Abstract
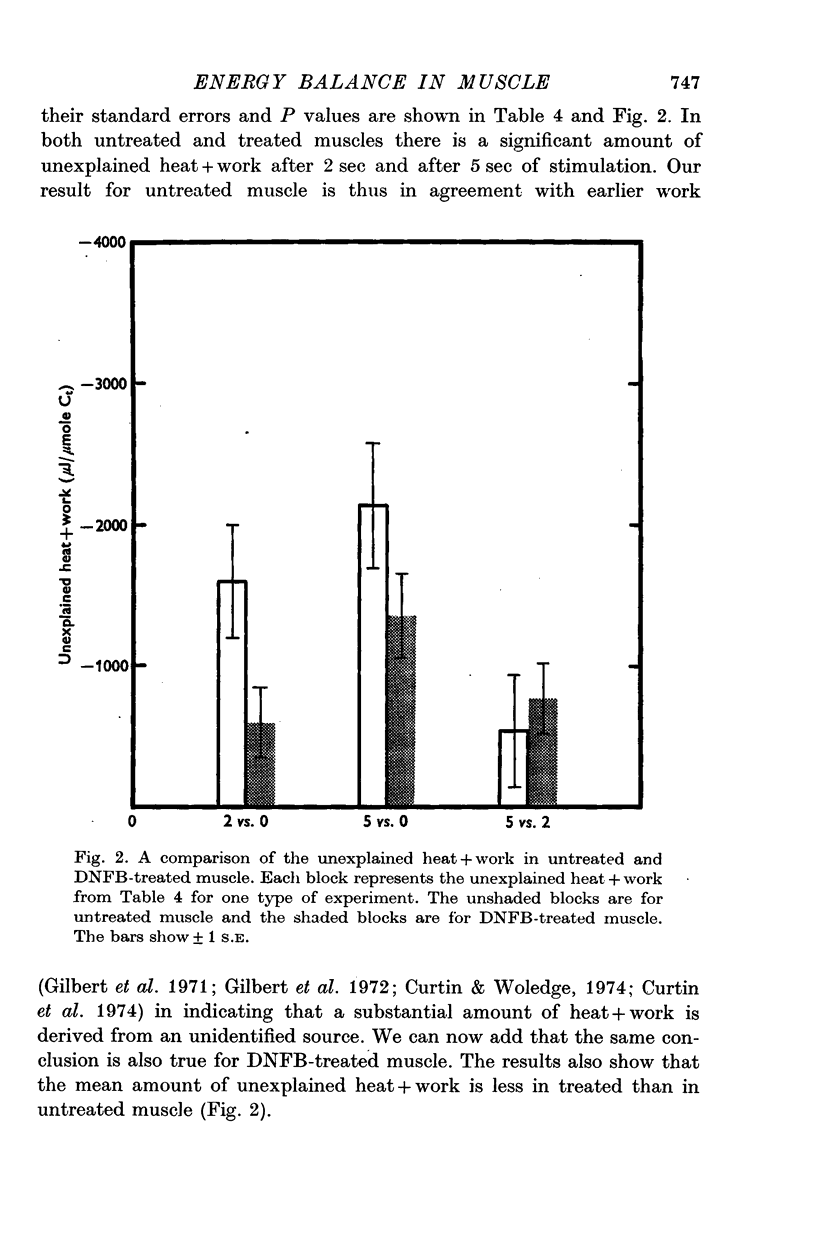
1. Heat production and chemical changes were measured in untreated and dinitrofluorobenzene (DNFB)-treated muscles during isometric tetani. Levels of total creatine (Ct), free creatine, ATP, ADP, AMP, inorganic phosphate, glucose-1-phosphate, glucose-6-phosphate, fructose-6-phosphate, fructose-1,6-diphosphate, pyruvate, phosphoenolpyruvate, and lactate were measured. Changes in inosinic acid (IMP) were also measured. 2. DNFB effectively inhibited the creatine kinase reaction (Lohmann reaction). 3. Our major finding is that even after effective treatment with DNFB the observed heat plus work after 2 sec and 5 sec of stimulation is significantly greater than the enthalpy change produced by the measured chemical changes. This confirms that an unidentified exothermic process occurs during muscle contraction; this conclusion was reached previously from studies of untreated muscle. 4. The unexplained heat plus work is unlikely to be derived from glycolytic reactions since under anaerobic conditions no formation of lactate, pyruvate, phosphoenolpyruvate or fructose-1,6-diphosphate could be detected in either untreated or DNFB-treated muscles even 34 sec after a series of three 5 sec isometric tetani. 5. In the first 2 sec of stimulation the unexplained heat plus work is less in DNFB-treated muscles than in untreated muscles. However from 2 to 5 sec of stimulation the unexplained heat plus work is the same in treated and untreated muscles.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bárány M., Bárány K. Change in the reactivity of myosin during muscle contraction. J Biol Chem. 1970 May 25;245(10):2717–2721. [PubMed] [Google Scholar]
- CAIN D. F., DAVIES R. E. Breakdown of adenosine triphosphate during a single contraction of working muscle. Biochem Biophys Res Commun. 1962 Aug 7;8:361–366. doi: 10.1016/0006-291x(62)90008-6. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Gilbert C., Kretzschmar K. M., Wilkie D. R. The effect of the performance of work on total energy output and metabolism during muscular contraction. J Physiol. 1974 May;238(3):455–472. doi: 10.1113/jphysiol.1974.sp010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energetics of relaxation in frog muscle. J Physiol. 1974 Apr;238(2):437–446. doi: 10.1113/jphysiol.1974.sp010535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson V. A., Woledge R. C. The thermal effects of shortening in tetanic contractions of frog muscle. J Physiol. 1973 Sep;233(3):659–671. doi: 10.1113/jphysiol.1973.sp010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dydyńska M., Wilkie D. R. The chemical and energetic properties of muscles poisoned with fluorodinitrobenzene. J Physiol. 1966 Jun;184(3):751–769. doi: 10.1113/jphysiol.1966.sp007946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Chemical change and energy output during muscular contraction. J Physiol. 1971 Oct;218(1):163–193. doi: 10.1113/jphysiol.1971.sp009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. A., Davies R. E. The effect of 2,4-dinitrofluorobenzene on the activity of striated muscle. J Biol Chem. 1965 Oct;240(10):3996–4001. [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Perry S. V. The structure and interactions of myosin. Prog Biophys Mol Biol. 1967;17:325–381. doi: 10.1016/0079-6107(67)90010-7. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. A new enzymic method for inorganic phosphate determination. Anal Biochem. 1972 Sep;49(1):88–94. doi: 10.1016/0003-2697(72)90244-8. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Automated fluorometric analysis of biological compounds. Anal Biochem. 1972 Sep;49(1):73–87. doi: 10.1016/0003-2697(72)90243-6. [DOI] [PubMed] [Google Scholar]
- WOLEDGE R. C. Heat production and energy liberation in the early part of a muscular contraction. J Physiol. 1963 Apr;166:211–224. doi: 10.1113/jphysiol.1963.sp007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLEDGE R. C. The thermoelastic effect of change of tension in active muscle. J Physiol. 1961 Jan;155:187–208. doi: 10.1113/jphysiol.1961.sp006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Shimizu H., Suga H. A kinetic study of the energy storing enzyme-product complex in the hydrolysis of ATP by heavy meromyosin. Biochim Biophys Acta. 1973 Jun 28;305(3):642–653. doi: 10.1016/0005-2728(73)90083-2. [DOI] [PubMed] [Google Scholar]


