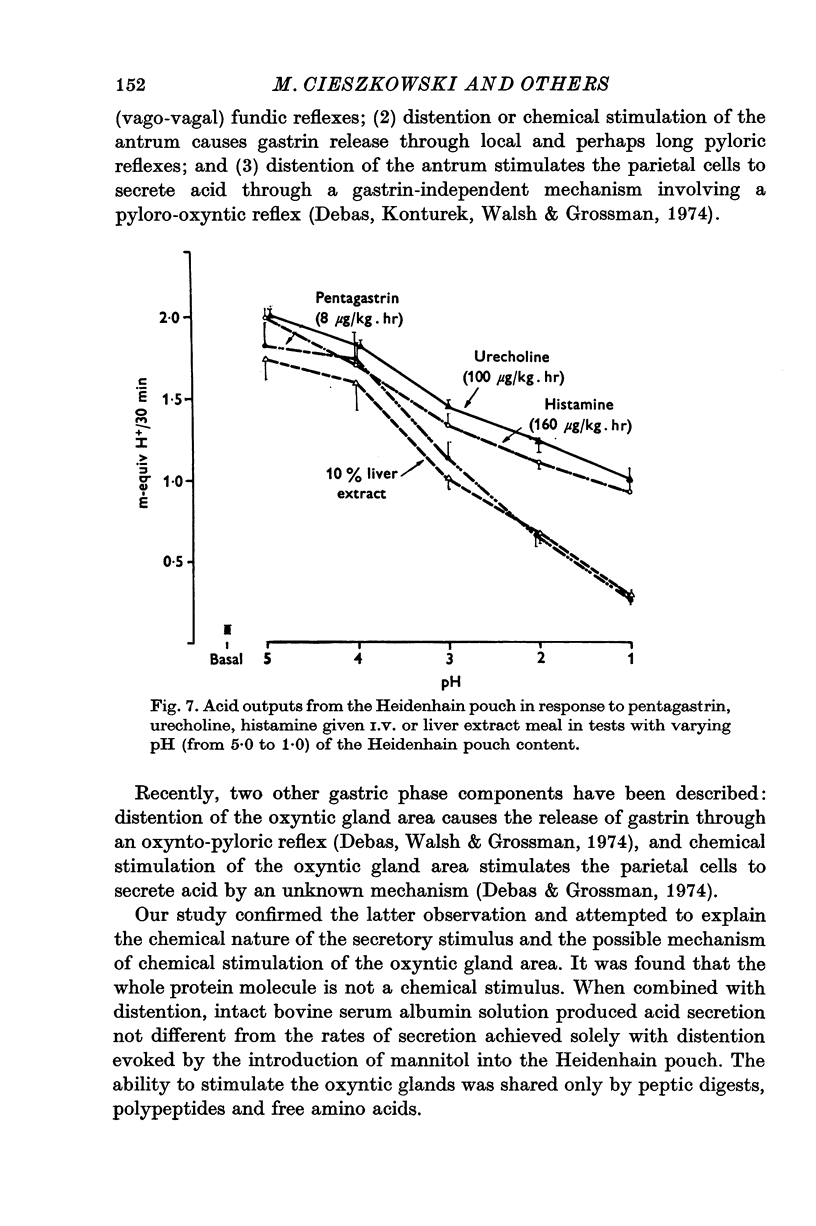
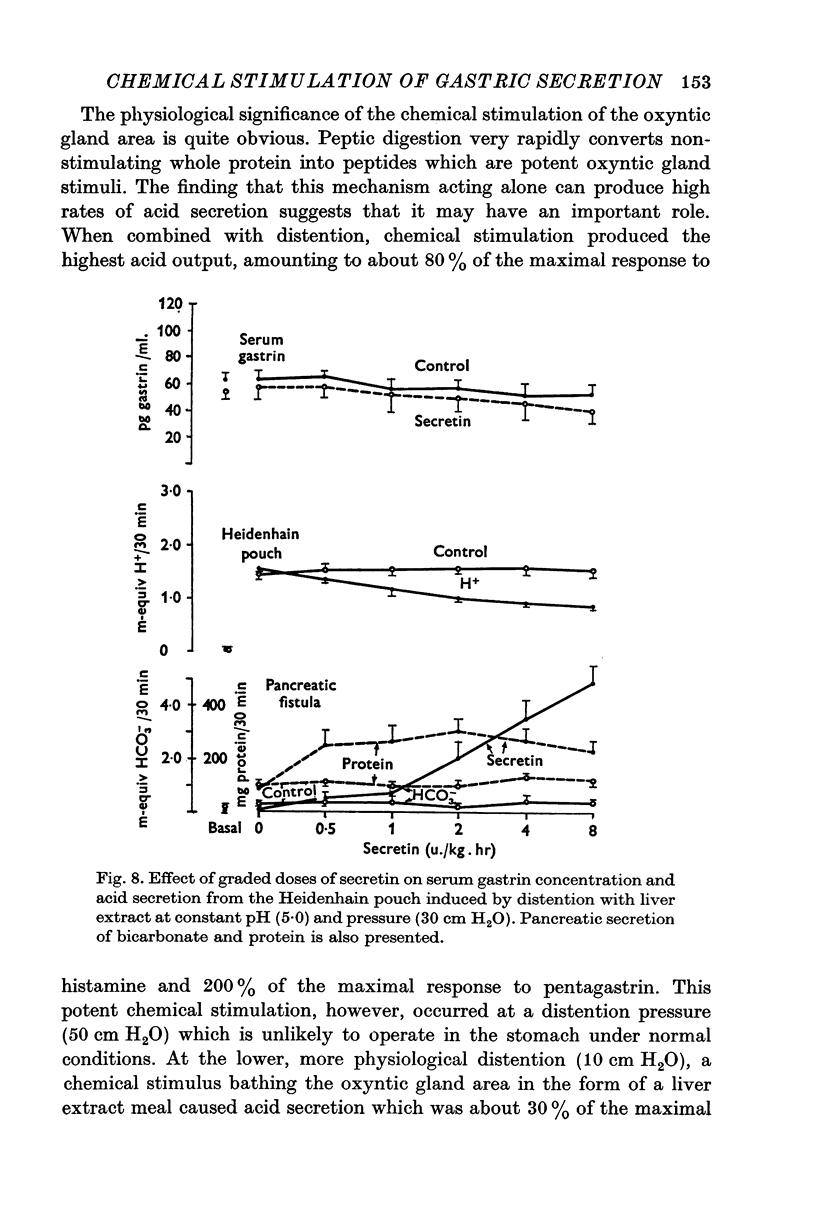
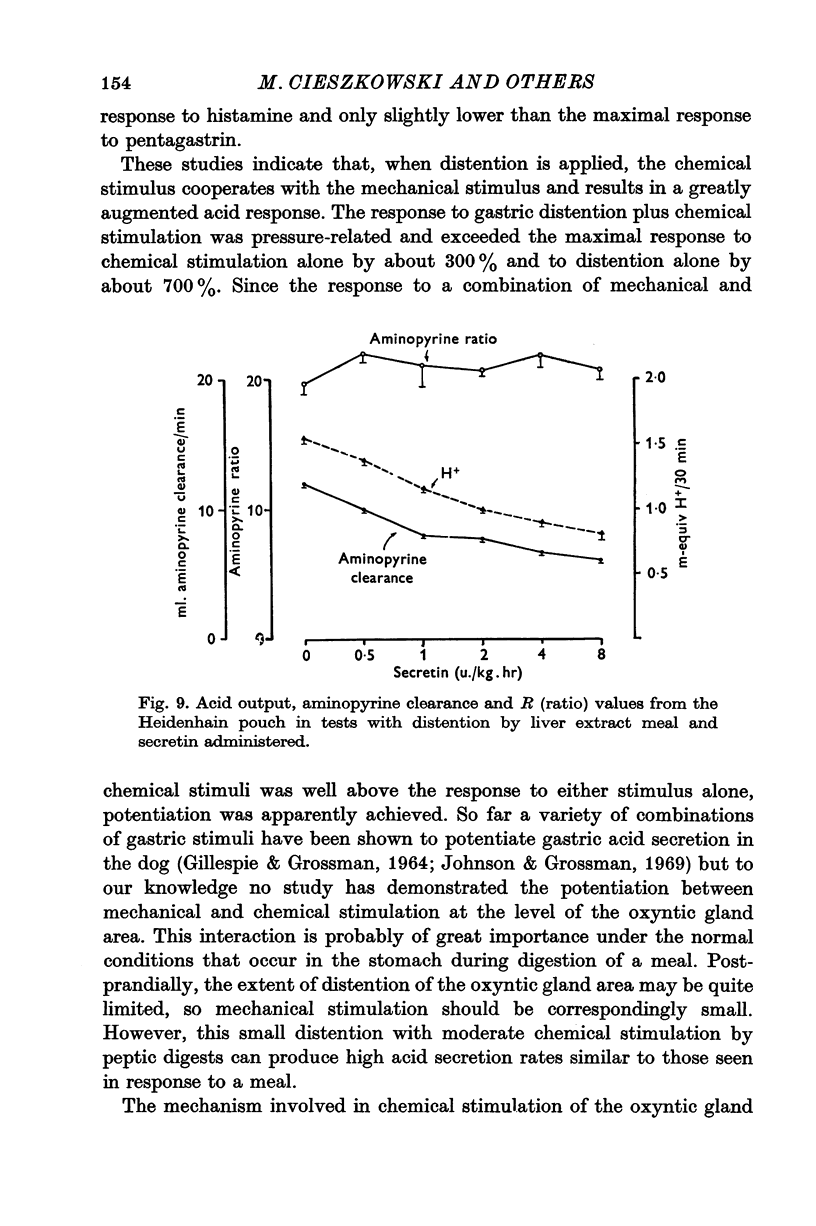
1. The serum gastrin level, gastric mucosal blood flow and acid secretion from the canine Heidenhain pouch have been measured in response to the introduction of bovine serum albumin, pepsin-digested albumin, an amino acid mixture, liver extract and mannitol used as control. 2. Distention of the Heidenhain pouch with mannitol or albumnin at pH 5-0 produced a similar pressure-related increase of acid secretion reaching a peak of only 10 percent of the maximal response to histamine. Pepsin-digested albumin was capable of producing larger acid outputs than undigested albumin.The highest acid output, attaining about 80 percent of the maximal response to histamine, was obtained with liver extract both before and after exhaustive dialysis to remove all the amino acids and short peptide fragments. An amino acid mixture containing all essential amino acids was also found to stimulate acid secretion but a lesser degree than liver extract. 3. This concluded that it is not the intact protein but the products of its digestion, the polypeptides and free amino acids, which are potent chemical stimulants of acid secretion from the oxyntic gland area. Since the serum gastrin level was not changed during acid secretion induced by peptic digests bathing the oxyntic gland area, the mechanism of chemical stimulation appears to be gastrin-independent. 4. The response to chemical stimulation by peptic digests can be greatly potentiated by combining this with distention of the oxyntic gland area. Topical application of xylocaine or atropine causes a marked decrease of Heidenhain pouch response to peptic digests, suggesting a possible neural reflex component in the mechanism of chemical stimulation of the oxyntic gland area. 5. When the pH of the liver extract in the Heidenhain pouch was gradually decreased in sequential order from 5-0 to 1-0, this resulted in a pH-related decrease in acid secretion and in the mucosal blood flow falling to the basal level at pH 1-0. Exogenous secretion given in graded doses from 0-5 to 8-0 u./kg. hr caused a small but dose-related inhibition of acid response to liver extract accompanied by a decrease of mucosal blood flow but without any significant change in the serum gastrin level. 6. The results indicate that the chemical stimulation of the oxyntic gland area by peptic digests is capable of inducing acid secretion by a local, gastrin-independent, partially neural reflex mechanism; sensitive to pH, pressure and secretin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Debas H. T., Konturek S. J., Walsh J. H., Grossman M. I. Proof of a pyloro-oxyntic reflex for stimulation of acid secretion. Gastroenterology. 1974 Apr;66(4):526–532. [PubMed] [Google Scholar]
- Fordtran J. S., Walsh J. H. Gastric acid secretion rate and buffer content of the stomach after eating. Results in normal subjects and in patients with duodenal ulcer. J Clin Invest. 1973 Mar;52(3):645–657. doi: 10.1172/JCI107226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE I. E., GROSSMAN M. I. POTENTIATION BETWEEN URECHOLINE AND GASTRIN EXTRACT AND BETWEEN URECHOLINE AND HISTAMINE IN THE STIMULATIONS OF HEIDENHAIN POUCHES. Gut. 1964 Feb;5:71–76. doi: 10.1136/gut.5.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera F., Kemp D. R., Tsukamoto M., Woodward E. R., Dragstedt L. R. A new cannula for the study of pancreatic function. J Appl Physiol. 1968 Aug;25(2):207–209. doi: 10.1152/jappl.1968.25.2.207. [DOI] [PubMed] [Google Scholar]
- Jacobson E. D., Linford R. H., Grossman M. I. Gastric secretion in relation to mucosal blood flow studied by a clearance technic. J Clin Invest. 1966 Jan;45(1):1–13. doi: 10.1172/JCI105313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. D., Swan K. G., Grossman M. I. Blood flow and secretion in the stomach. Gastroenterology. 1967 Feb;52(2):414–420. [PubMed] [Google Scholar]
- Johnson L. R., Grossman M. I. Potentiation of gastric acid response in the dog. Gastroenterology. 1969 Apr;56(4):687–692. [PubMed] [Google Scholar]
- Konturek S. J., Biernat J., Grzelec T. Inhibition by secretin of the gastric acid responses to meals and to pentagastrin in duodenal ulcer patients. Gut. 1973 Nov;14(11):842–846. doi: 10.1136/gut.14.11.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek S. J., Radecki T., Konturek D., Dimitrescu T. Effect of calcitonin on gastric and pancreatic secretion and peptic ulcer formation in cats. Am J Dig Dis. 1974 Mar;19(3):235–241. doi: 10.1007/BF01072540. [DOI] [PubMed] [Google Scholar]
- Thompson J. C., Reeder D. D., Bunchman H. H., Becker H. D., Brandt E. N., Jr Effect of secretin on circulating gastrin. Ann Surg. 1972 Sep;176(3):384–393. doi: 10.1097/00000658-197209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Radioimmunoassay of gastrin. Gastroenterology. 1970 Jan;58(1):1–14. [PubMed] [Google Scholar]


