Abstract
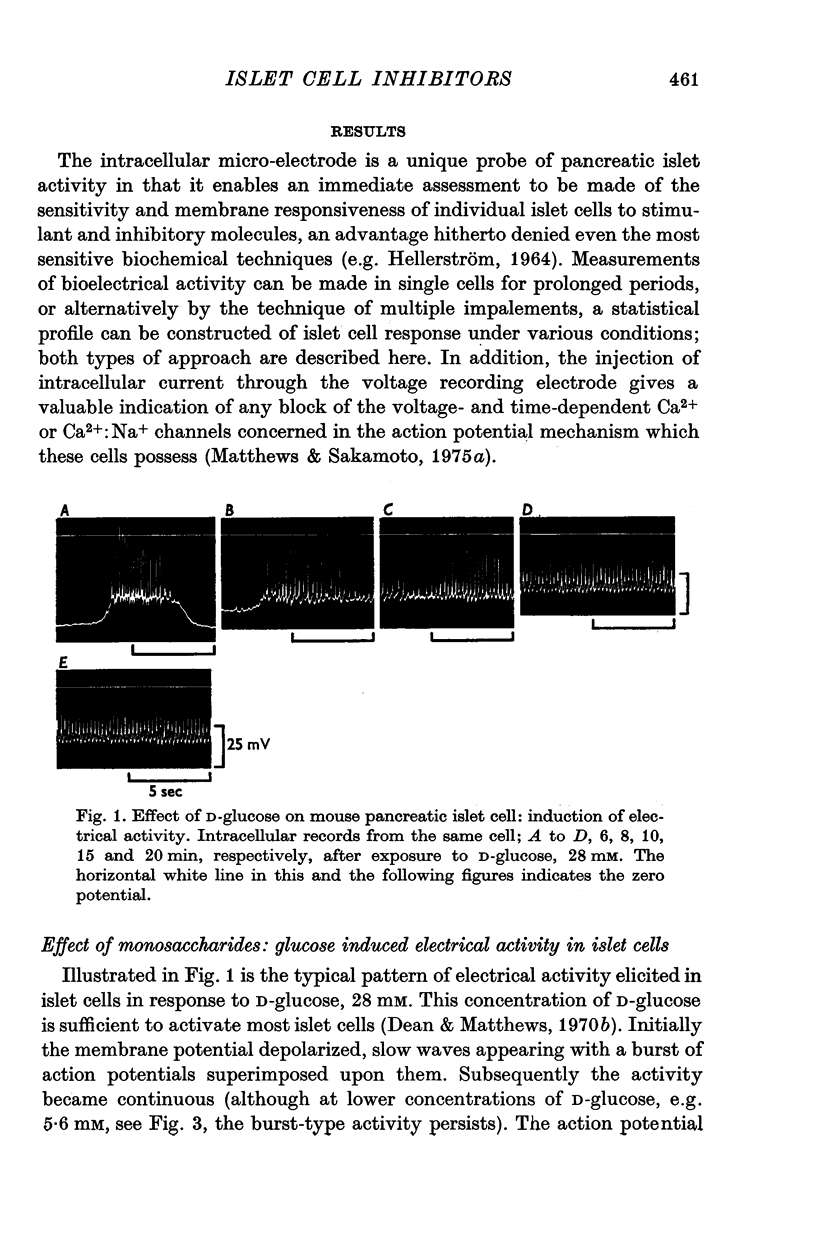
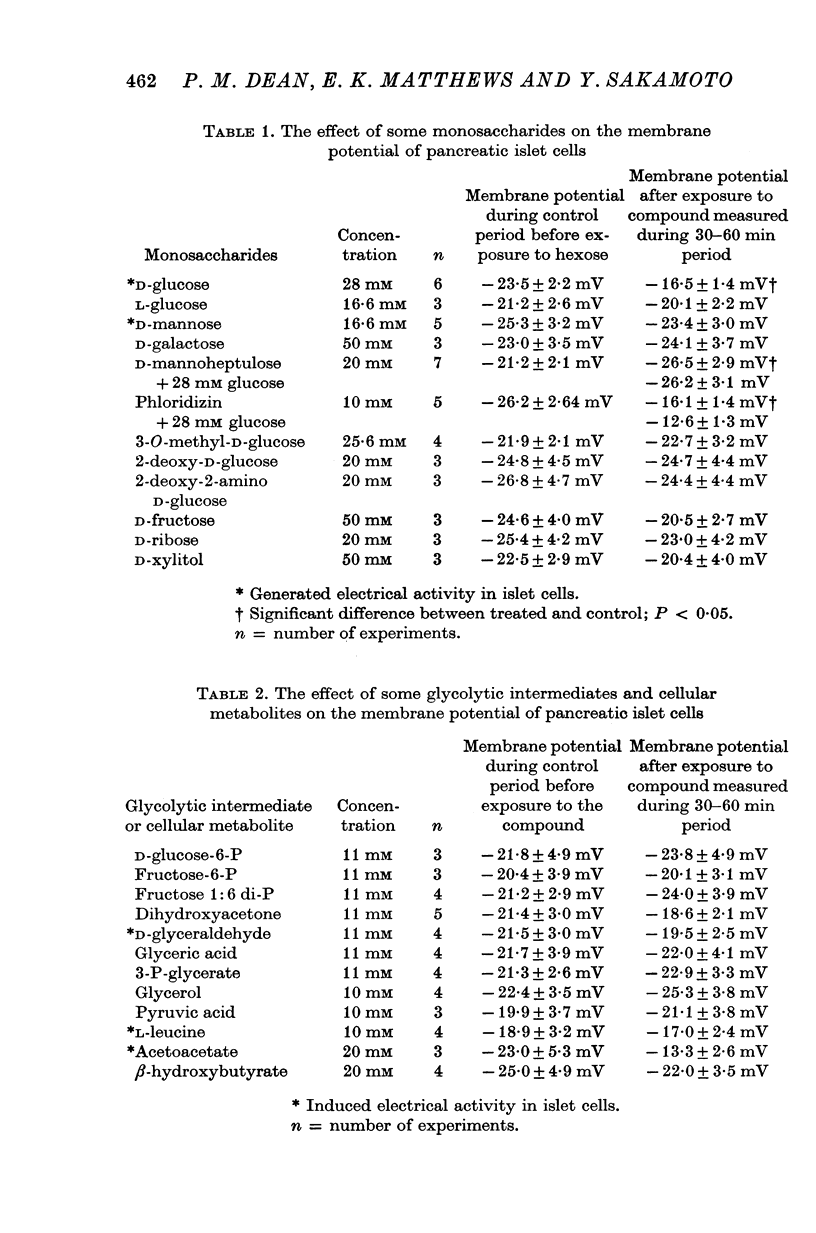
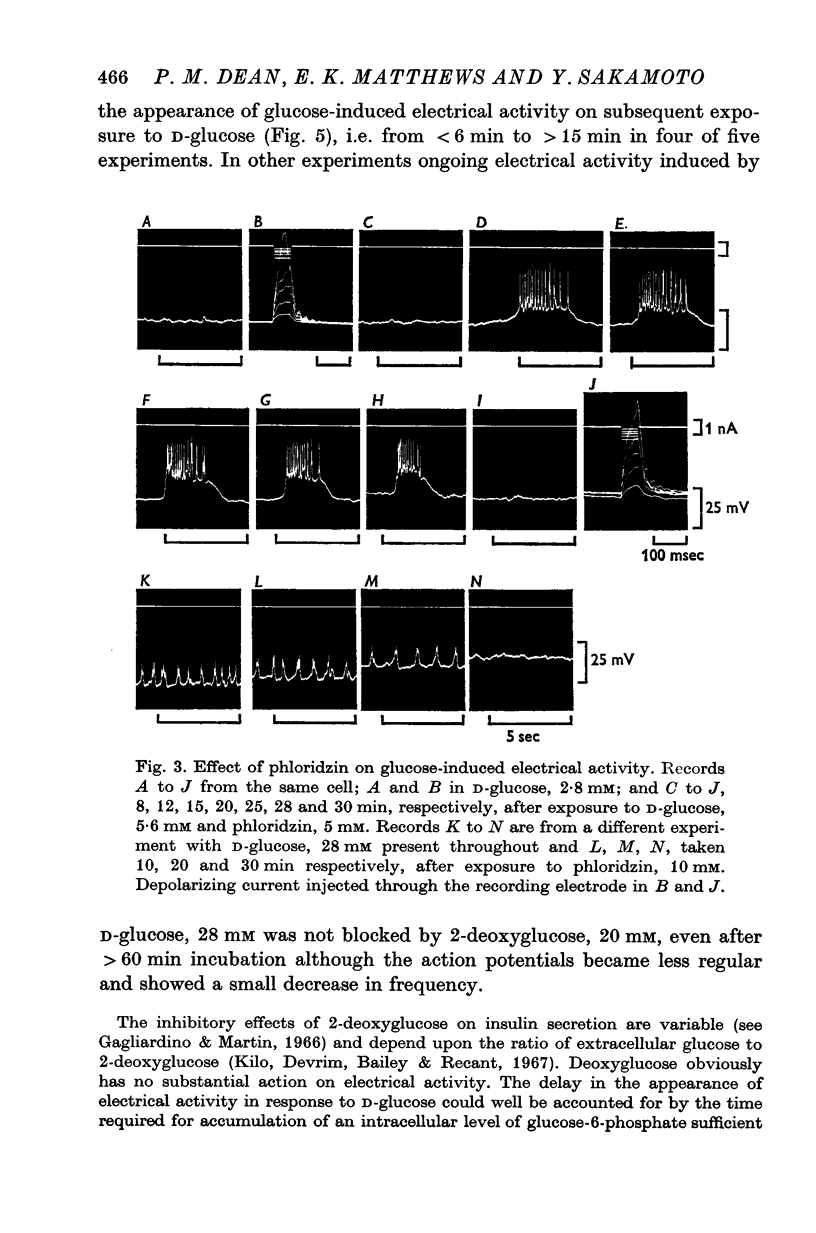
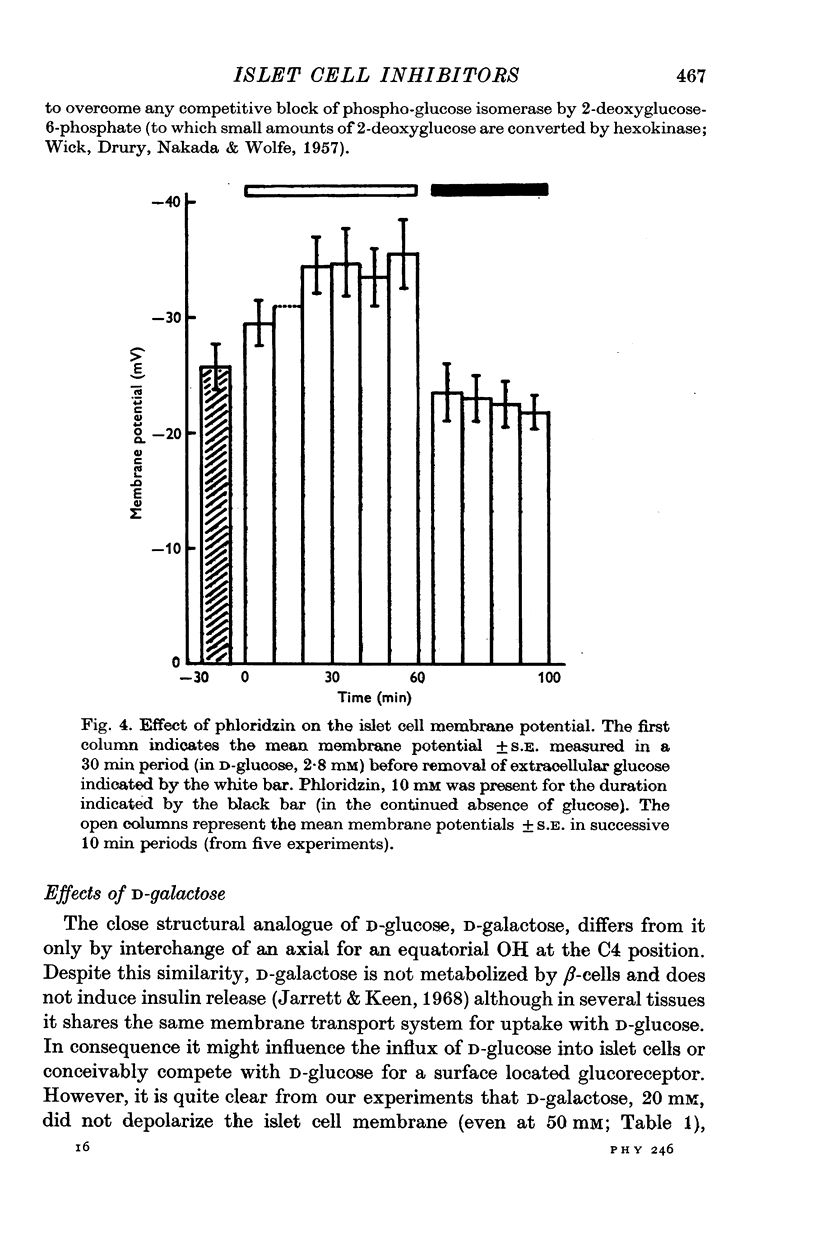
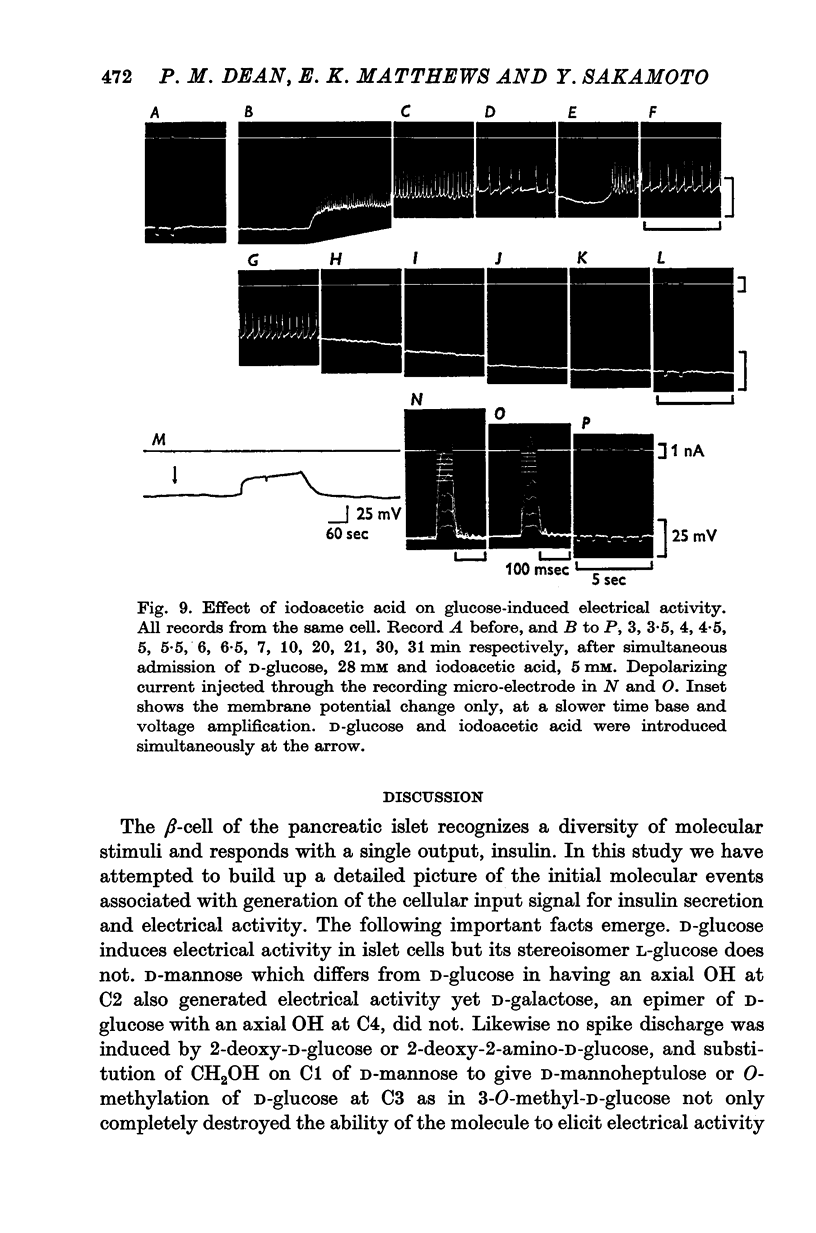
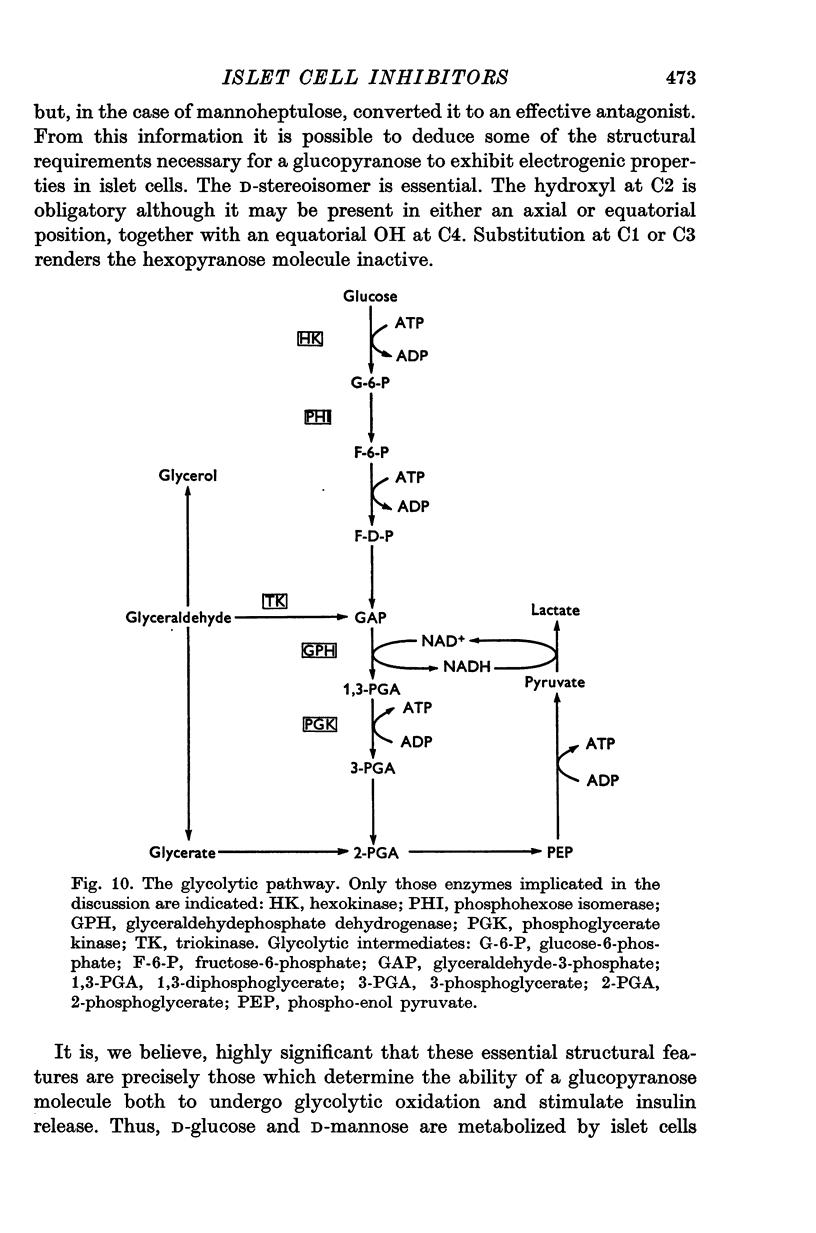
1. The effects of monosaccharides, glycolytic intermediates, metabolic inhibitors and anxia, have been studied on the membrane electrical activity of mouse pancreatic islet cells in vitro using a single intracellular micro-electrode for both voltage recording and current injection. 2. In addition to D-glucose (28mM), D-mannose (16-6mM), and L-leucin (10mM), the substances D-glyceraldehyde (11mM), and acetoacetate (20 mM), induced action potentials in islet cells but other glucos analogues and metabolic intermediates including L-glucose dod not. 3. Mannoheptulose 20 mM), but not D-galactose or 2-deoxy-D-glucose, antagonized the electrical activity induced in islet cells by D-glucose, 28mM. Prior treatment of the cells with mannoheptulose caused them to hyperpolarize and completely prevented the appearance of electrical activity on subsequent exposure to D-glucose. 4. Electrical activity induced by D0glucose 28mM, was progressively inhibited by phloridzin, 10mM, if the cells were exposed to D-glucose and inhibitor simultaneously, and abolished on pretreatment with inhibitor for 30-60 min. Phloridzin also caused depolarization of the islet cells which was independent of extracellular glucose. 5. Anoxia completely blocked the electrical activity induced by glucose but not that evoked by D-glyceraldehyde, L-leucine, tolbutamide or glibenclamide. 6. Iodoacetic acid, 5 mM, rapidly blocked glucose-induced electrical activity whilst that elicited by tolbutamide was relatively resistant to inhibition. 7. The nature and possible location of the glucoreceptor in pancreatic islet cells is discussed in relation to the origin and functional significance of glucose-induced electrical activity and insulin secretion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Guinto E. The reduction of glyceraldehyde by human erythrocytes. L-hexonate dehydrogenase activity. J Clin Invest. 1974 May;53(5):1258–1264. doi: 10.1172/JCI107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasi E., Luft R. Diabetes mellitus--a disorder of cellular information transmission? Horm Metab Res. 1970 Jul;2(4):246–249. doi: 10.1055/s-0028-1095082. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells. Nature. 1968 Jul 27;219(5152):389–390. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. The bioelectrical properties of pancreatic islet cells: effects of diabetogenic agents. Diabetologia. 1972 Jul;8(3):173–178. doi: 10.1007/BF01212257. [DOI] [PubMed] [Google Scholar]
- Gagliardino J. J., Martin J. M. Studies on the mechanism of insulin release. Metabolism. 1966 Dec;15(12):1068–1075. doi: 10.1016/0026-0495(66)90095-3. [DOI] [PubMed] [Google Scholar]
- Georg R. H., Sussman K. E., Leitner J. W., Kirsch W. M. Inhibition of glucose and tolbutamide-induced insulin release by iodoacetate and antimycin A. Endocrinology. 1971 Jul;89(1):169–176. doi: 10.1210/endo-89-1-169. [DOI] [PubMed] [Google Scholar]
- Hellman B., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic -cell recognition of insulin secretagogues. V. Binding and stimulatory action of phlorizin. Mol Pharmacol. 1972 Nov;8(6):759–769. [PubMed] [Google Scholar]
- Hellman B. Methodological approaches to studies on the pancreatic islets. Diabetologia. 1970 Apr;6(2):110–120. doi: 10.1007/BF00421438. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Evidence for mediated transport of glucose in mammalian pancreatic -cells. Biochim Biophys Acta. 1971 Jul 6;241(1):147–154. doi: 10.1016/0005-2736(71)90312-9. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. The pancreatic -cell recognition of insulin secretagogues. IV. Islet uptake of sulfonylureas. Diabetologia. 1973 Jun;9(3):210–216. doi: 10.1007/BF01219785. [DOI] [PubMed] [Google Scholar]
- Jarrett R. J., Keen H. Oxidation of sugars, other than glucose, by isolated mammalian islets of Langerhans. Metabolism. 1968 Feb;17(2):155–157. doi: 10.1016/0026-0495(68)90142-x. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. The proteins of the erythrocyte membrane. Biochim Biophys Acta. 1973 Dec 28;300(4):341–378. doi: 10.1016/0304-4157(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y., Orci L., Lambert A. E. Organ culture of fetal rat pancreas. IV. Effects of metabolic inhibitors on insulin release. Endocrinology. 1971 Aug;89(2):576–583. doi: 10.1210/endo-89-2-576. [DOI] [PubMed] [Google Scholar]
- Kilo C., Devrim S., Bailey R., Recant L. Studies in vivo and in vitro of glucose-stimulated insulin release. The effects of metabolizable sugars, tolbutamide, and 2-deoxyglucose. Diabetes. 1967 Jun;16(6):377–385. doi: 10.2337/diab.16.6.377. [DOI] [PubMed] [Google Scholar]
- Lernmark A. [Isolated mouse islets as a model for studying insulin release]. Acta Diabetol Lat. 1971 Jul-Aug;8(4):649–679. doi: 10.1007/BF01550894. [DOI] [PubMed] [Google Scholar]
- Lipton P. Effects of membrane depolarization on nicotinamide nucleotide fluorescence in brain slices. Biochem J. 1973 Dec;136(4):999–1009. doi: 10.1042/bj1360999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Mattews E. K., Sakamoto Y. Pancreatic islet cells: electrogenic and electrodiffusional control of membrane potential. J Physiol. 1975 Mar;246(2):439–457. doi: 10.1113/jphysiol.1975.sp010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975 Mar;246(2):421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Sakamoto Y. Inhibition of glucose-induced electrical activity in pancreatic islet cells by phloridzin, mannoheptulose, and anoxia. J Physiol. 1973 Apr;230(1):38P–40P. [PubMed] [Google Scholar]
- Matthews E. K. [Electrical activity in islet cells and insulin secretion]. Acta Diabetol Lat. 1970 Sep;7 (Suppl 1):83–101. [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Islet-cell metabolism during insulin release. Effects of glucose, citrate, octanoate, tolbutamide, glucagon and theophylline. Biochem J. 1969 Nov;115(2):257–262. doi: 10.1042/bj1150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panten U., Dal Ri H., Poser W., Hasselblatt A. Eine Methode der Gewebsumströmung für Fluorescenzmessungen. Pflugers Arch. 1971;323(1):86–90. doi: 10.1007/BF00586569. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Hoffman J. F. The role of membrane phosphoglycerate kinase in the control of glycolytic rate by active cation transport in human red blood cells. J Gen Physiol. 1967 Mar;50(4):893–916. doi: 10.1085/jgp.50.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein P. S., Cooney D. A., McMenamin M. G., Anderson T. Streptozotocin diabetes--further studies on the mechanism of depression of nicotinamide adenine dinucleotide concentrations in mouse pancreatic islets and liver. Biochem Pharmacol. 1973 Oct 15;22(20):2625–2631. doi: 10.1016/0006-2952(73)90071-3. [DOI] [PubMed] [Google Scholar]
- Taylor K. W. Metabolic changes in the islets of Langerhans in relationship to the onset of diabetes mellitus. Diabetologia. 1972 Aug;8(4):236–243. doi: 10.1007/BF01225566. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Watkins J. C., Curtis D. R., Felix D. Effect of intracellular injection of pyridine nucleotides on electrical responses of cat spinal motoneurones. Brain Res. 1971 Dec 24;35(2):570–572. doi: 10.1016/0006-8993(71)90503-8. [DOI] [PubMed] [Google Scholar]


