Abstract
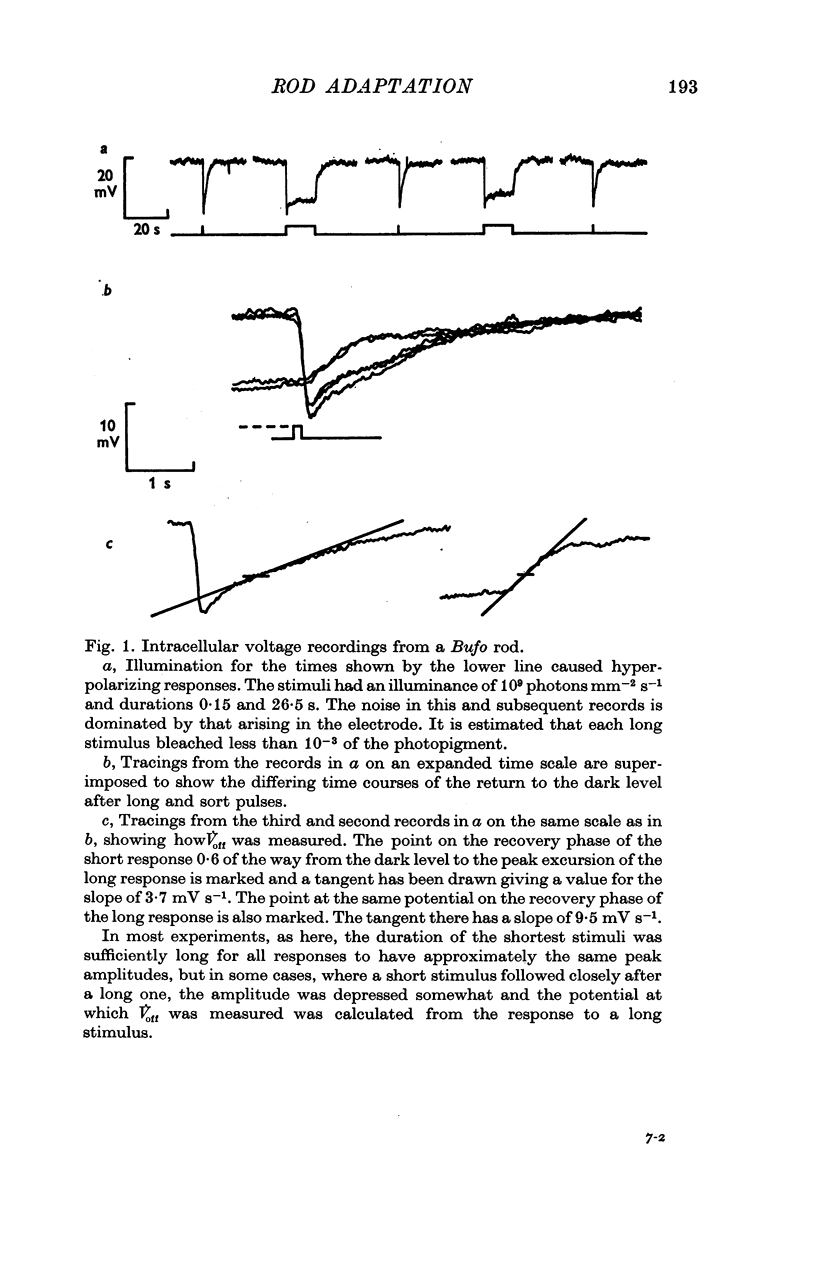
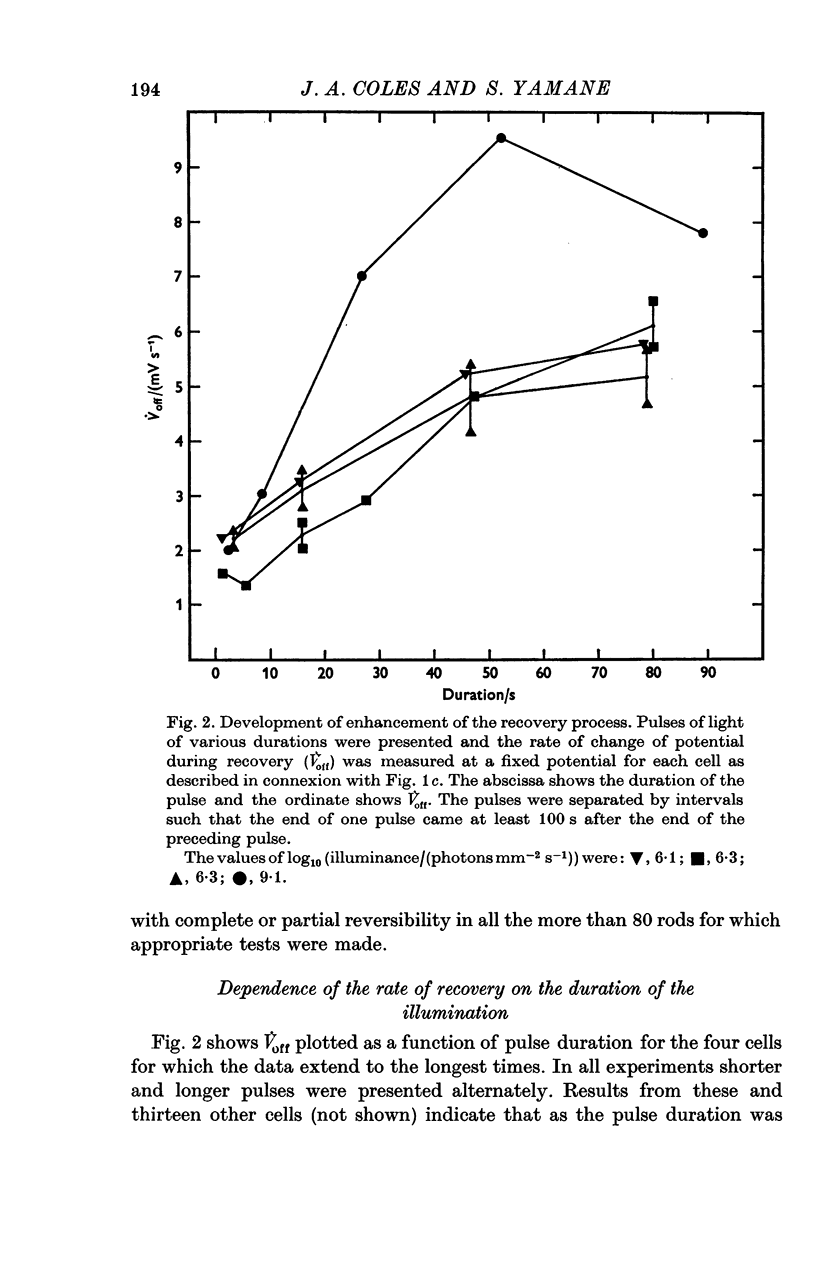
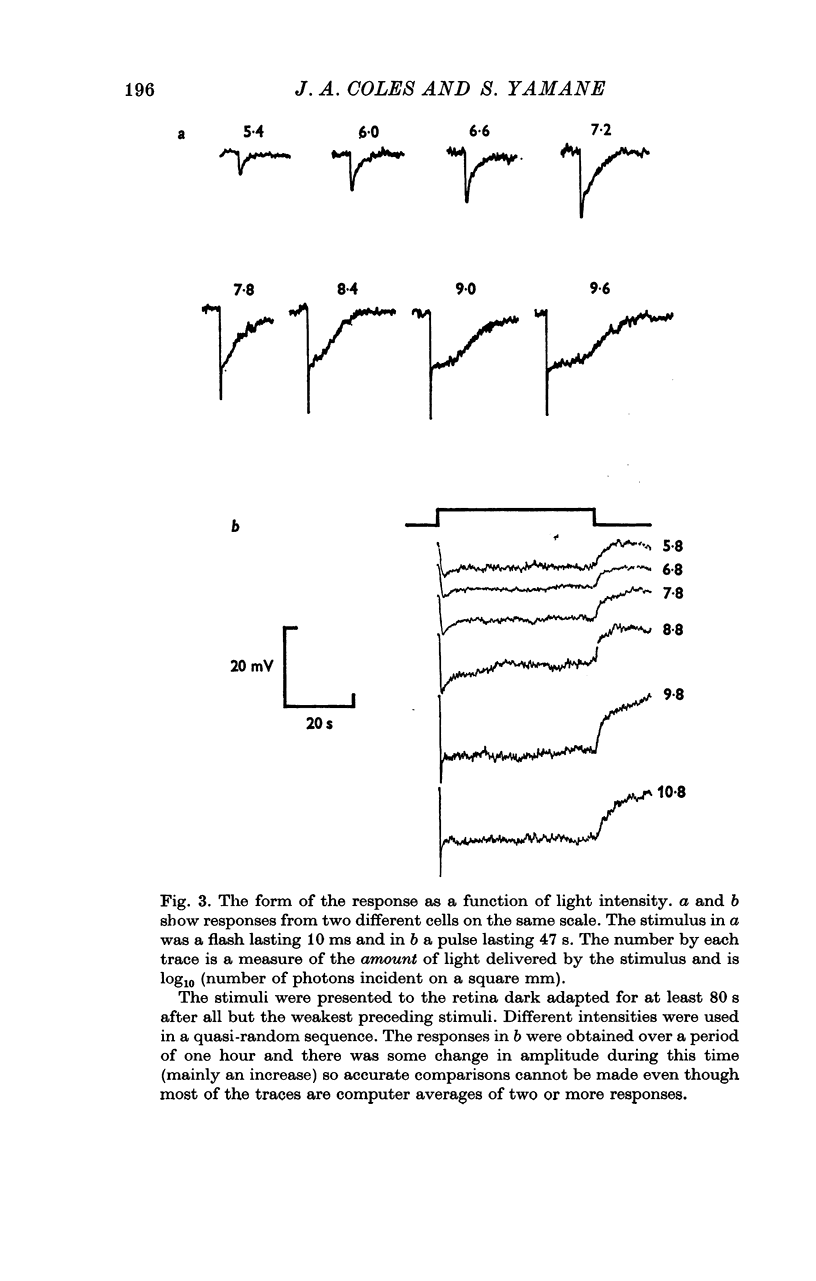
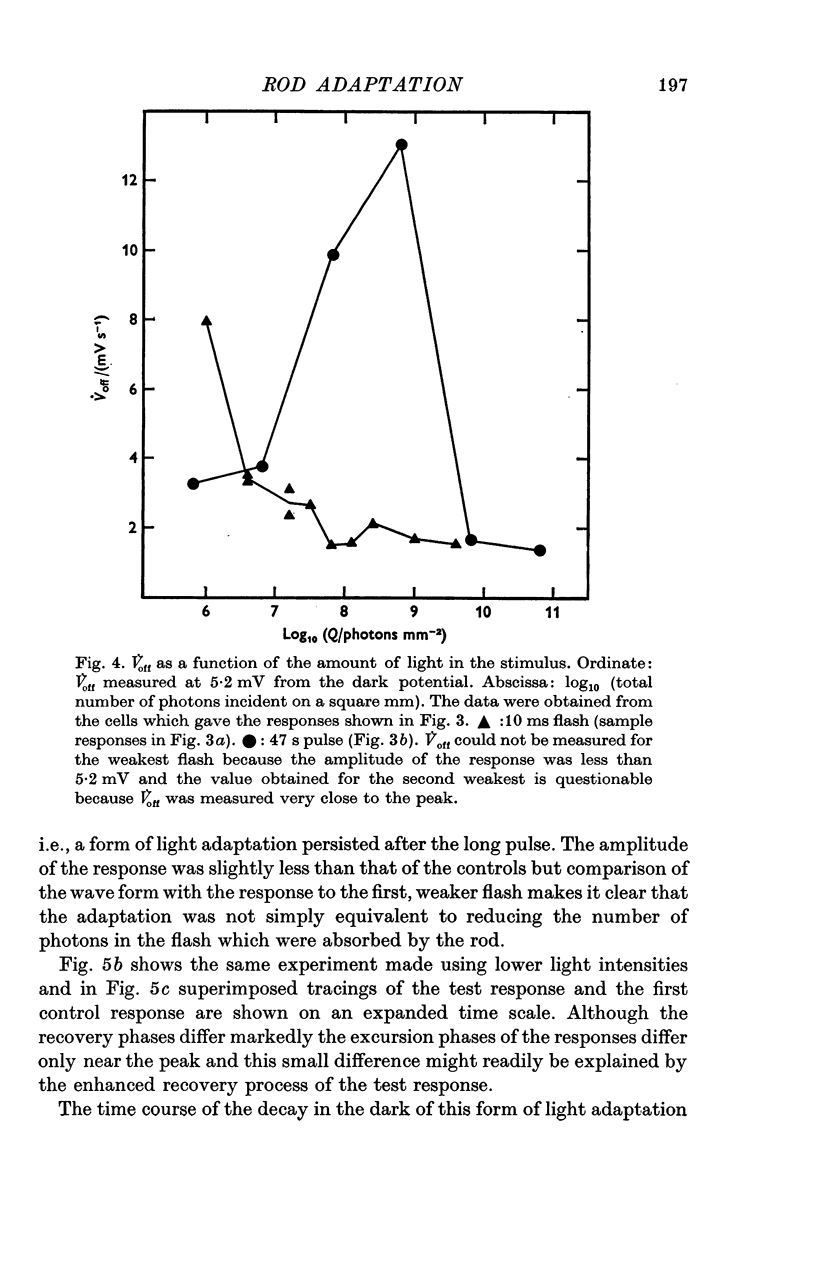
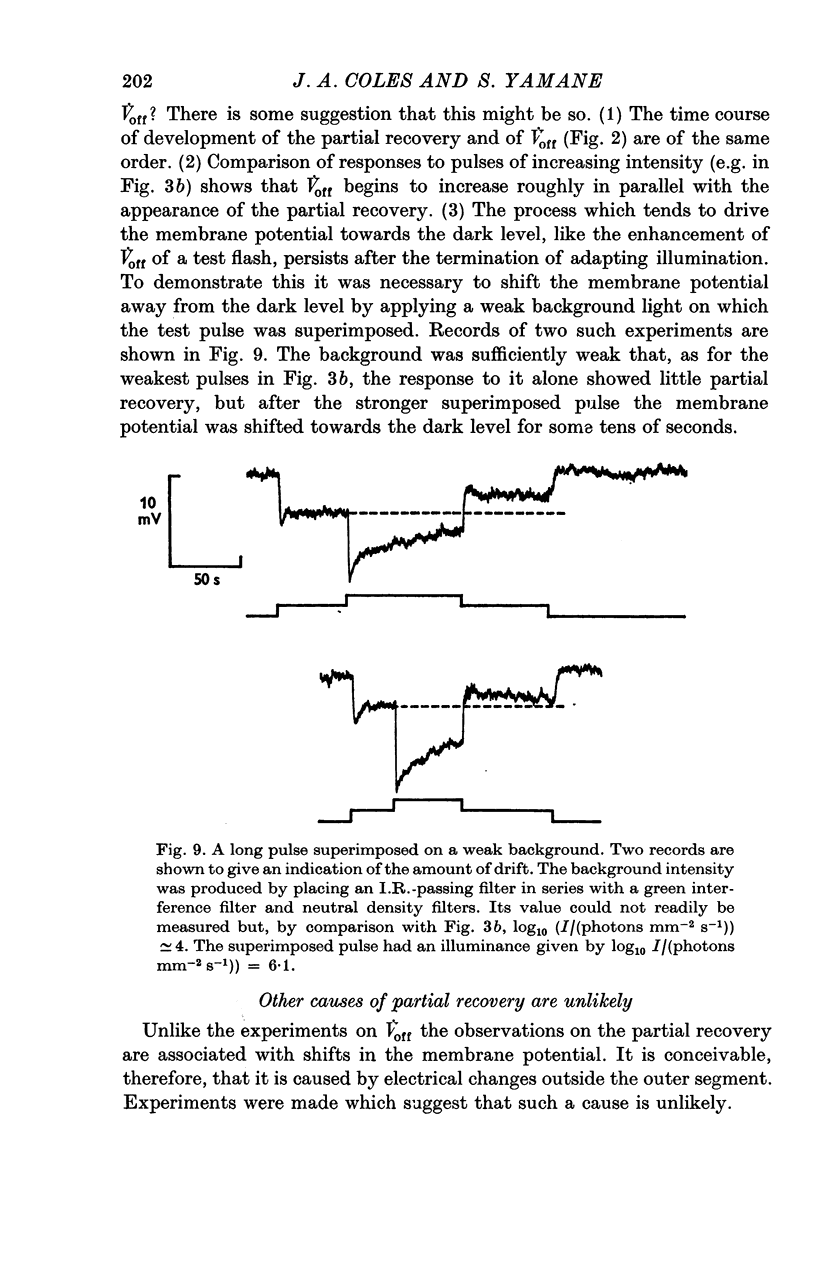
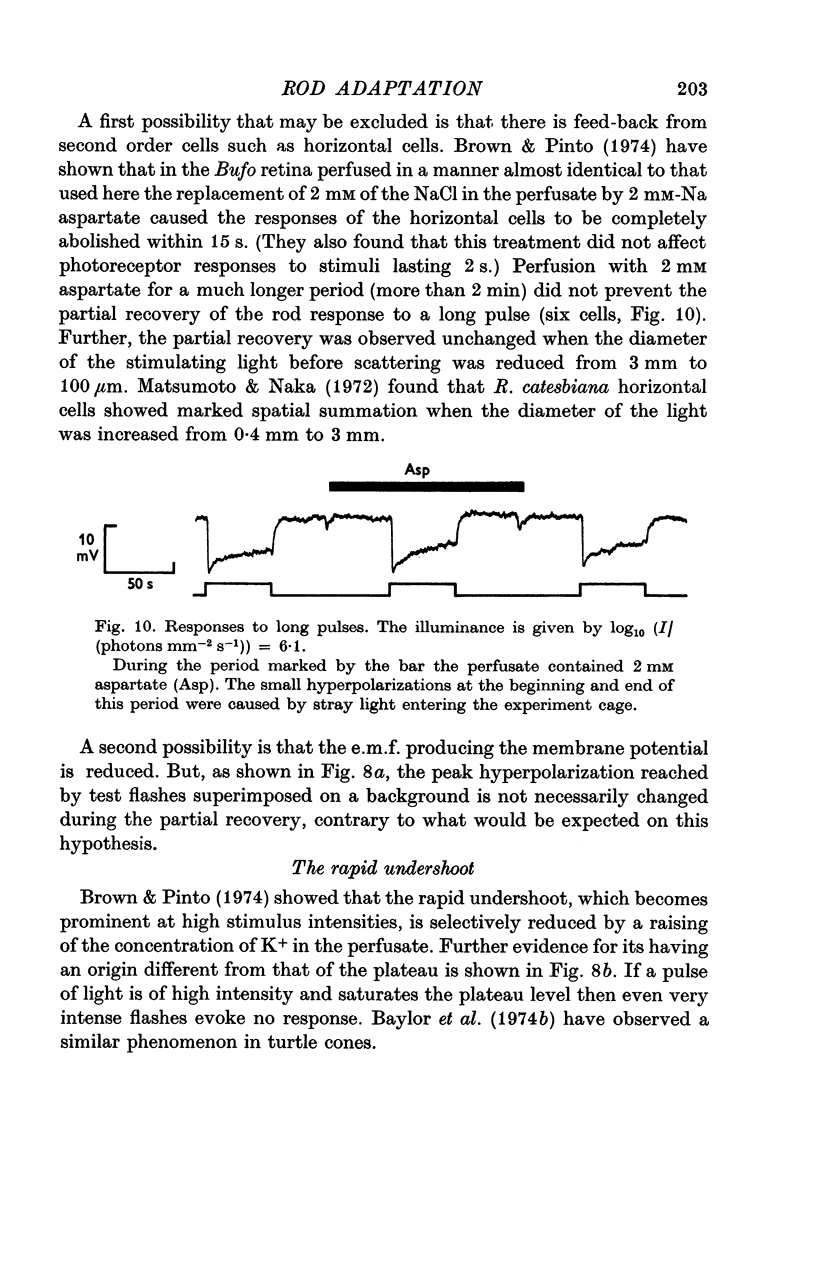
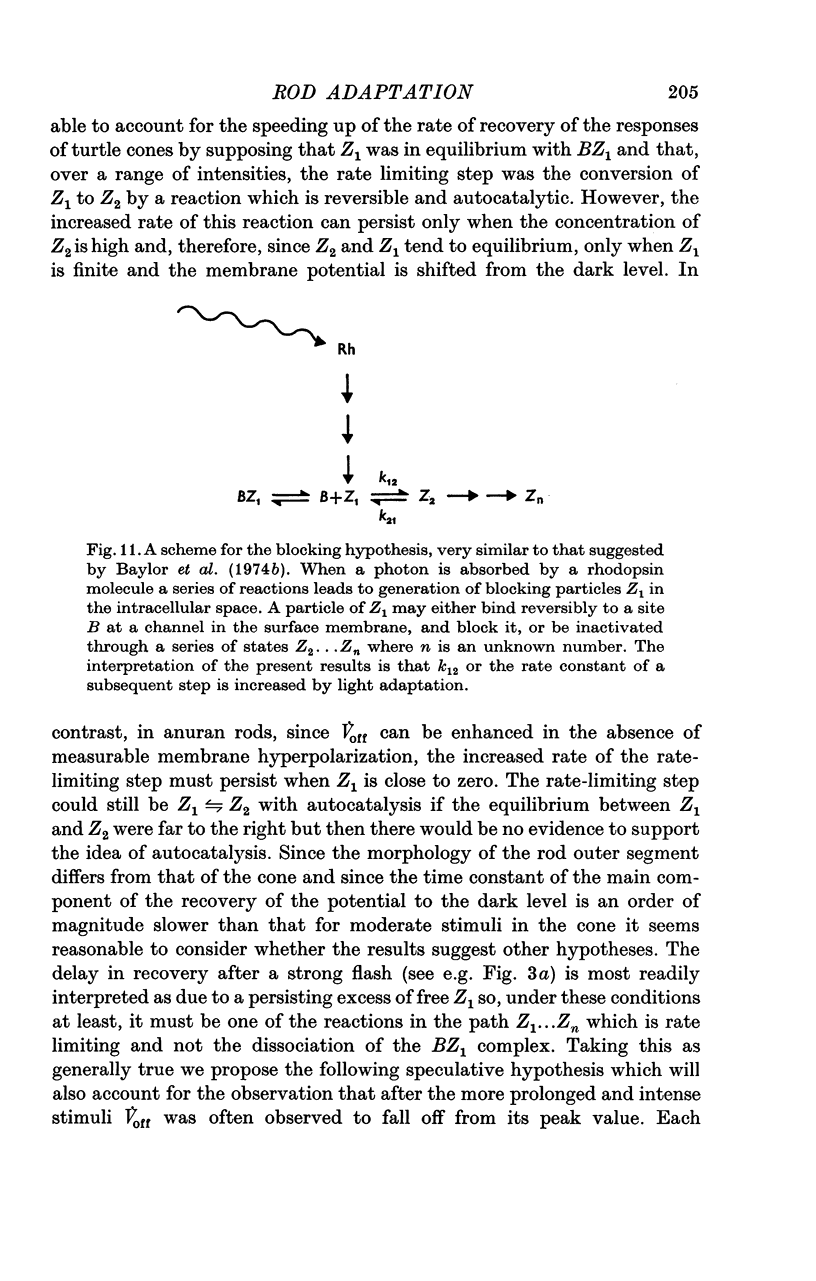
1. The intracellular receptor potential of the retinal rod cell was recorded in the unperfused, isolated retina of Rana catesbiana and in the perfused, isolated retina of Bufo marinus. Qualitatively, the responses from the two preparations were similar. 2. The rate at which the receptor potential returned to the dark level at the termination of a pulse of light (Voff) was measured at a fixed potential chosen to be about 0-6 of the way from the dark level to the peak of the response. 3. When the light intensity was such that less than about 10-minus 5 of the photopigment was bleached per second, Voff increased as the duration of the pulse was increased, reaching a maximum in 50-100 s. 4. When a brief test flash was presented at various intervals after an adapting pulse lasting about 50 s, Voff for the test flash was greater than the value in the dark adapted state for times up to about 80 s after the adapting pulse. 5. It has been hypothesized that in the vertebrate rod light causes release from the disk sacs of particles which block conducting channels in the surface membrane (Yoshikami & Hagins, 1971, 1973). A modification is proposed in which the blocking particles are converted to an inactive state can be increased by light adaptation. 6. This modified hypothesis will account qualitatively for the further observations that (a) during the response to illumination lasting several seconds the membrane potential recovers part of the way to the dark level and (b) if a second light pulse is superimposed on this background illumination then after the superimposed pulse the depolarization is increased.
Full text
PDF




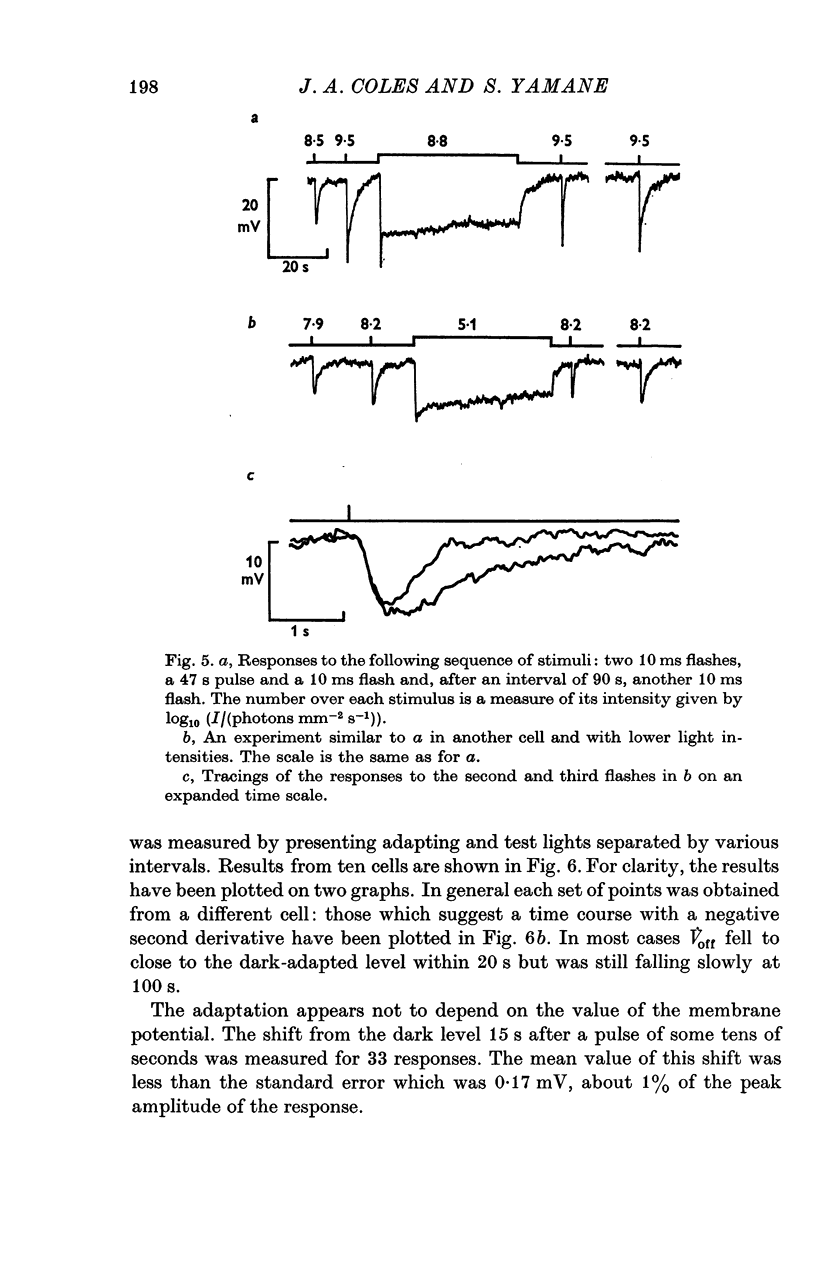
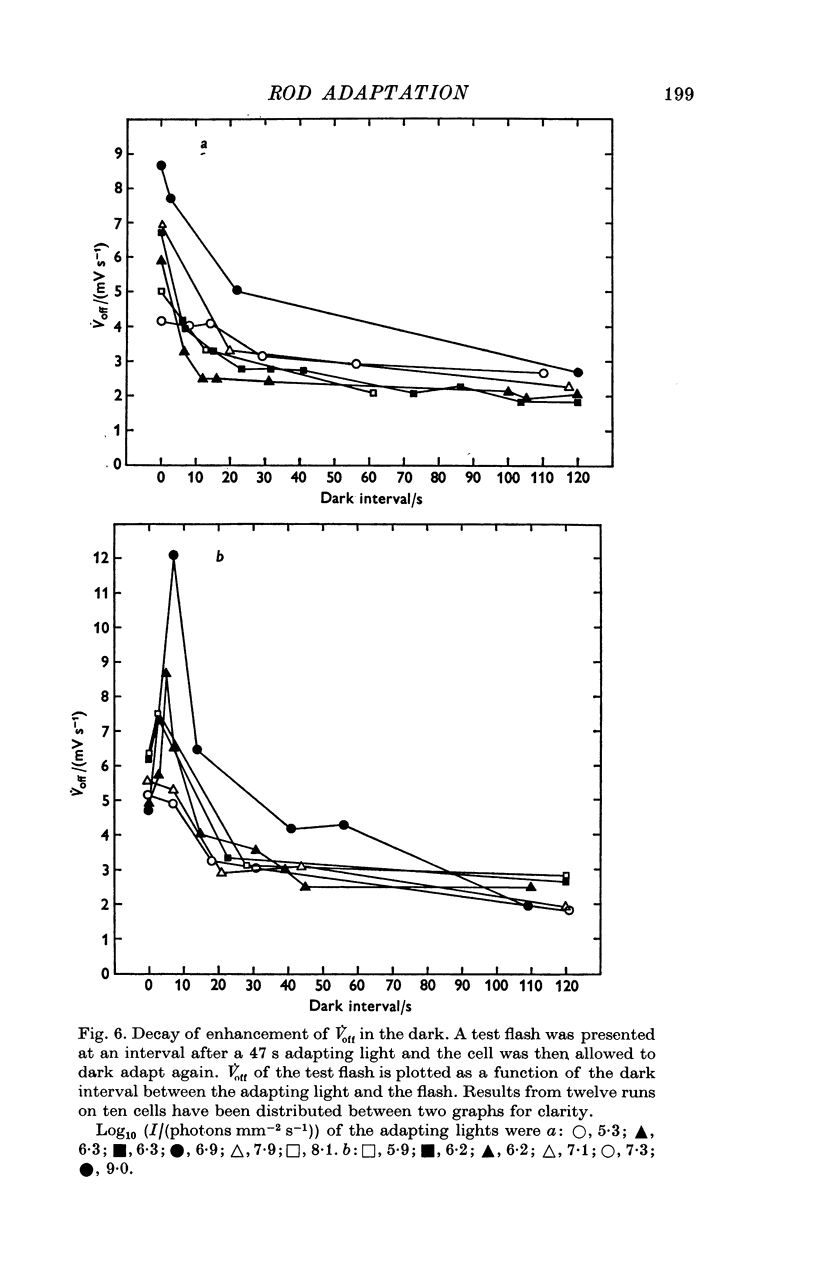
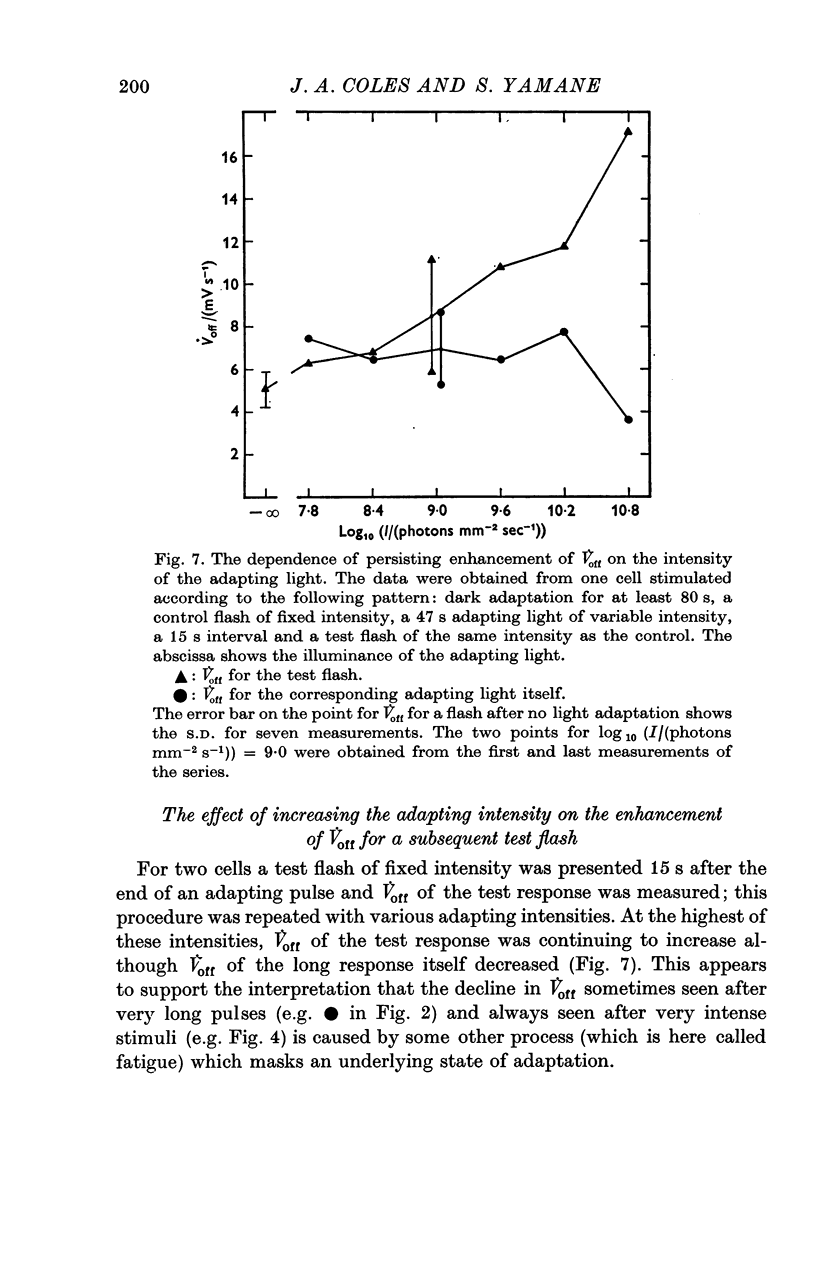
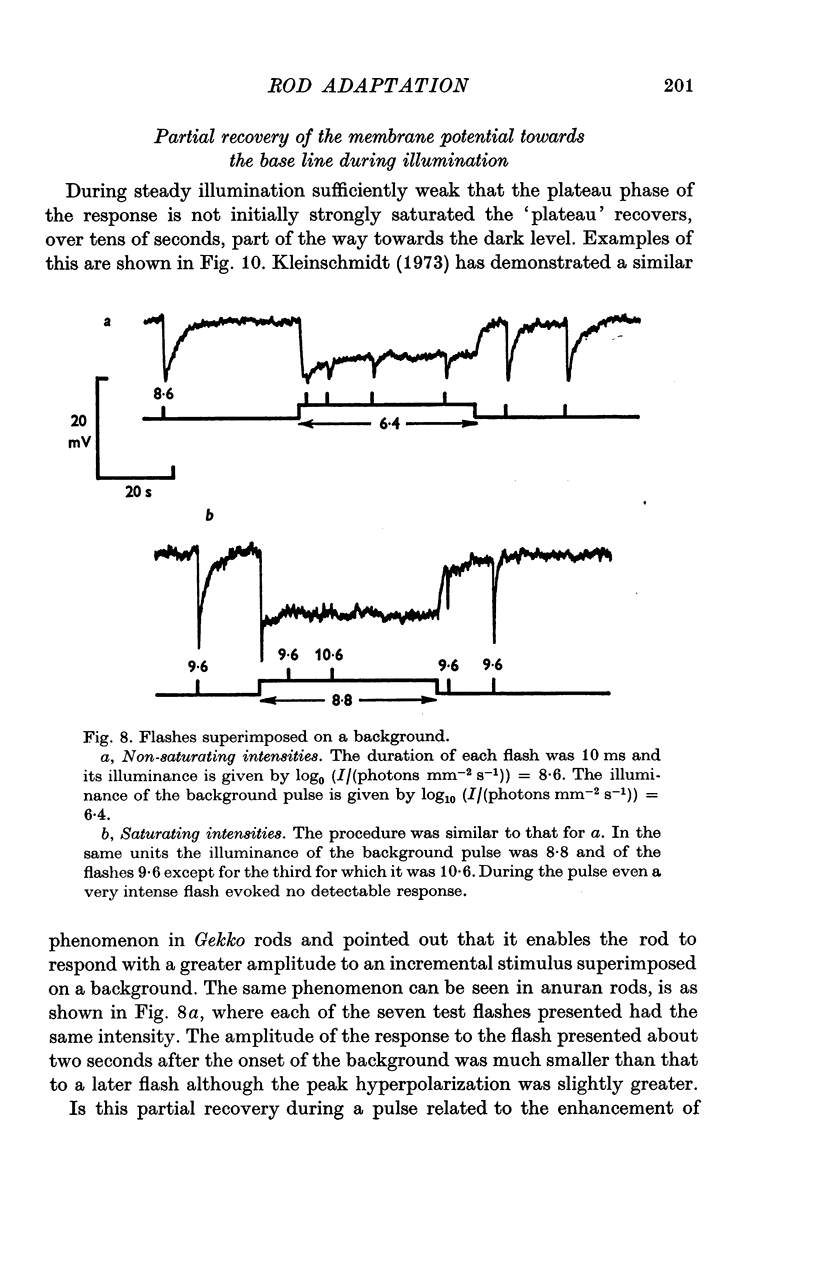



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Falk G., Fatt P. An analysis of light-induced admittance changes in rod outer segments. J Physiol. 1973 Feb;229(1):185–220. doi: 10.1113/jphysiol.1973.sp010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Naka K. I. Identification of intracellular responses in the frog retina. Brain Res. 1972 Jul 13;42(1):59–71. doi: 10.1016/0006-8993(72)90042-x. [DOI] [PubMed] [Google Scholar]
- Murakami M., Otsu K., Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol. 1972 Dec;227(3):899–913. doi: 10.1113/jphysiol.1972.sp010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter T. E., White R. H., Wald G. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J Gen Physiol. 1971 Oct;58(4):351–371. doi: 10.1085/jgp.58.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A. W., Hamer M. The light-capture area of a photoreceptor. Vision Res. 1972 Oct;12(10):1749–1753. doi: 10.1016/0042-6989(72)90045-4. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A., Murakami M., Pautler E. L. Spectral response curves of single cones in the carp. Vision Res. 1967 Jul;7(7):519–531. doi: 10.1016/0042-6989(67)90061-2. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Coles J. A. [Proceedings: 348. Some properties of the quantal response in the frog rod (author's transl)]. Nihon Seirigaku Zasshi. 1973 Aug-Sep;35(8):528–529. [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]


