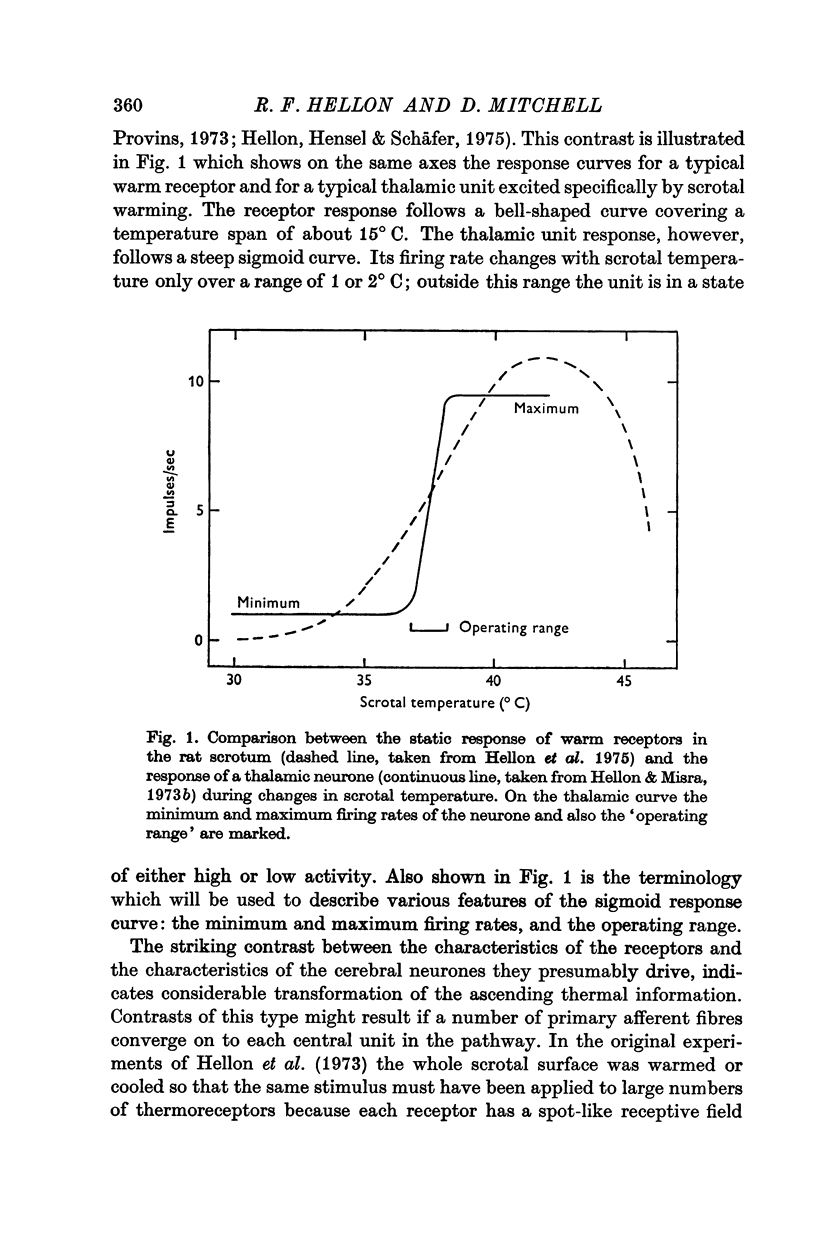
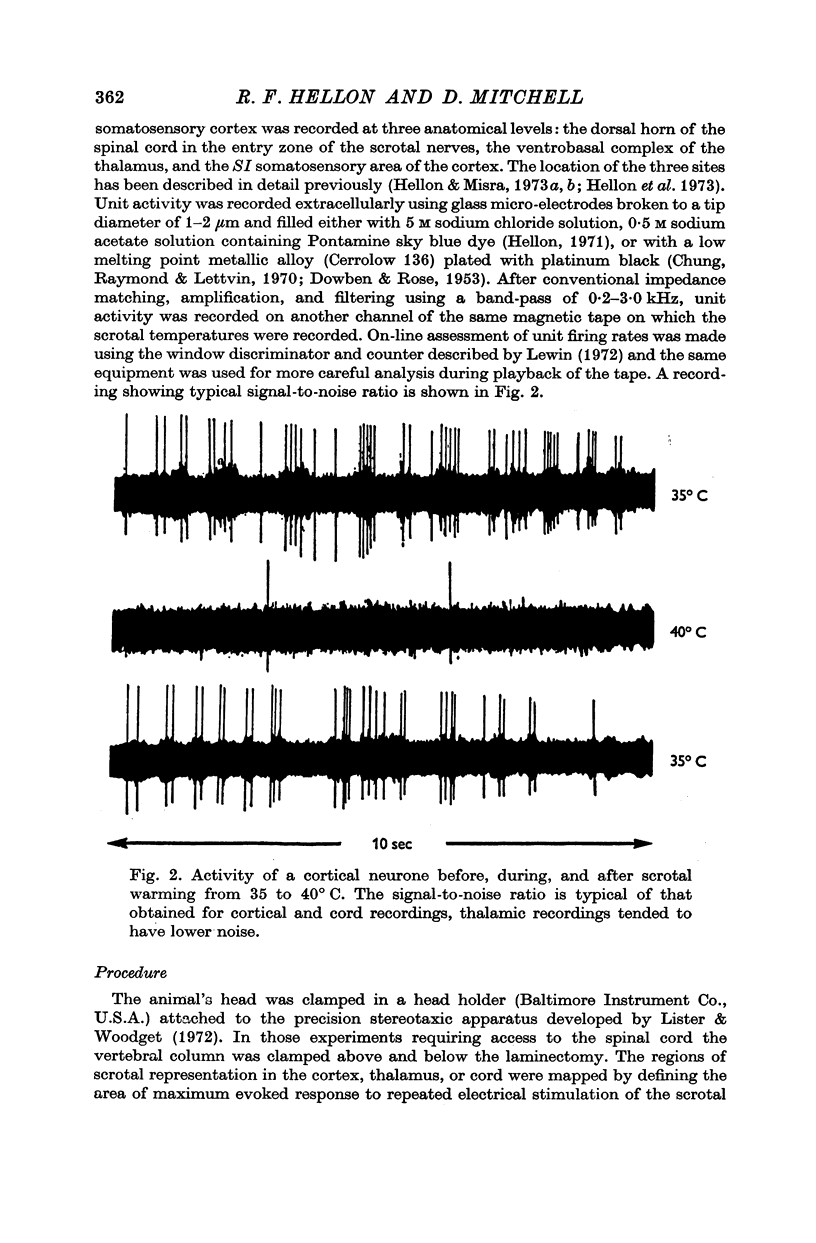
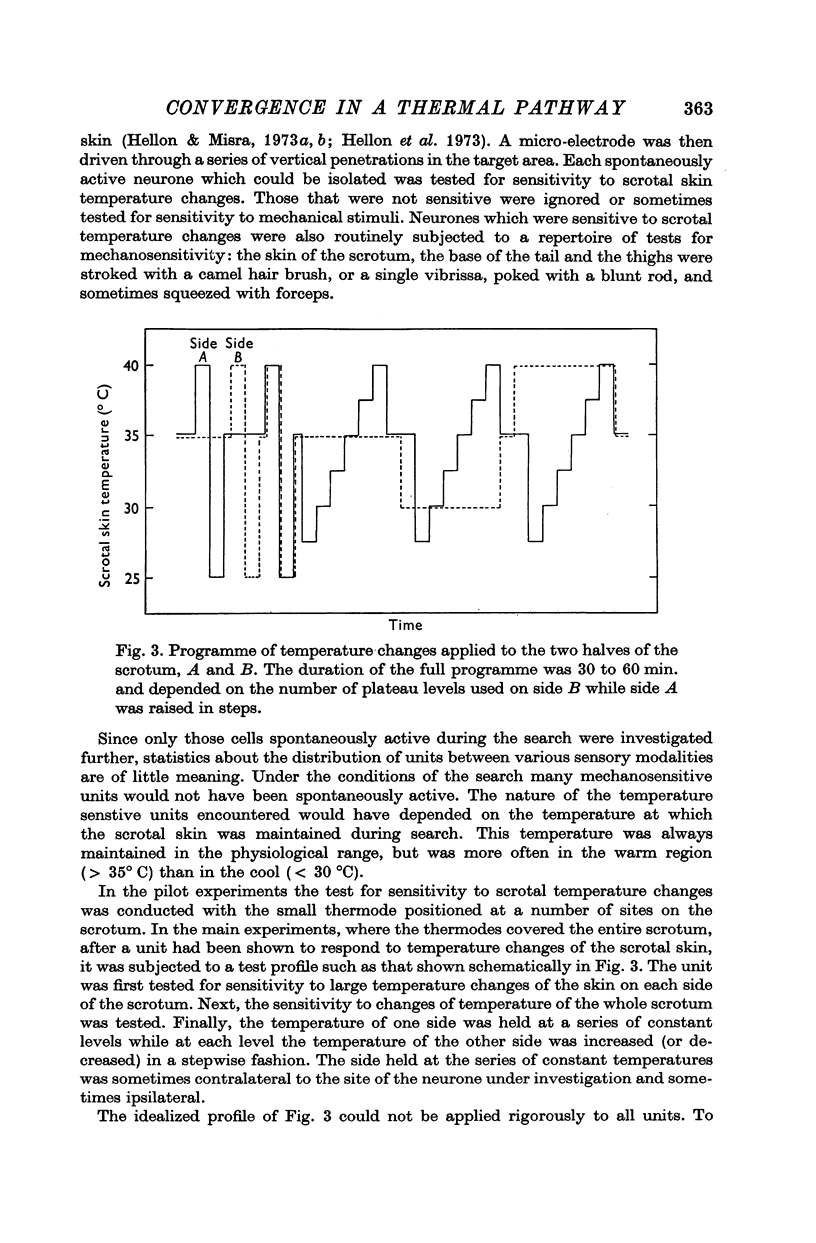
Abstract
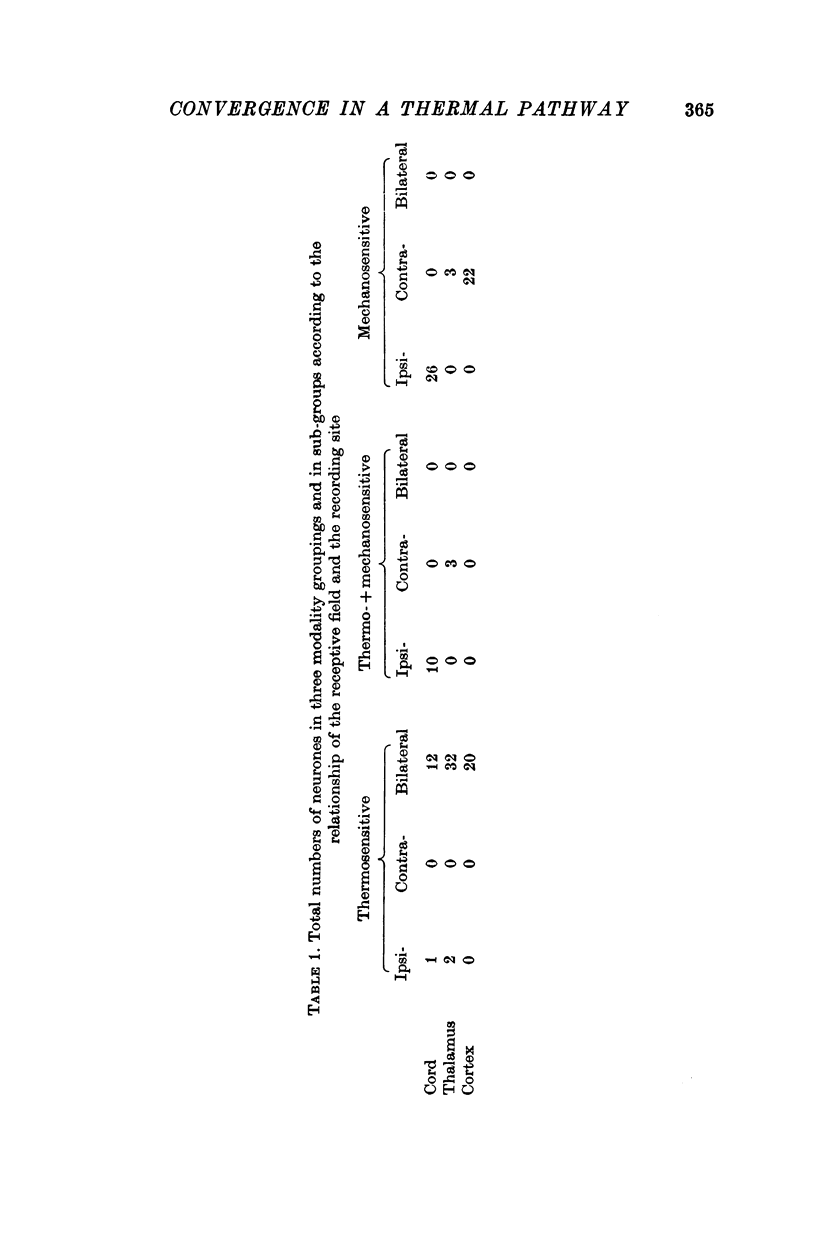
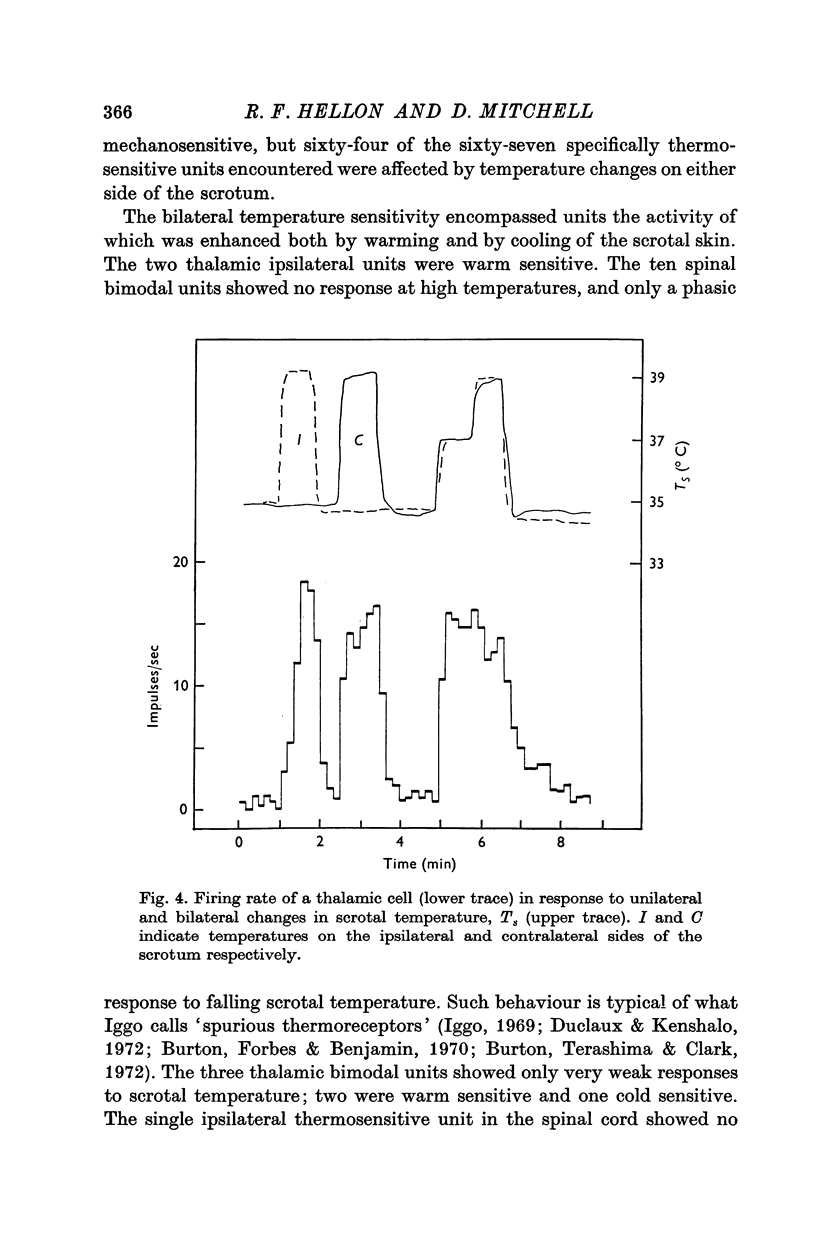
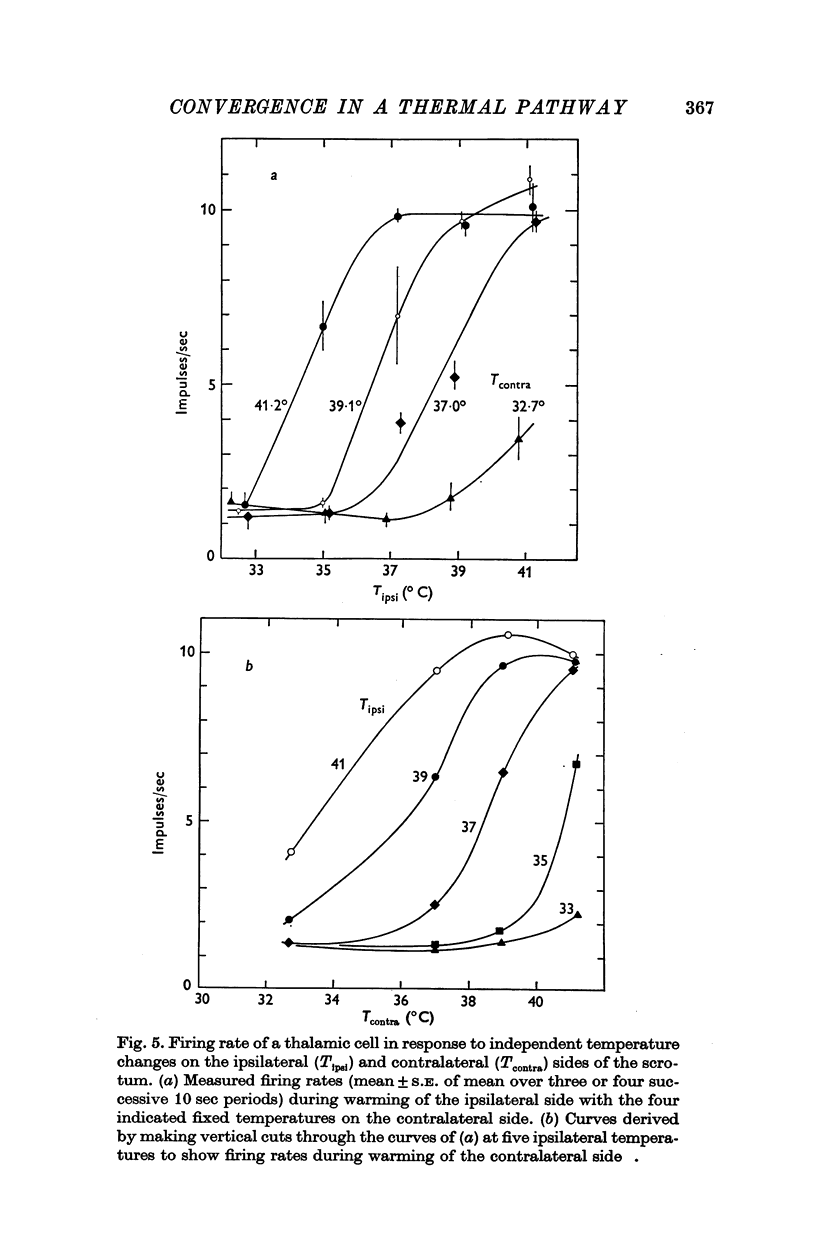
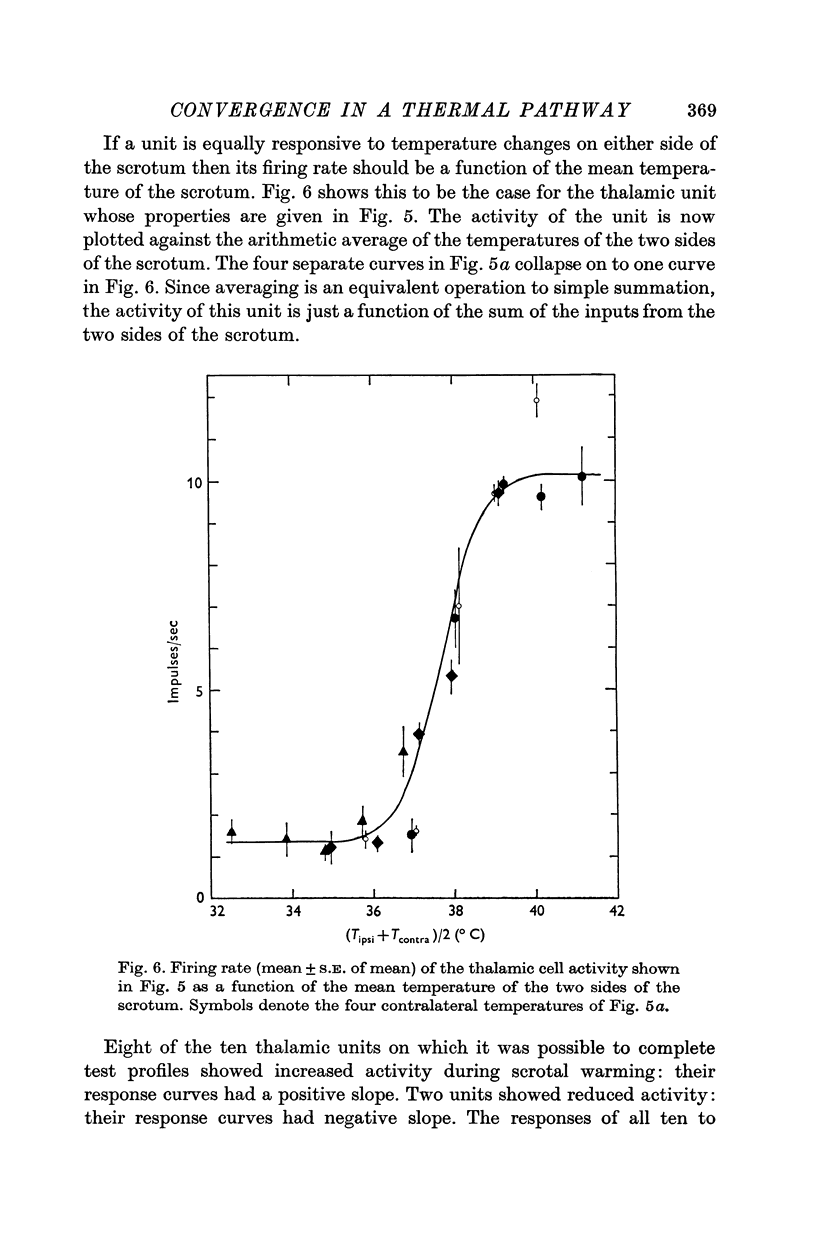
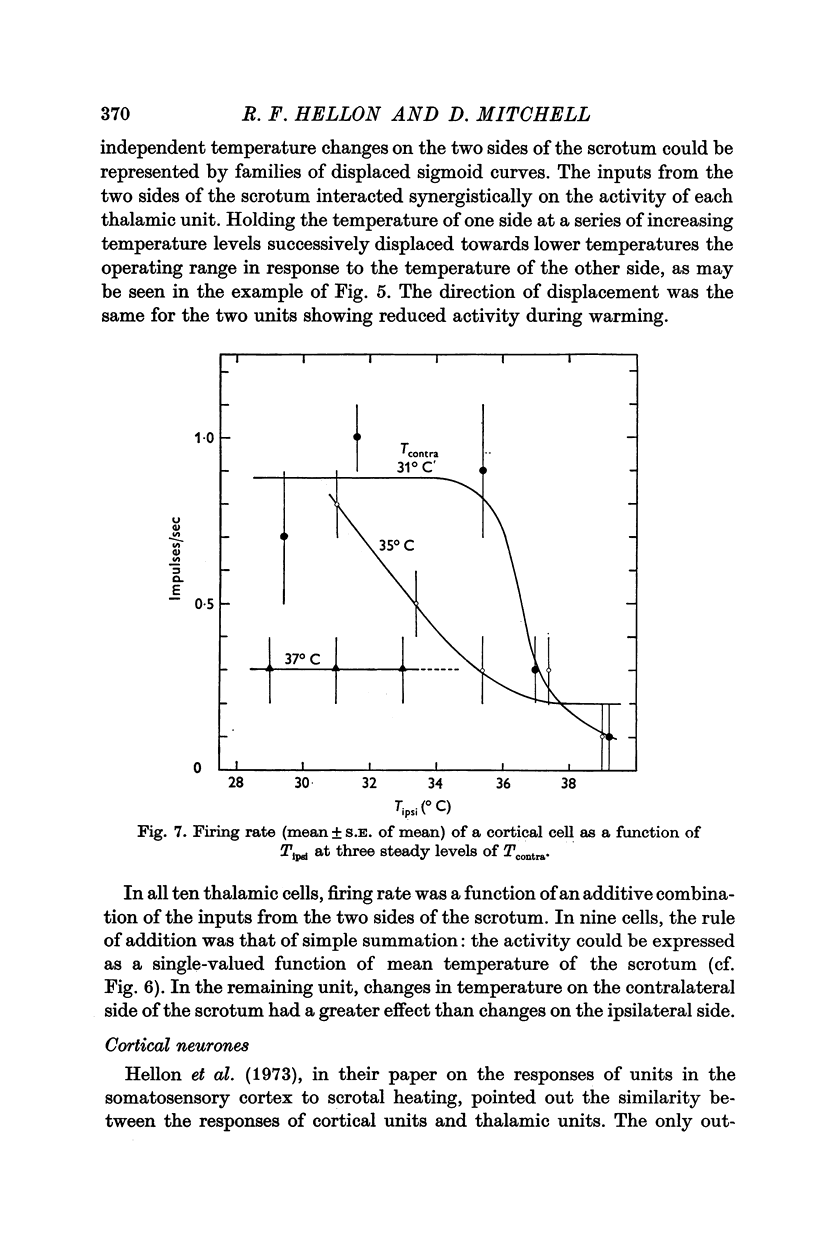
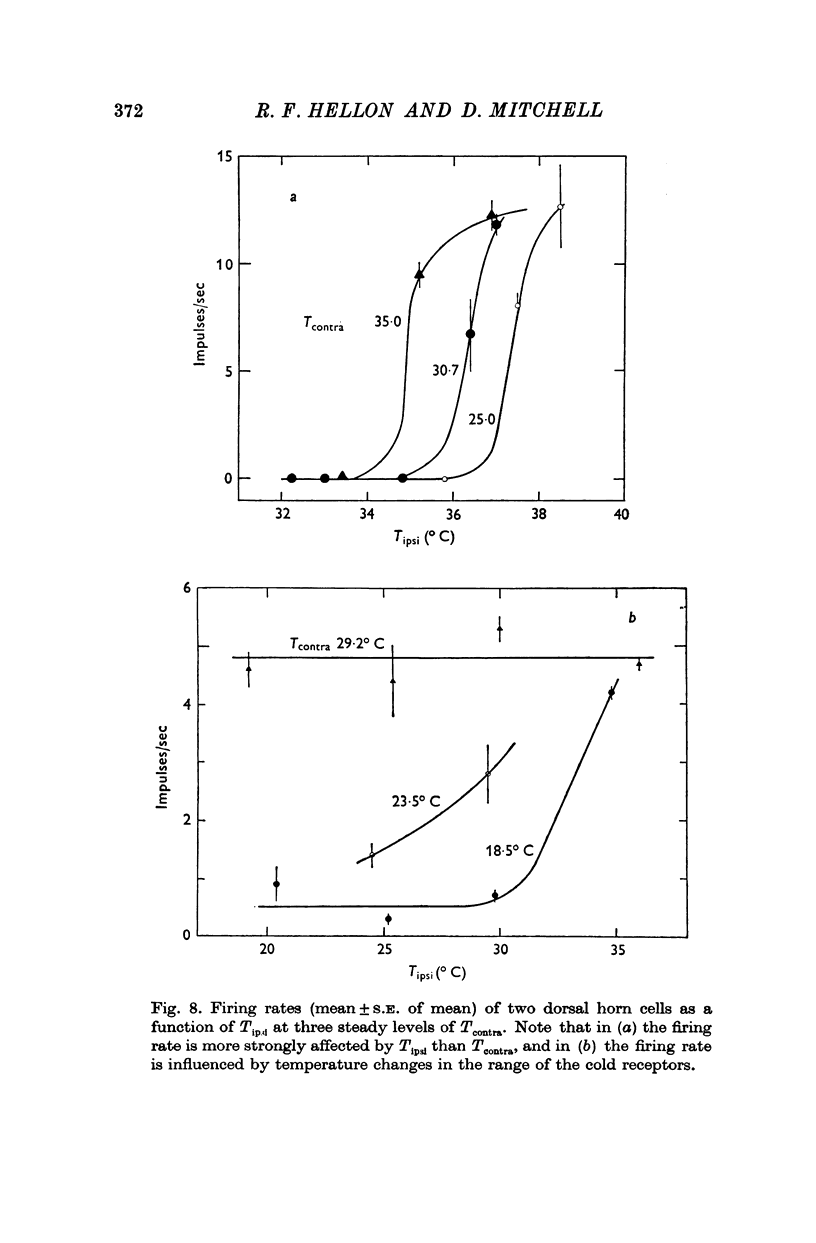
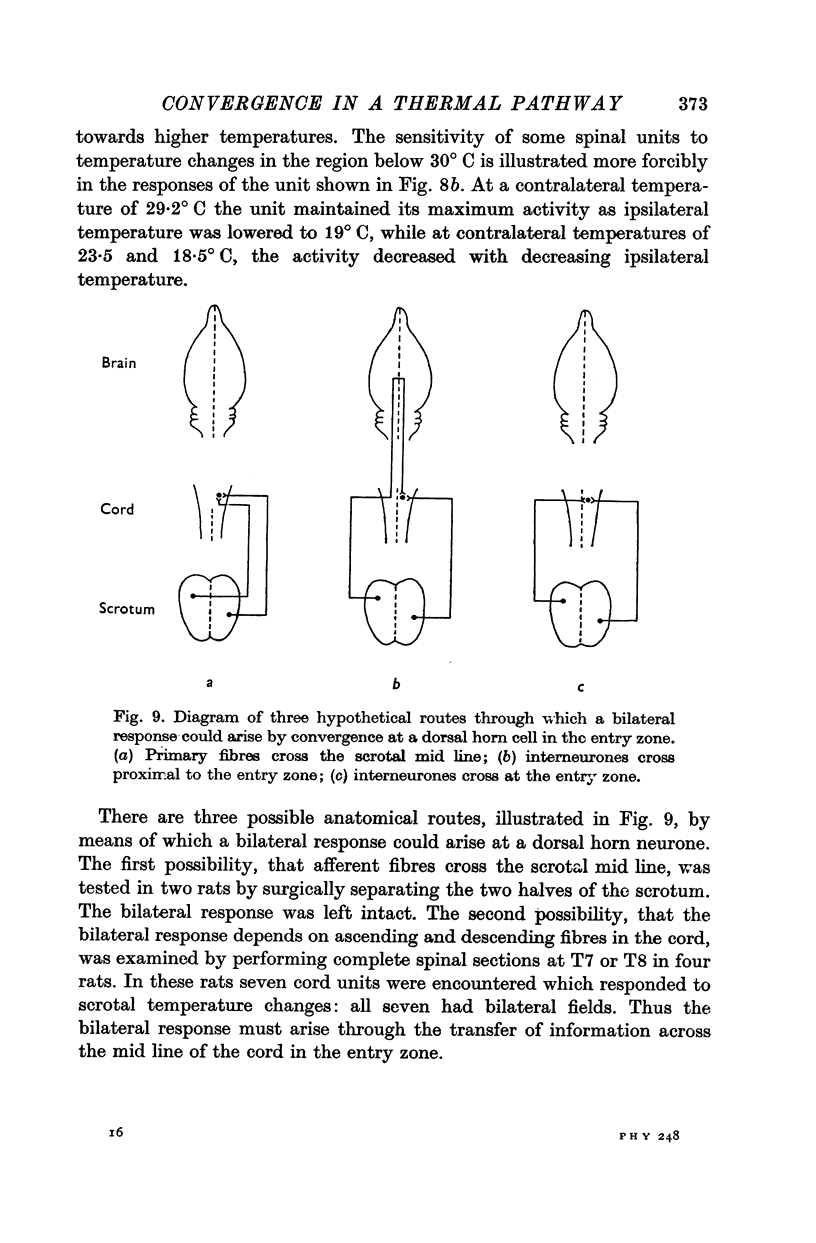
1. In anaesthetized rats, unit activity was recorded in the afferent somatosensory pathway leading from the scrotum. Recording sites were in the dorsal horn near the entry zone of the scrotal nerve, in the ventrobasal complex of the thalamus and in the somatosensory (SI) cortex. During recording, the temperatures of the left and right sides of the scrotum were varied independently. 2. Almost all (64/67) the units in dorsal horn, thalamus and cortex responding specifically to scrotal temperature were equally affected by temperature changes on either side of the scrotum. The receptive fields of these units were bilateral and large, implying a massive convergence of fibres from thermoreceptors on to each central unit. In contrast, mechanosensitive units responded only to unilateral stimulation. 3. As a consequence of the convergence in the thermal pathway, the firing rate of each central unit was a function of an additive combination, often simply the sum, of the temperatures of the two sides of the scrotum. 4. The relationship between firing rate and the temperature of one side of the scrotum was sigmoid, the position, but not the shape, of the curve depending on the temperature at which the opposite side was maintained. An increase in the maintained temperature shifted the sigmoid response curve towards lower temperatures and vice versa. 5. The convergence which this pathway exhibits would be well suited to integration of the temperature of the scrotal skin, but not to spatial discrimination.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton H., Forbes D. J., Benjamin R. M. Thalamic neurons responsive to temperature changes of glabrous hand and foot skin in squirrel monkey. Brain Res. 1970 Dec 1;24(2):179–190. doi: 10.1016/0006-8993(70)90099-5. [DOI] [PubMed] [Google Scholar]
- Burton H., Terashima S. I., Clark J. Response properties of slowly adapting mechanoreceptors to temperature stimulation in cats. Brain Res. 1972 Oct 27;45(2):401–416. doi: 10.1016/0006-8993(72)90471-4. [DOI] [PubMed] [Google Scholar]
- Chung S. H., Raymond S. A., Lettvin J. Y. Multiple meaning in single visual units. Brain Behav Evol. 1970;3(1):72–101. doi: 10.1159/000125464. [DOI] [PubMed] [Google Scholar]
- DOWBEN R. M., ROSE J. E. A metal-filled microelectrode. Science. 1953 Jul 3;118(3053):22–24. doi: 10.1126/science.118.3053.22. [DOI] [PubMed] [Google Scholar]
- Duclaux R., Kenshalo D. R. The temperature sensitivity of the type I slowly adapting mechanoreceptors in cats and monkeys. J Physiol. 1972 Aug;224(3):647–664. doi: 10.1113/jphysiol.1972.sp009917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstorfer H., Hensel H. Thermal cutaneous afferents in the trigeminal nucleus of the cat. Naturwissenschaften. 1973 Apr;60(4):209–209. doi: 10.1007/BF00599448. [DOI] [PubMed] [Google Scholar]
- Hellon R. F., Hensel H., Schäfer K. Thermal receptors in the scrotum of the rat. J Physiol. 1975 Jun;248(2):349–357. doi: 10.1113/jphysiol.1975.sp010978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the dorsal horn of the rat responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):375–388. doi: 10.1113/jphysiol.1973.sp010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K. Neurones in the ventrobasal complex of the rat thalamus responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):389–399. doi: 10.1113/jphysiol.1973.sp010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Misra N. K., Provins K. A. Neurones in the somatosensory cortex of the rat responding to scrotal skin temperature changes. J Physiol. 1973 Jul;232(2):401–411. doi: 10.1113/jphysiol.1973.sp010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellon R. F., Mitchell D. Proceedings: Convergence in a thermal afferent pathway. J Physiol. 1974 May;239(1):61P–62P. [PubMed] [Google Scholar]
- Hellon R. F. The marking of electrode tip positions in nervous tissue. J Physiol. 1971;214 (Suppl):12P–12P. [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Ramsey R. L. Proceedings: Dorsal horn neurons excited by cutaneous cold receptors in primates. J Physiol. 1974 Oct;242(2):132P–133P. [PubMed] [Google Scholar]
- Lewin J. E. A counter for recording the rate of firing of neurones. J Physiol. 1972 Apr;222(2):132P–133P. [PubMed] [Google Scholar]
- Lister W. C., Woodget L. L. Precision stereotaxic equipment. J Physiol. 1972 Apr;222(2):130P–132P. [PubMed] [Google Scholar]
- Poulos D. A., Benjamin R. M. Response of thalamic neurons to thermal stimulation of the tongue. J Neurophysiol. 1968 Jan;31(1):28–43. doi: 10.1152/jn.1968.31.1.28. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res. 1968 Jun;9(1):32–58. doi: 10.1016/0006-8993(68)90256-4. [DOI] [PubMed] [Google Scholar]


