Abstract
1. The volume of distribution of [14-C]carboxyl inulin has been studied in slices of outer and inner medulla from rat kidney incubated in Krebs phosphate-bicarbonate Ringer, modified to render it iso-osmolal with the tissue fluids in these zones, under three conditions, (a) aerobically at 37 degrees C (control), (b) anoxically at 37 degrees C, and (c) aerobically at 0 degrees C. 2. Under control conditions near steady-state volumes of approximately 24 and 42 mul./100 mg wet weight slice were obtained for outer and inner medulla respectively during the period 10-30 min from the start of incubation. In the outer medulla the volumes of distribution in anoxic and hypothermic slices exceeded that in control slices during this time, but control values increased from 30 to 100 min so that after 100 min the distribution volumes were approximately 30 mul./100 mg under each set of conditions. 3. In the inner medulla control and anoxic slices had inulin distribution volumes of approximately 42 mul./100 mg during 10-30 min, rising to over 50 mul./u99 mg by 100 min. Slices incubated hypothermically reached a steady-state value of approximately 40 mul./100 mg by 30 min, which did not increase further for up to 100 min. 4. All slices lost about 10% of their initial weight during the first 3 min of incubation. Thereafter control slices maintained weight constancy for at least 30 min (outer medulla) or 100 min (inner medulla); slices incubated anoxically or hypothermically gained weight, the gains being greatest in anoxic outer and hypothermic inner medulla. 5. The K concentration within control slices (both zones), hypothermic outer and anoxic inner medulla attained equilibrium when slice [K] was approximately 8 times medium [K] (5-9 mM). In anoxic outer and hypothermic inner medullary slices [K] fell to a significantly greater extent, but interpretation of these findings in terms of slice K loss is subject to modification in respect of the increases in slice weight (water content) accompanying the [K] decreases. 6. There was a transient (1-3 min) rise in [Na] in all slices. This was followed by a [Na] decrease, which was most apparent in control slices, and finally a gradual increase towards medium [Na] (141 and 180 mM for outer and inner medulla respectively).
Full text
PDF




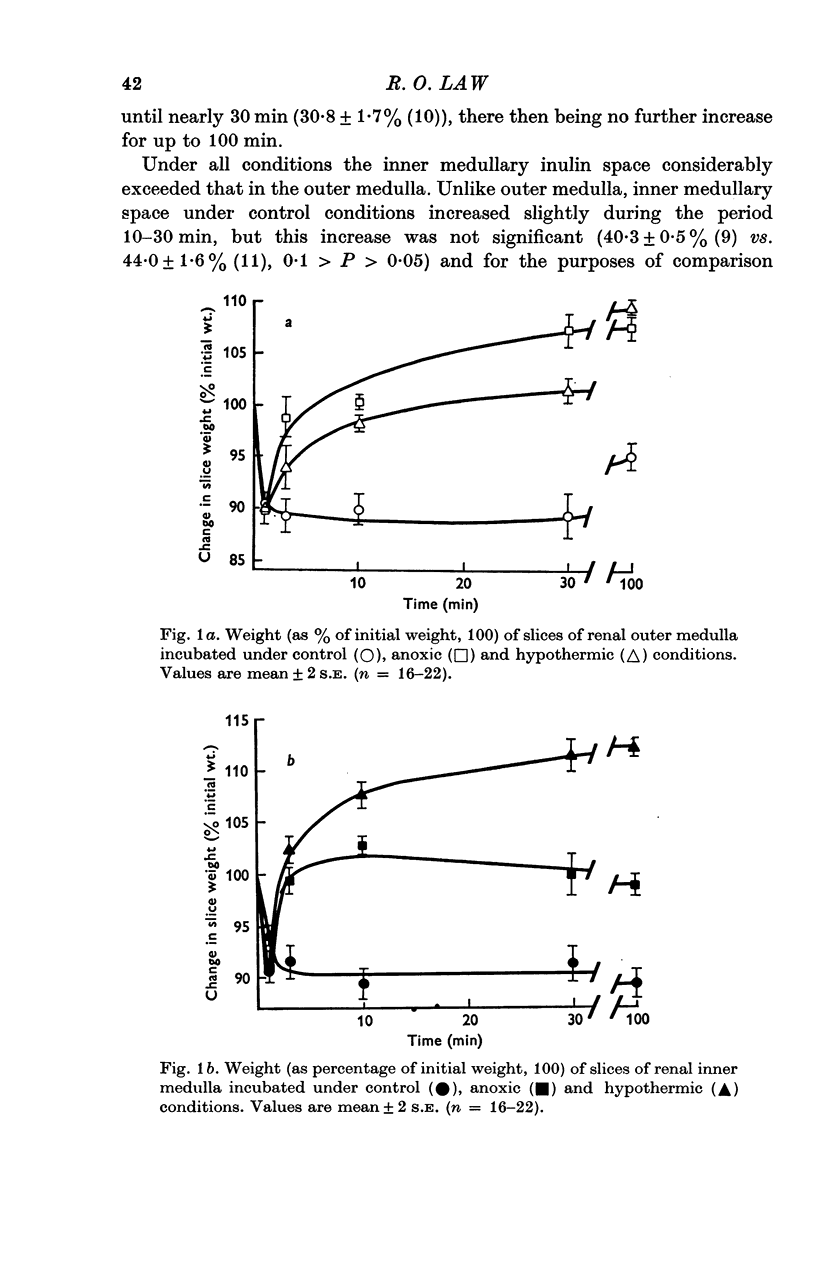
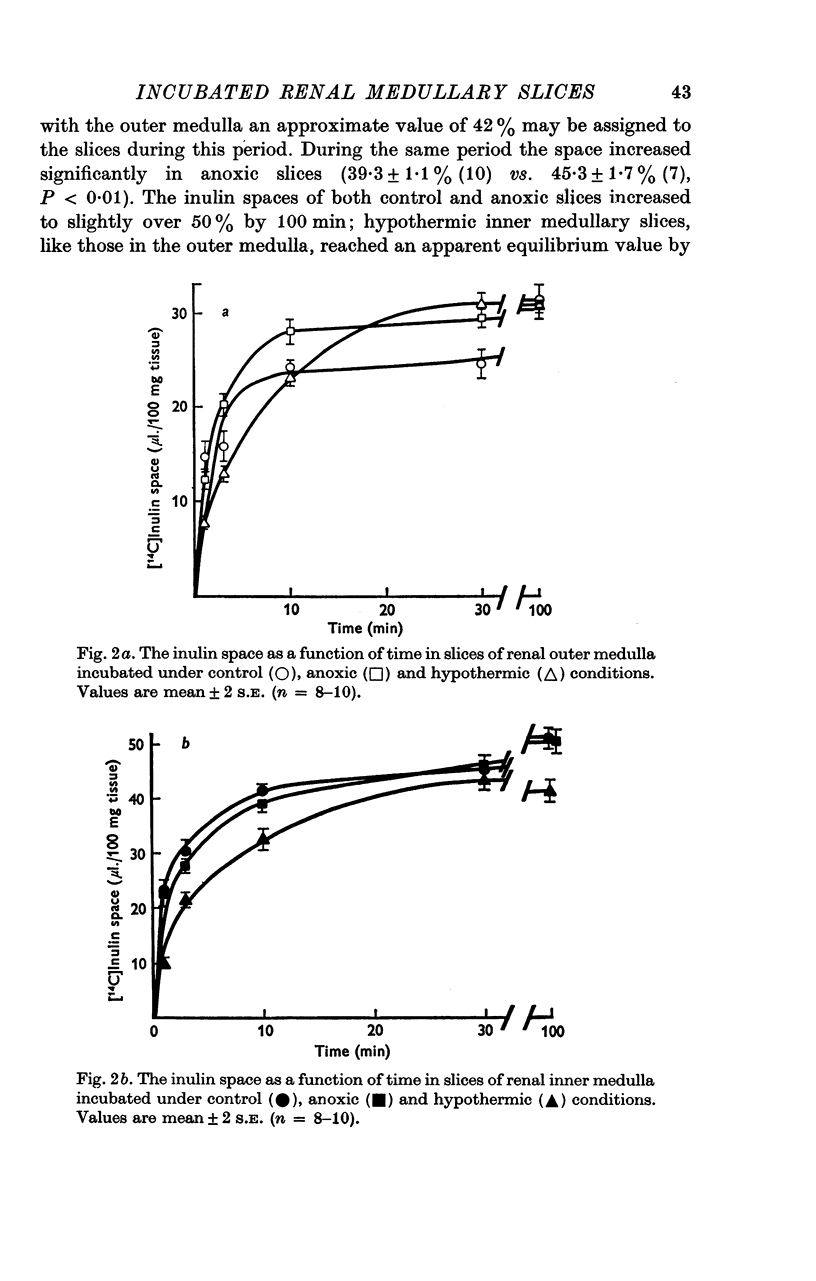

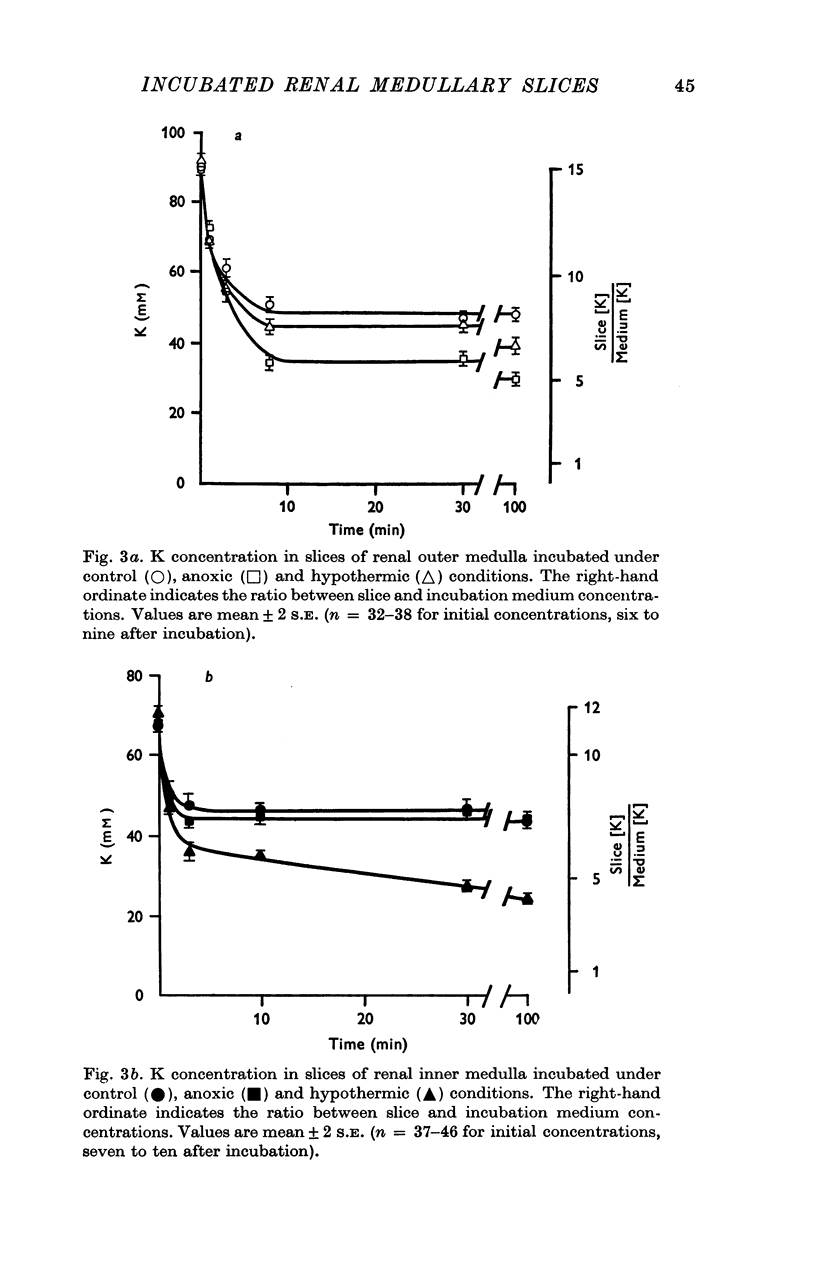
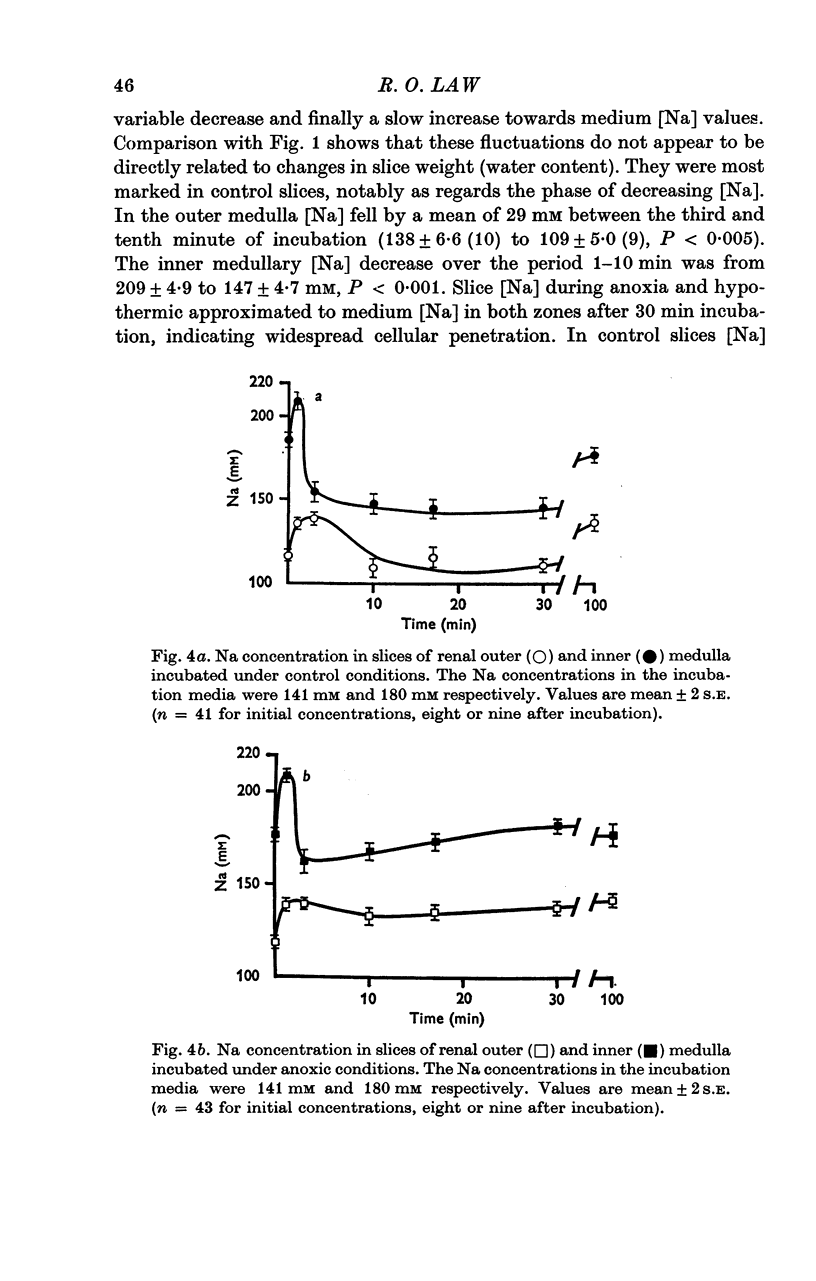






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
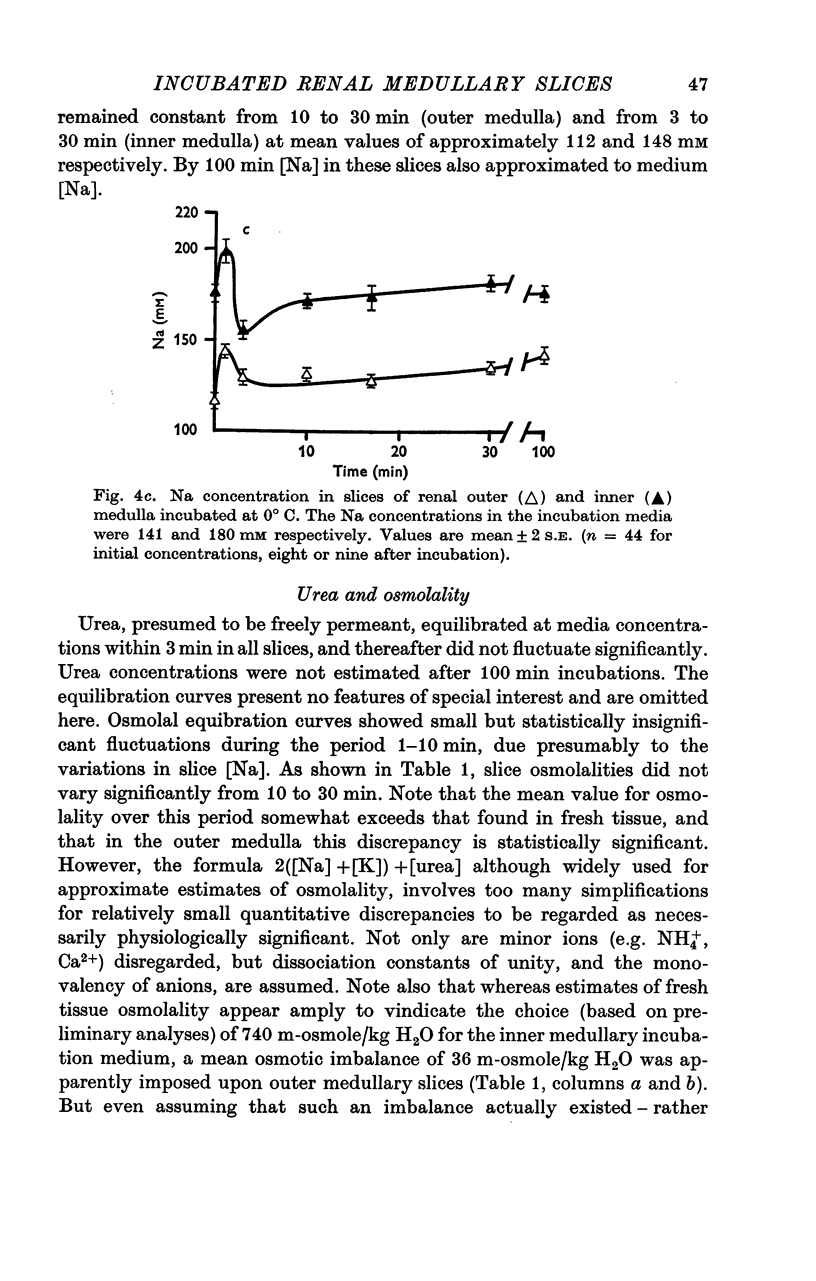
- Abodeely D. A., Lee J. B. Fuel of respiration of outer renal medulla. Am J Physiol. 1971 Jun;220(6):1693–1700. doi: 10.1152/ajplegacy.1971.220.6.1693. [DOI] [PubMed] [Google Scholar]
- Alexander J. C., Lee J. B. Effect of osmolality on Na plus-K plus-ATPase in outer renal medulla. Am J Physiol. 1970 Dec;219(6):1742–1745. doi: 10.1152/ajplegacy.1970.219.6.1742. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Hai M. A., Thomas S. The time course of changes in renal tissue composition duruig water diuresis in the rat. J Physiol. 1968 Jul;197(2):429–443. doi: 10.1113/jphysiol.1968.sp008568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans J. H., Bailie M. D., Biggs D. L. In vitro metabolism of C-14-labeled amino acids by sheep kidney cortex and medulla. Am J Physiol. 1966 Jul;211(1):249–254. doi: 10.1152/ajplegacy.1966.211.1.249. [DOI] [PubMed] [Google Scholar]
- Gutman Y., Wald H., Czaczkes W. Urea and sodium: effect on microsomal ATPase in different parts of the kidney. Pflugers Arch. 1973 Dec 7;345(1):81–92. doi: 10.1007/BF00587064. [DOI] [PubMed] [Google Scholar]
- Holmes E. W., Jr, DiScala V. A. Oxygen consumption, glycolysis, and sodium reabsorption in the hypothyroid rat kidney. Am J Physiol. 1971 Sep;221(3):839–843. doi: 10.1152/ajplegacy.1971.221.3.839. [DOI] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest. 1974 Feb;53(2):393–402. doi: 10.1172/JCI107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEAN E. L., ADAMS P. H., WINTERS R. W., DAVIES R. E. Energy metabolism of the renal medulla. Biochim Biophys Acta. 1961 Dec 23;54:474–478. doi: 10.1016/0006-3002(61)90087-7. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- LEE J. B., VANCE V. K., CAHILL G. F., Jr Metabolism of C14-labeled substrates by rabbit kidney cortex and medulla. Am J Physiol. 1962 Jul;203:27–36. doi: 10.1152/ajplegacy.1962.203.1.27. [DOI] [PubMed] [Google Scholar]
- LITTLE J. R. DETERMINATION OF WATER AND ELECTROLYTES IN TISSUE SLICES. Anal Biochem. 1964 Jan;7:87–95. doi: 10.1016/0003-2697(64)90122-8. [DOI] [PubMed] [Google Scholar]
- Law R. O., Phelps C. F. The size of the sucrose, raffinose, and inulin spaces in the gastrocnemius muscle of the rat. J Physiol. 1966 Oct;186(3):547–557. doi: 10.1113/jphysiol.1966.sp008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. The distribution of [14C]sucrose within the skeletal muscle of the rat in vitro. J Physiol. 1967 May;190(1):71–79. doi: 10.1113/jphysiol.1967.sp008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Volume adjustment by renal medullary cells in hypo- and hyperosmolal solutions containing permeant and impermeant solutes. J Physiol. 1975 May;247(1):55–70. doi: 10.1113/jphysiol.1975.sp010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. The effects of 11-deoxycorticosterone and antidiuretic hormone (pitressin) on fluid exchange and electrolyte excretion by normal and starved polyuric-polydipsic rabbits. Pflugers Arch. 1973 Dec 18;345(3):249–263. doi: 10.1007/BF00586338. [DOI] [PubMed] [Google Scholar]
- Lee J. B., Peterhm Effect of oxygen tension on glucose metabolism in rabbit kidney cortex and medulla. Am J Physiol. 1969 Nov;217(5):1464–1471. doi: 10.1152/ajplegacy.1969.217.5.1464. [DOI] [PubMed] [Google Scholar]
- Lowenstein L. M., Hagopian L. Amino acid transport in stored renal medulla. Transplantation. 1969 Nov;8(5):558–565. doi: 10.1097/00007890-196911000-00002. [DOI] [PubMed] [Google Scholar]
- Lowenstein L. M., Smith I., Segal S. Amino acid transport in the rat renal papilla. Biochim Biophys Acta. 1968 Jan 3;150(1):73–81. doi: 10.1016/0005-2736(68)90010-2. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. The extracellular space in rat renal cortical slices incubated at 0.5 degrees and 25 degrees. Biochim Biophys Acta. 1968 Aug;163(1):85–92. doi: 10.1016/0005-2736(68)90035-7. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Macknight A. D. Extracellular space in some isolated tissues. J Physiol. 1974 May;239(1):31–49. doi: 10.1113/jphysiol.1974.sp010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulski P., Angielski S., Rogulski J. Metabolism of tricarboxylic acid cycle in rat kidney medulla in vitro. Am J Physiol. 1972 Sep;223(3):485–491. doi: 10.1152/ajplegacy.1972.223.3.485. [DOI] [PubMed] [Google Scholar]
- Paradise R. R., Morrow R. J. Constancy of intracellular water as a function of dry weight. Proc Soc Exp Biol Med. 1972 Dec;141(3):836–838. doi: 10.3181/00379727-141-36884. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 2. The effects of insulin, anaerobiosis and cell poisons on the penetration of isolated rat diaphragm by sugars. Biochem J. 1958 Nov;70(3):501–508. doi: 10.1042/bj0700501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG L. E., DOWNING S. J., SEGAL S. Extracellular space estimation in rat kidney slices using C saccharides and phlorizin. Am J Physiol. 1962 Apr;202:800–804. doi: 10.1152/ajplegacy.1962.202.4.800. [DOI] [PubMed] [Google Scholar]
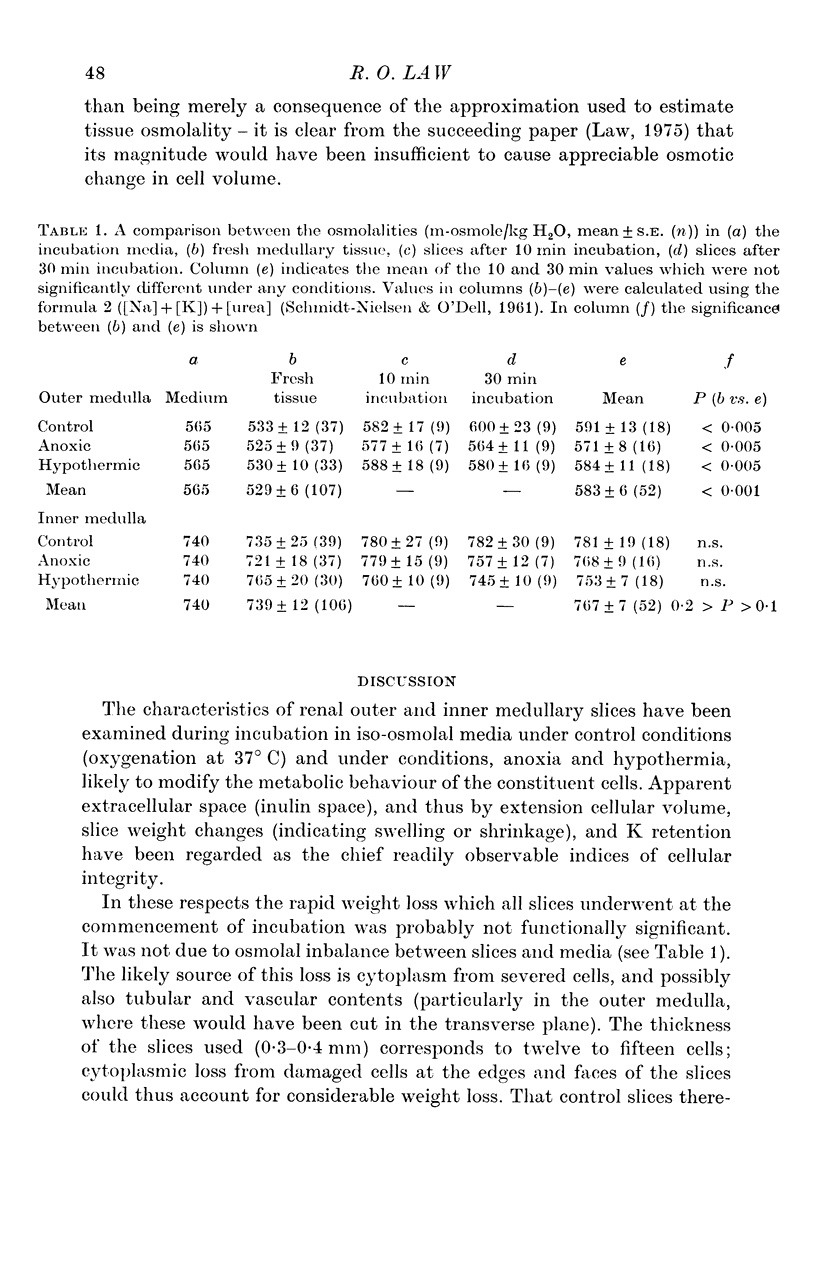
- SCHMIDT-NIELSEN B., O'DELL R. Structure and concentrating mechanism in the mammalian kidney. Am J Physiol. 1961 Jun;200:1119–1124. doi: 10.1152/ajplegacy.1961.200.6.1119. [DOI] [PubMed] [Google Scholar]
- Weinschelbaum de Jairala, Vieyra A., MacLaughlin M. Influence of ethacrynic acid and ouabain on the oxygen consumption and potassium and sodium content of the kidney external medulla of the dog. Biochim Biophys Acta. 1972 Sep 15;279(2):320–330. doi: 10.1016/0304-4165(72)90150-x. [DOI] [PubMed] [Google Scholar]
- de Jairala S., Vieyra A., Garcia A. P., Rasia M. L. Kinetics of inulin penetration in cortex and medullary kidney slices. Can J Physiol Pharmacol. 1973 Jul;51(7):511–515. doi: 10.1139/y73-075. [DOI] [PubMed] [Google Scholar]


