Abstract
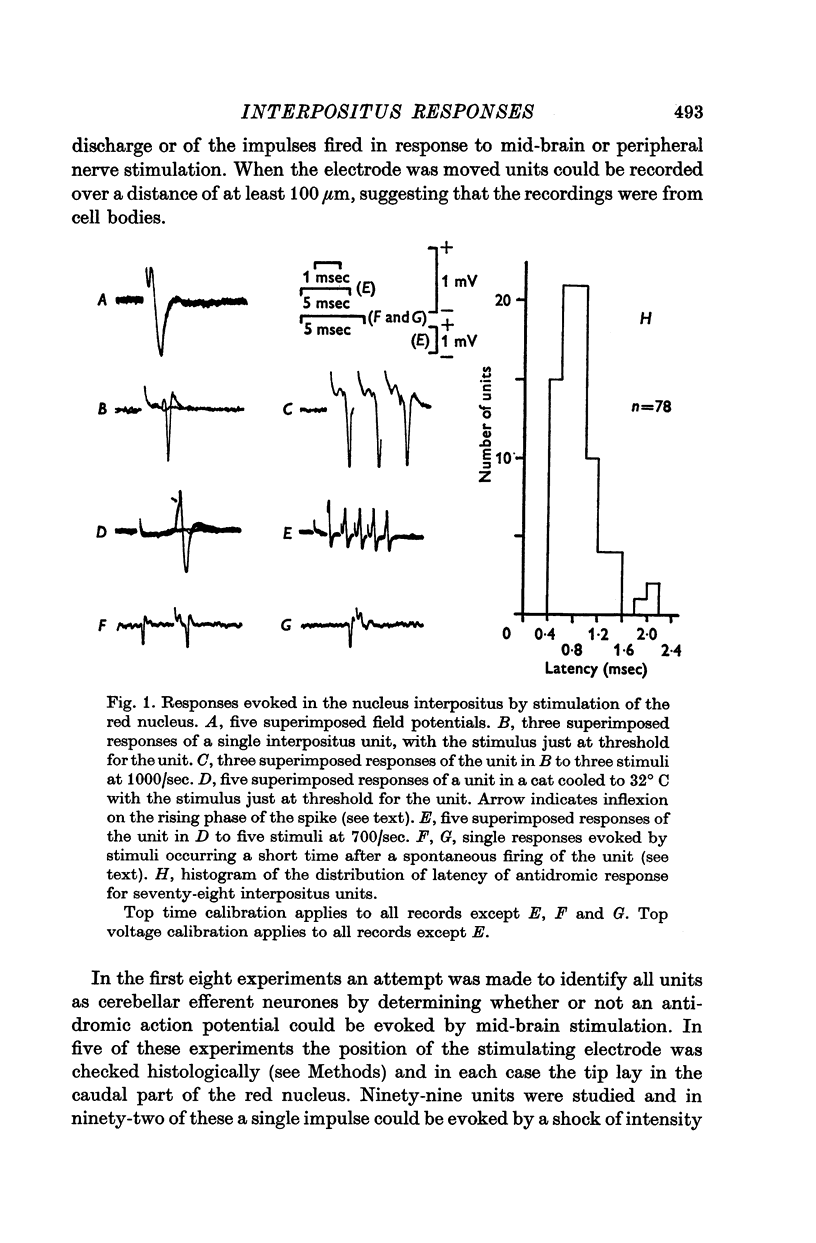
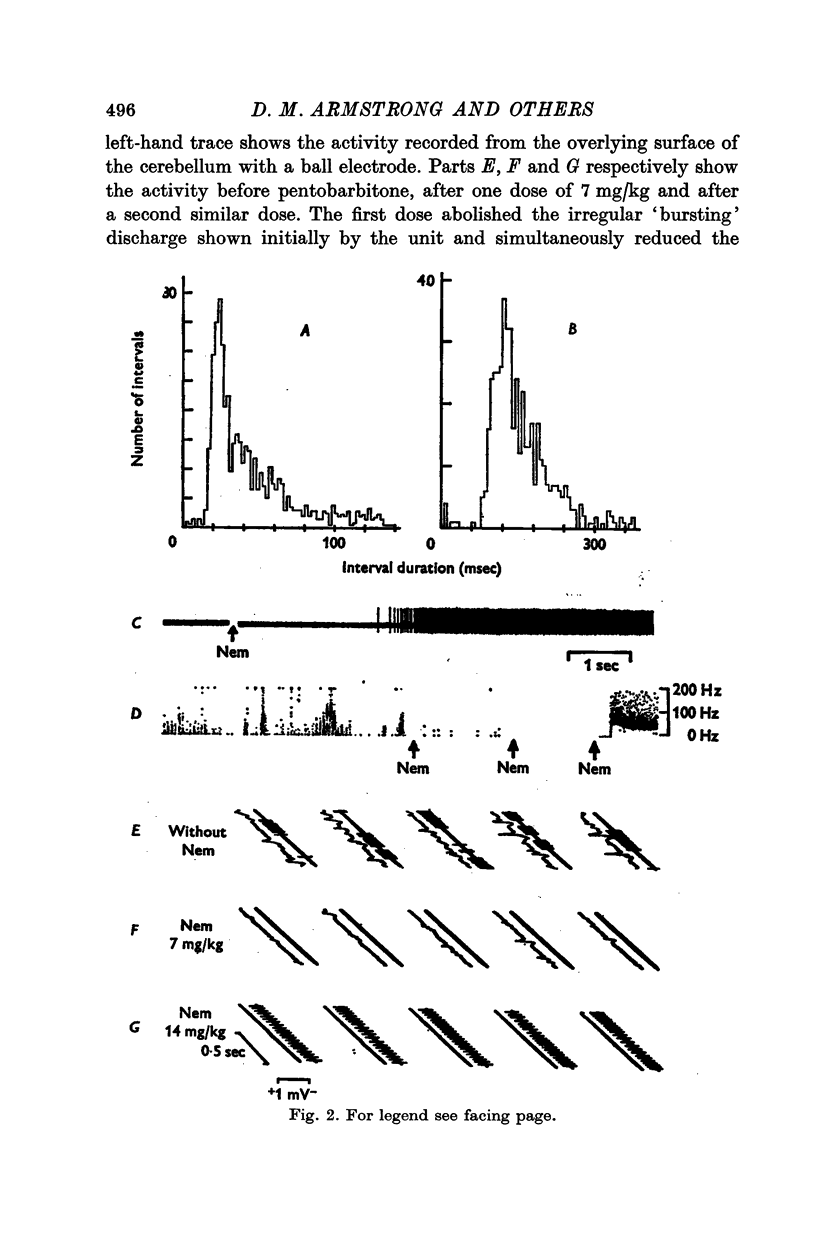
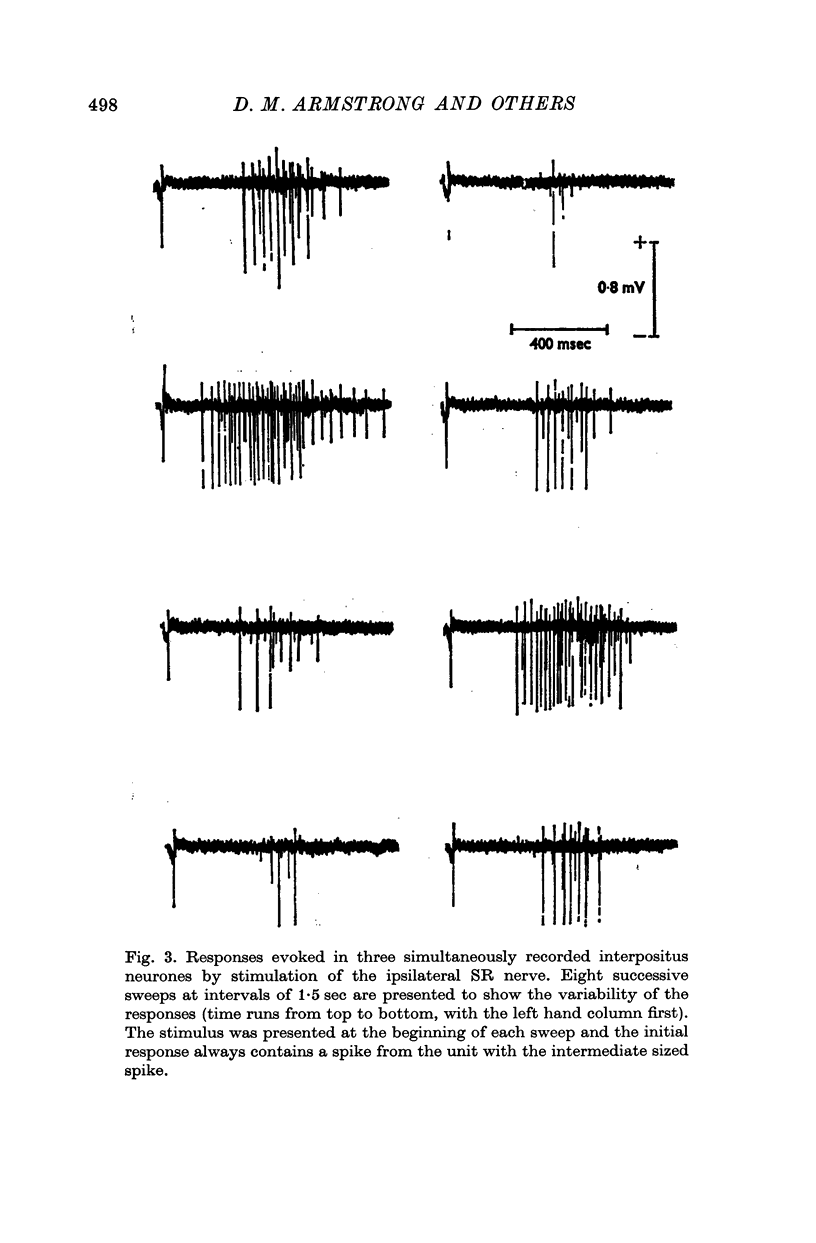
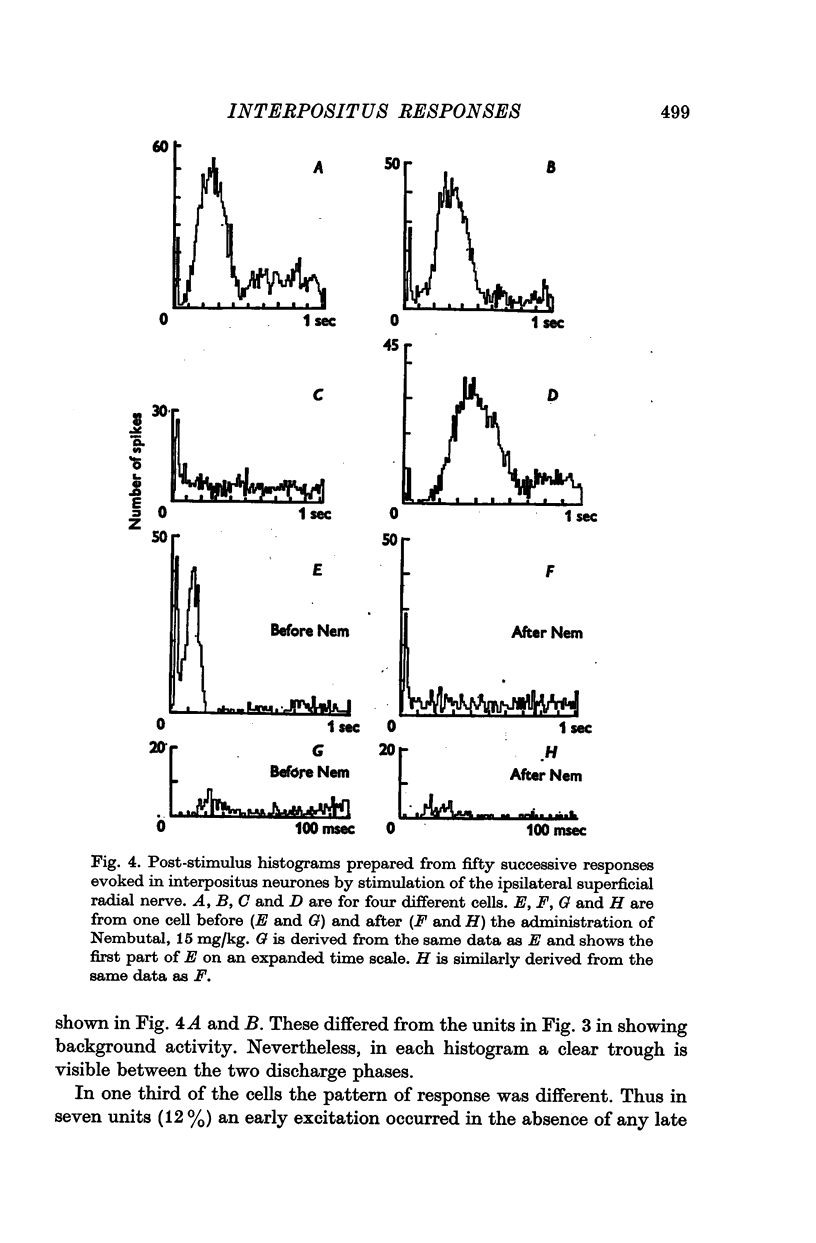
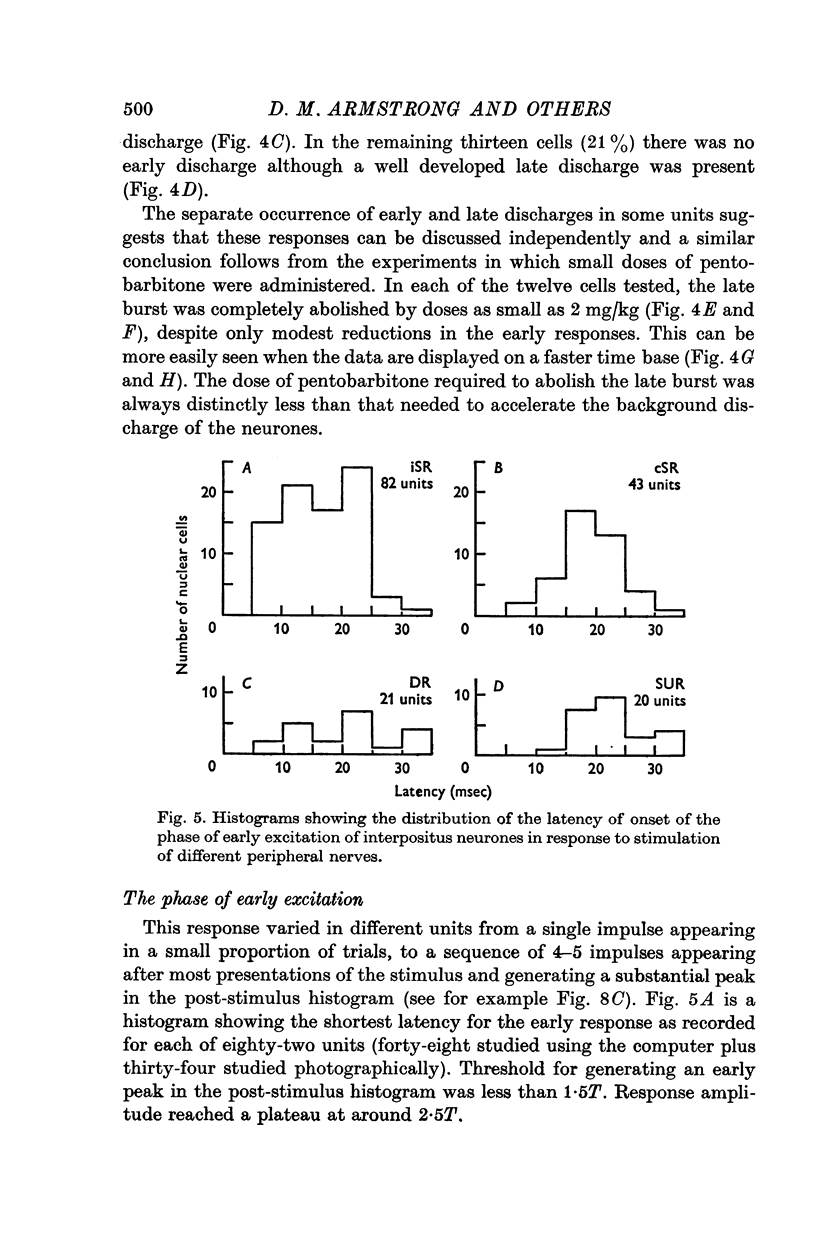
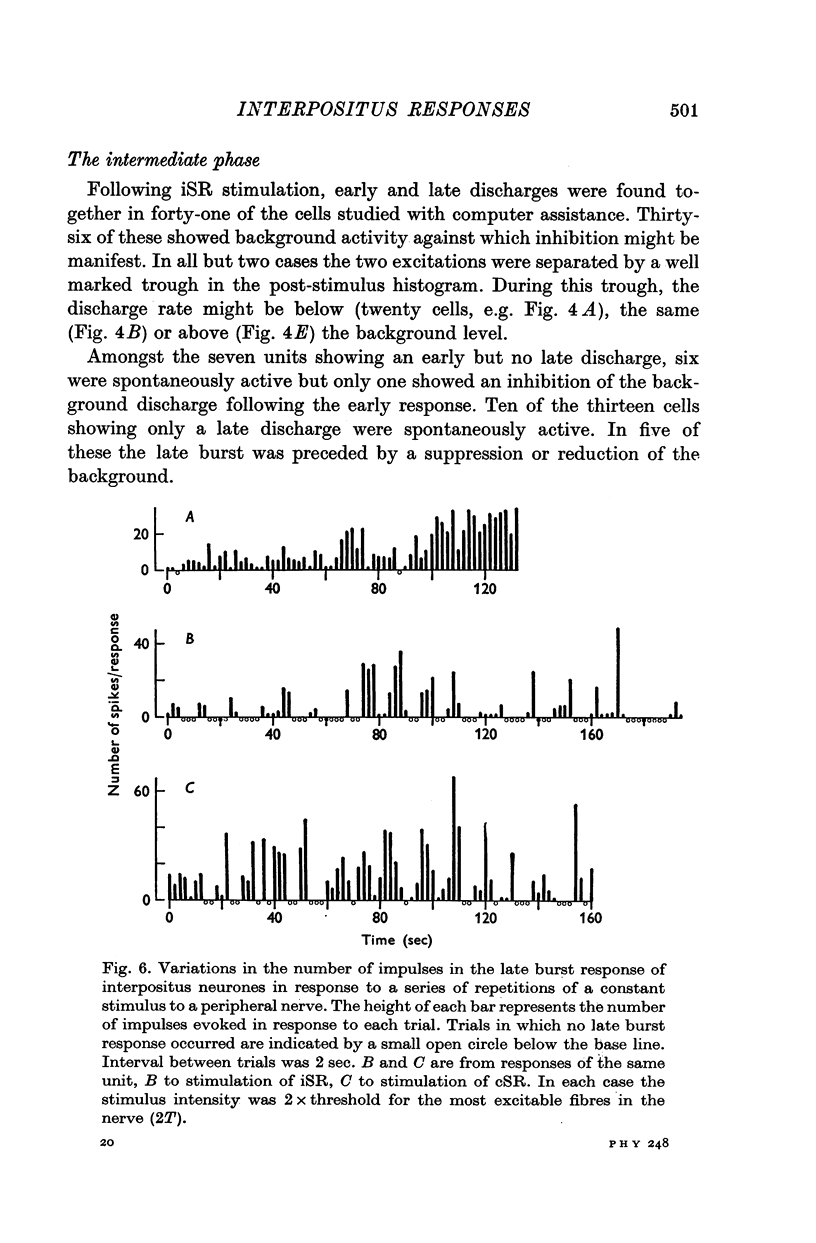
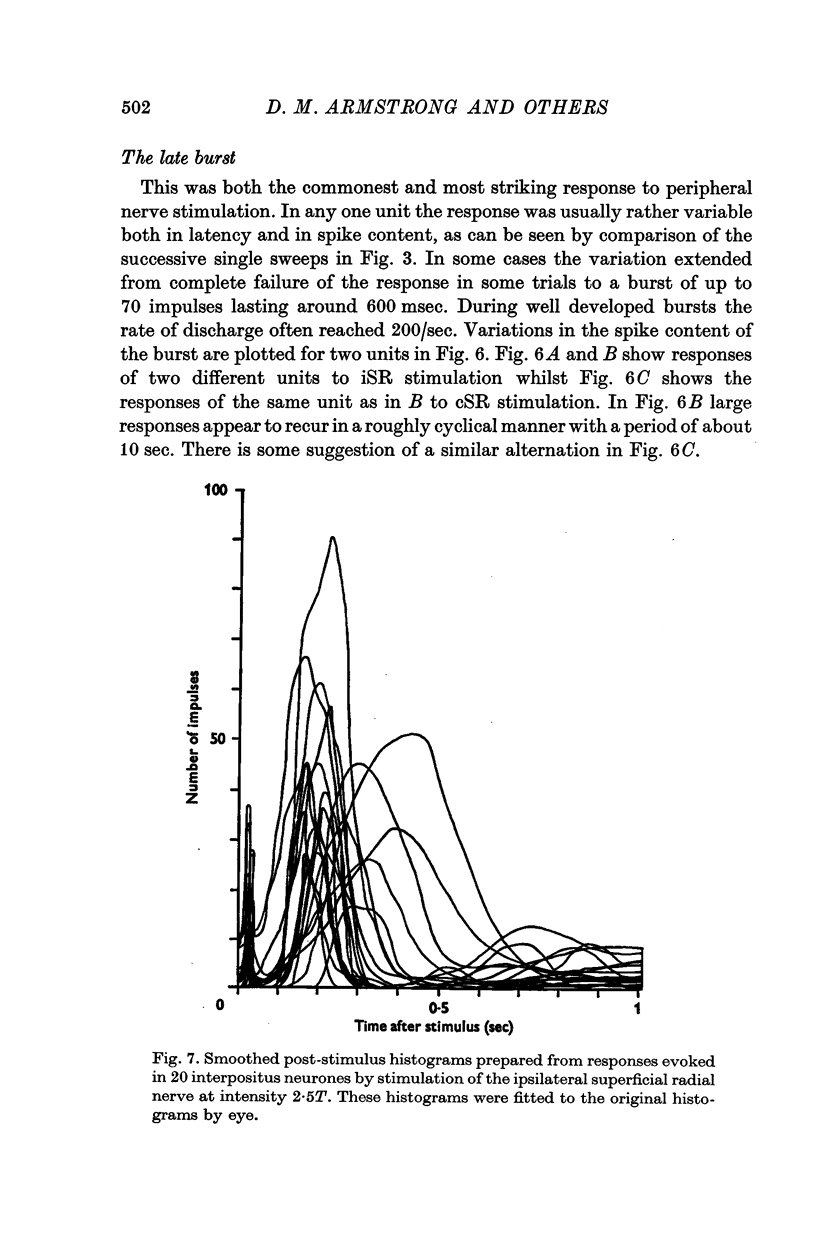
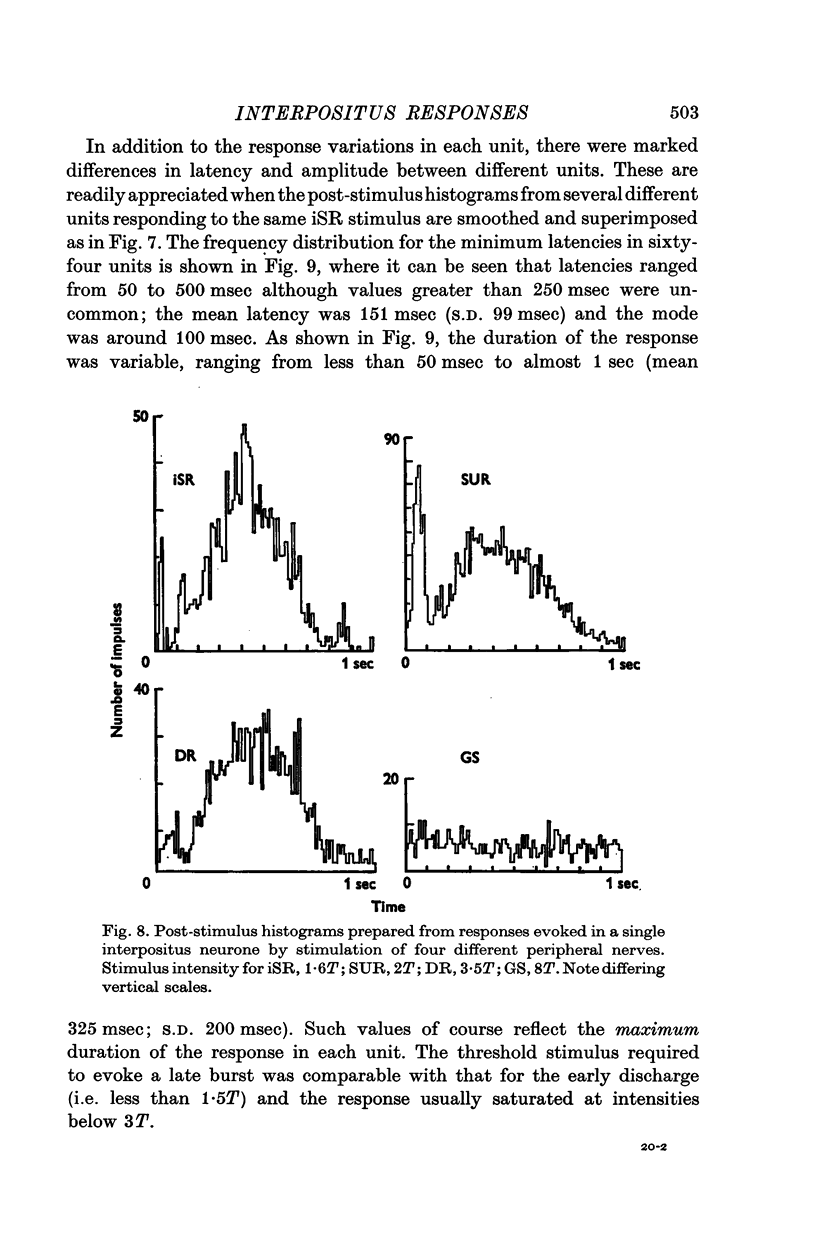
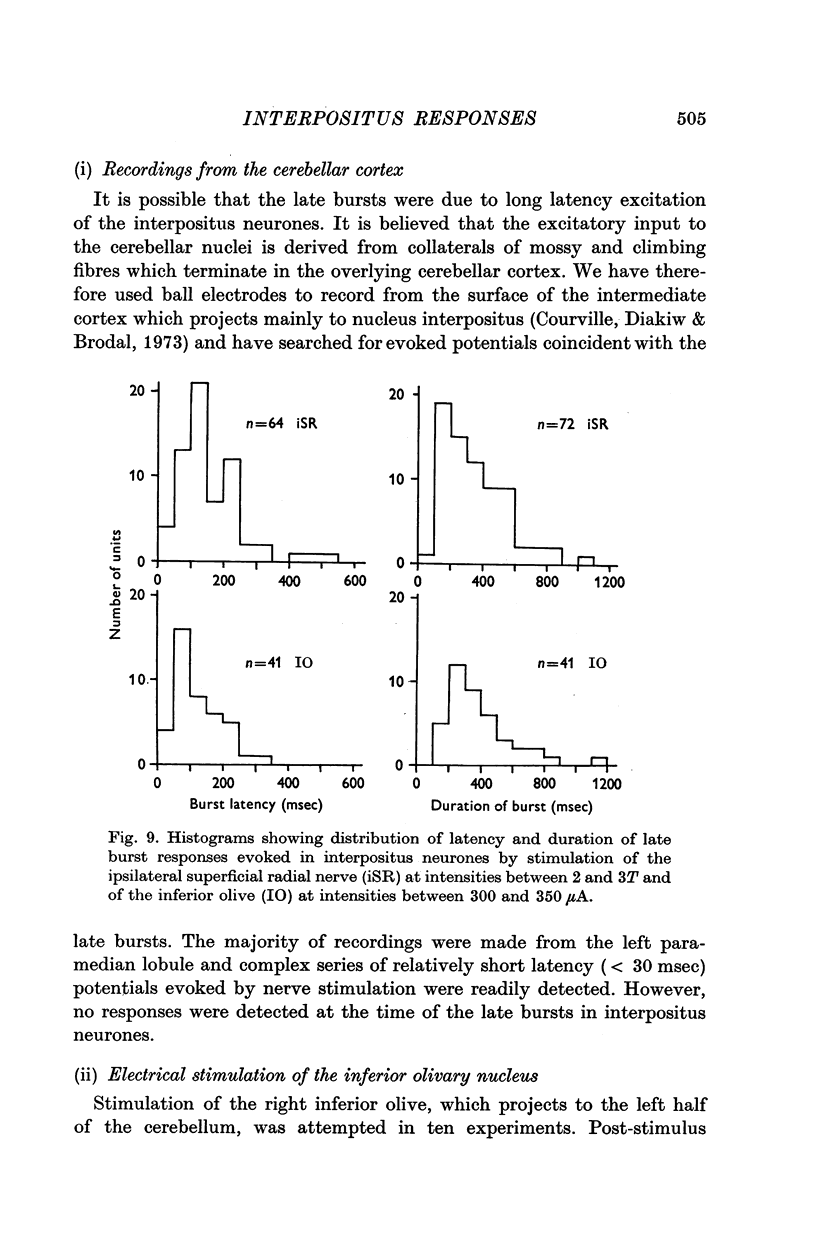
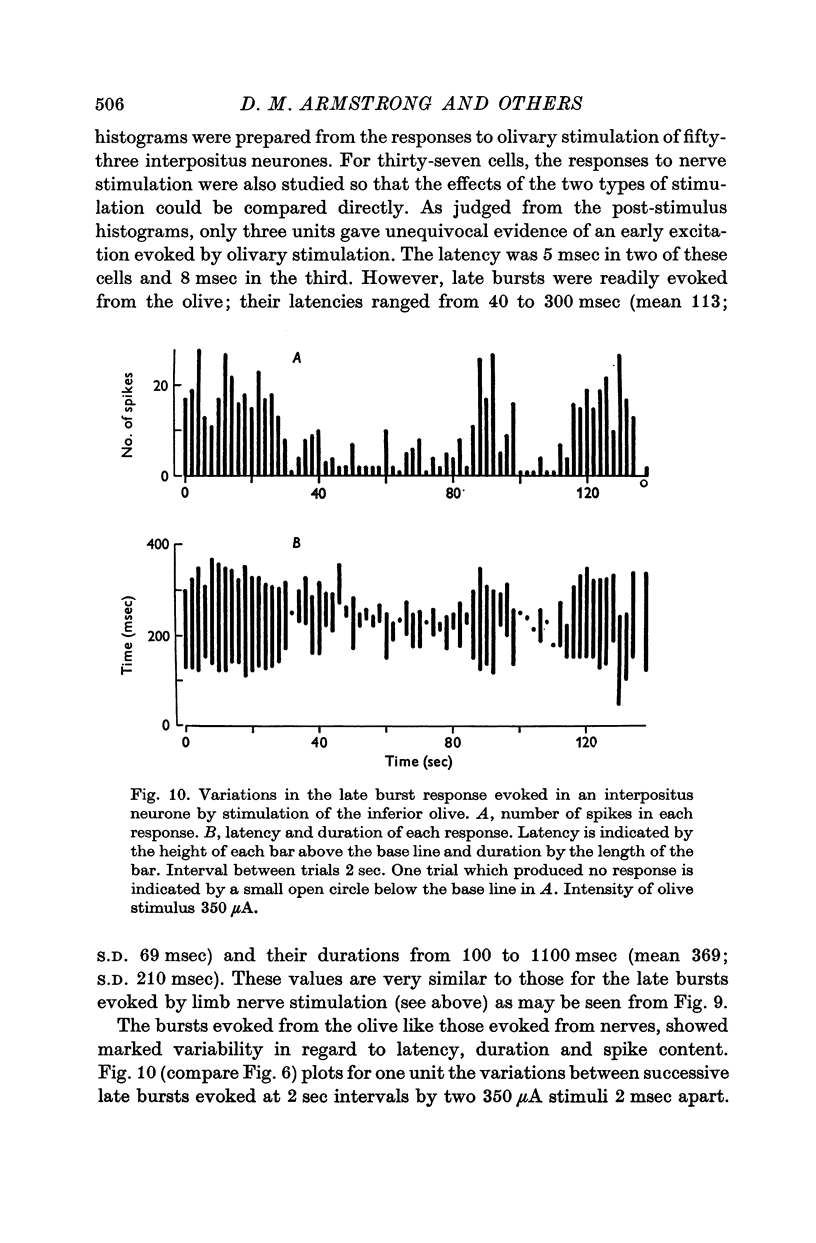
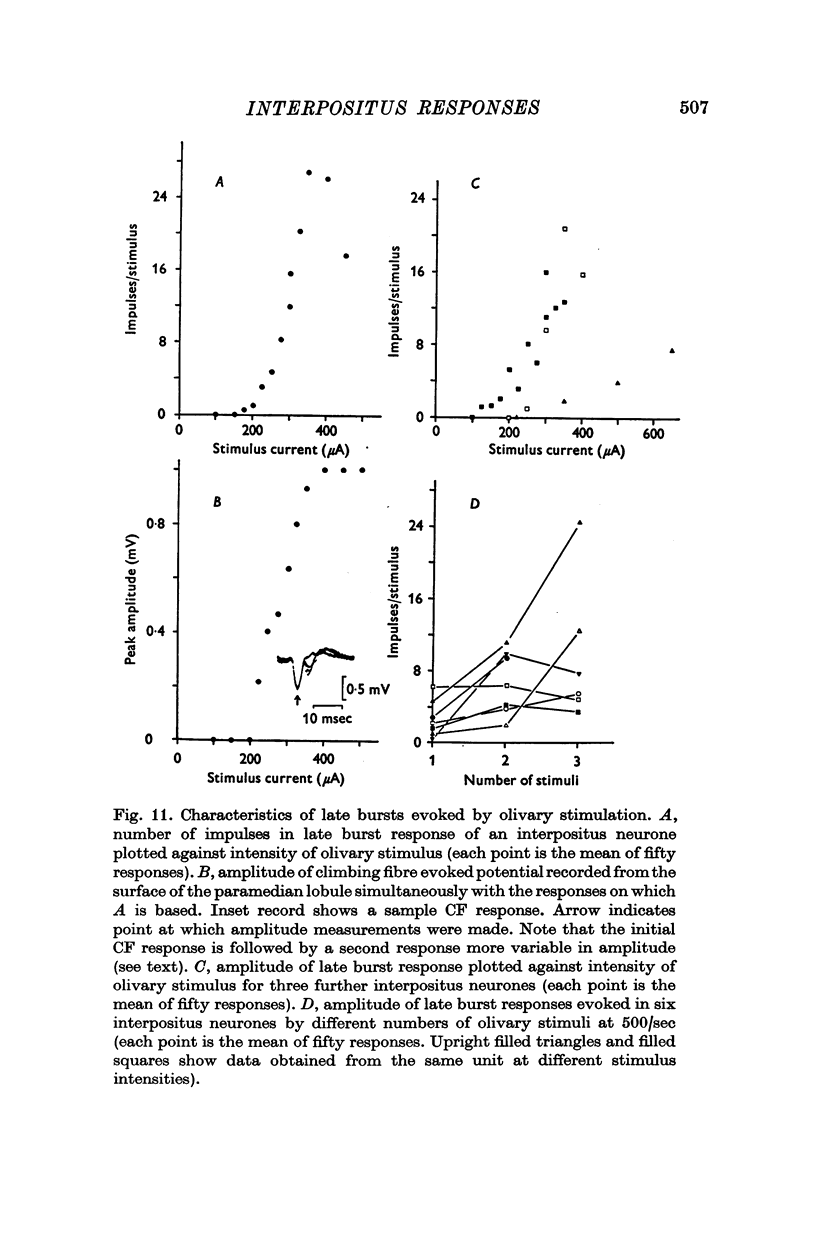
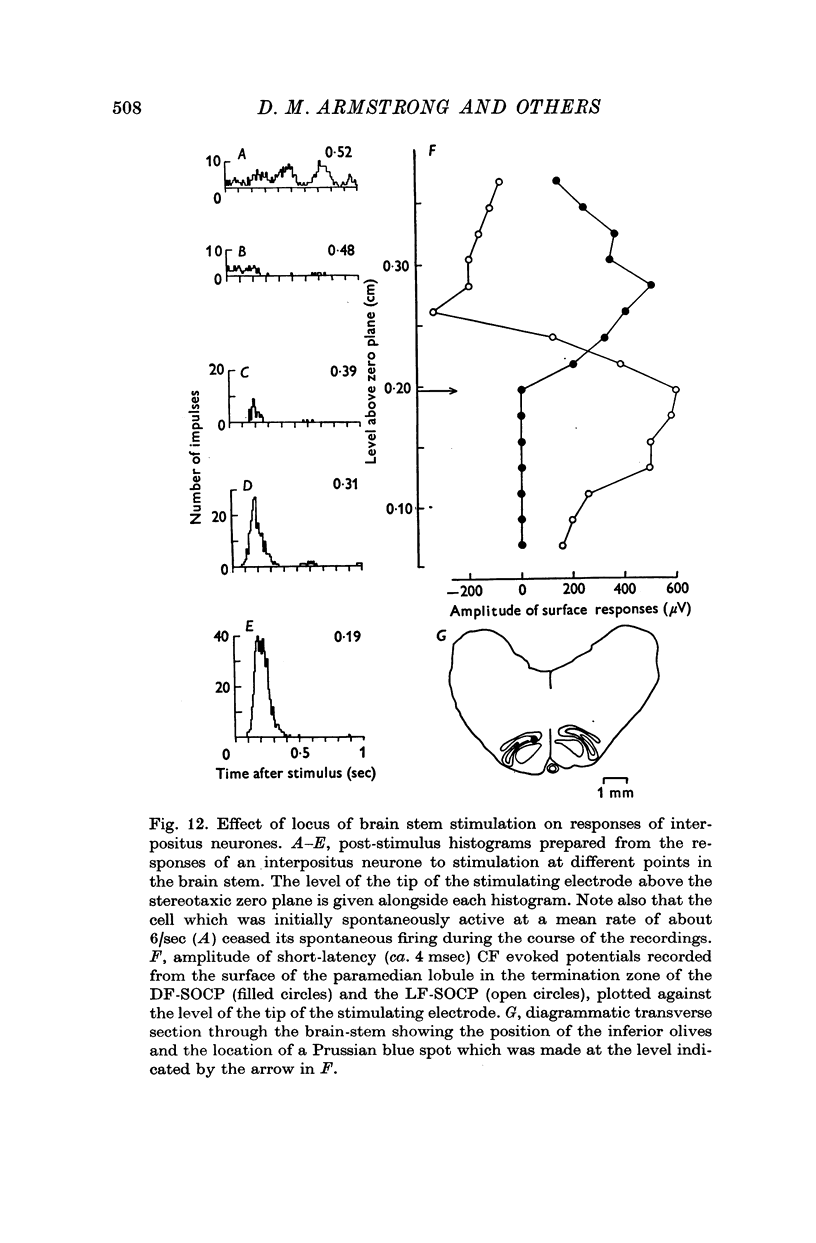
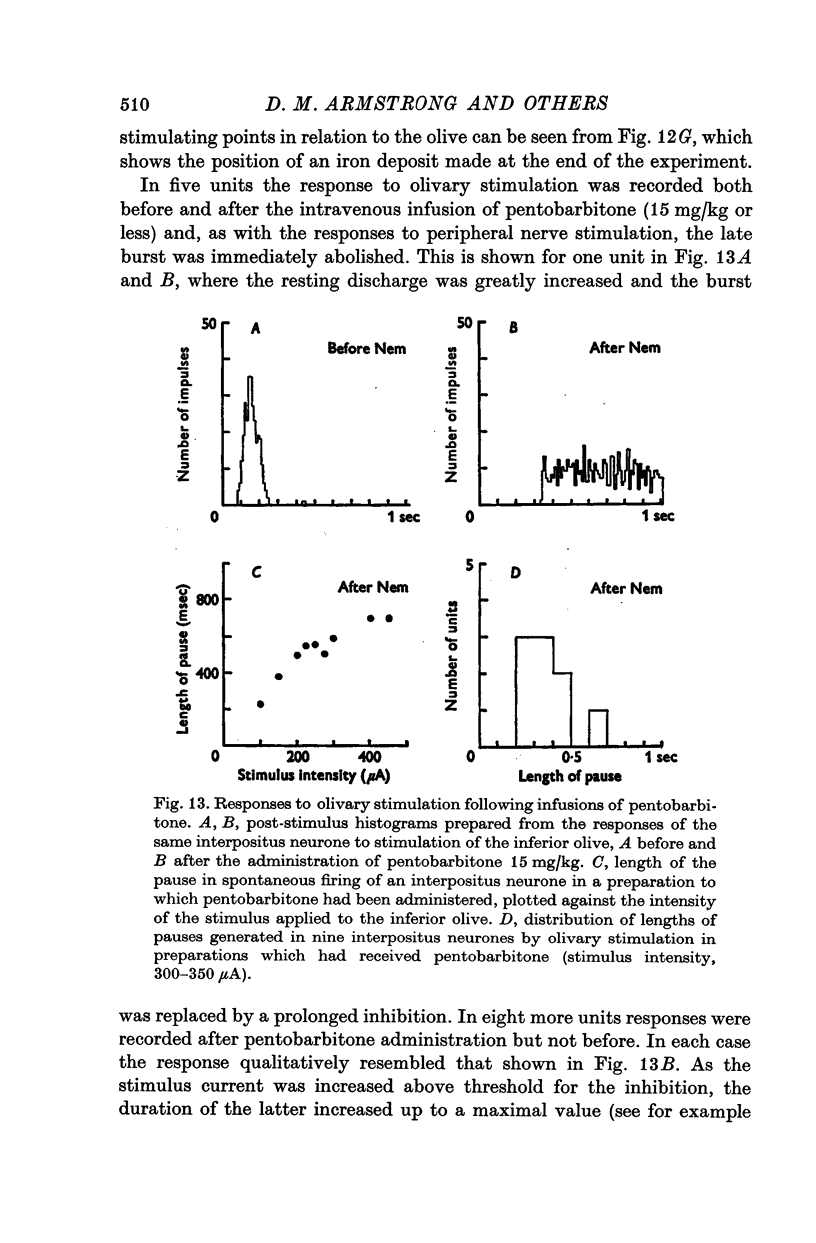
1. In cats anaesthetized with alpha-chloralose, micro-electrodes have been used to record the discharge patterns of single neurones in the region of the nucleus interpositus. 2. Almost all cells tested could be antidromically invaded following electrical stimulation of the contralateral red nucleus, showing that they were cerebellar efferent neurones. 3. A little over half of the interpositus neurones were spontaneously active, usually at rates of less than 20 impulses/sec. 4. About 40% of the cells had no spontaneous activity, although they gave brisk responses to electrical stimulation of cutaneous nerves. Such silent units were encountered most frequently in the earlier stages of an experiment, but a number were found more than 15 hr after the beginning of an experiment. 5. Stimulation of cutaneous and mixed nerves of the fore and hind limbs provoked impulse discharges of the cells and also produced phases of deceleration of the resting discharge of spontaneously firing cells. 6. The typical response of an interpositus neurone consisted of a short latency (6-35 msec) discharge, usually separated from a long latency (50-500 msec) discharge by a period of inhibition or return to the resting discharge rate. The two phases of excitation appeared to be independently generated, since in a number of cells one phase appeared without the other. In addition, the later phase of excitation was abolished in all cells tested by a small dose of pentobarbitone which produced very little effect on the earlier phase. The long latency response was quantitatively much greater, sometimes consisting of 50 or more impulses in a response which lasted several hundred msec, but was very variable from one trial to another. 7. The long latency discharge and sometimes the preceding inhibition could readily be mimicked by single shock stimulation of the region of the contralateral inferior olive. Short latency discharges were, however, rarely evoked by olivary stimulation. 8. It is suggested that the short latency responses of the interpositus neurones were a result of synaptic excitation via cerebellar afferents, while the ensuing inhibition was a result of post-synaptic inhibition resulting from the Purkinje cell excitation due to the afferent volleys. It is suggested that the long latency excitation is due to the afferent volleys. It is suggested that the long latency excitation is due at least in part to disinhibition resulting from long pauses in Purkinje cell firing following their activation by climbing fibre afferents. 9. The possibility that these long latency responses have a physiological significance in relation to locomotion is discussed.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALANIS J., MATTHEWS B. H. C. The mechano-receptor properties of central neurones. J Physiol. 1952 Aug;117(4):59P–60P. [PubMed] [Google Scholar]
- Adrian E. D., Moruzzi G. Impulses in the pyramidal tract. J Physiol. 1939 Dec 14;97(2):153–199. doi: 10.1113/jphysiol.1939.sp003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. I., Azzena G. B., Ono T. Responses of neurones of interpositus nucleus to stimulation of the sensorimotor cortex. Brain Res. 1972 Oct 27;45(2):585–589. doi: 10.1016/0006-8993(72)90487-8. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Cogdell B., Harvey R. J. Response of interpositus neurones to nerve stimulation in chloralose anesthetized cats. Brain Res. 1973 Jun 15;55(2):461–466. doi: 10.1016/0006-8993(73)90314-4. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Harvey R. J., Schild R. F. Spino-olivocerebellar pathways to the posterior lobe of the cat cerebellum. Exp Brain Res. 1973 Aug 31;18(1):1–18. doi: 10.1007/BF00236553. [DOI] [PubMed] [Google Scholar]
- Azzena G. B., Ohno T. Influence of spino-reticulo-cerebellar pathway on Purkyne cells of paramedian lobule. Exp Brain Res. 1973 Mar 29;17(1):63–74. doi: 10.1007/BF00234564. [DOI] [PubMed] [Google Scholar]
- Bell C. C., Grimm R. J. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969 Nov;32(6):1044–1055. doi: 10.1152/jn.1969.32.6.1044. [DOI] [PubMed] [Google Scholar]
- Bloedel J. R., Roberts W. J. Action of climbing fibers in cerebellar cortex of the cat. J Neurophysiol. 1971 Jan;34(1):17–31. doi: 10.1152/jn.1971.34.1.17. [DOI] [PubMed] [Google Scholar]
- Bloedel J. R., Roberts W. J. Functional relationship among neurons of the cerebellar cortex in the absence of anesthesia. J Neurophysiol. 1969 Jan;32(1):75–84. doi: 10.1152/jn.1969.32.1.75. [DOI] [PubMed] [Google Scholar]
- Courtney K. R., Fetz E. E. Unit responses recorded from cervical spinal cord of awake monkey. Brain Res. 1973 Apr 27;53(2):445–450. doi: 10.1016/0006-8993(73)90231-x. [DOI] [PubMed] [Google Scholar]
- Courville J., Diakiw N., Brodal A. Cerebellar corticonuclear projection in the cat. The paramedian lobule. An experimental study with silver methods. Brain Res. 1973 Feb 14;50(1):25–45. doi: 10.1016/0006-8993(73)90592-1. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Provini L., Strata P., Táboríková H. Analysis of electrical potentials evoked in the cerebellar anterior lobe by stimulation of hindlimb and forelimb nerves. Exp Brain Res. 1968;6(3):171–194. doi: 10.1007/BF00235123. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rantucci T., Sabah N. H., Táboríková H. Somatotopic studies on cerebellar fastigial cells. Exp Brain Res. 1974 Jan 22;19(1):100–118. doi: 10.1007/BF00233397. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Rosen I., Scheid P., Taborikova H. Cutaneous afferent responses in interpositus neurones of the cat. Brain Res. 1972 Jul 13;42(1):207–211. doi: 10.1016/0006-8993(72)90055-8. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Schmidt R. F., Táboríková H. Integration by Purkyne cells of mossy and climbing fiber inputs from cutaneous mechanoreceptors. Exp Brain Res. 1972 Oct 29;15(5):498–520. doi: 10.1007/BF00236405. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Sabah N. H., Táboríková H. Excitatory and inhibitory responses of neurones of the cerebellar fastigial nucleus. Exp Brain Res. 1974 Jan 22;19(1):61–77. doi: 10.1007/BF00233395. [DOI] [PubMed] [Google Scholar]
- Engberg I., Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969 Apr;75(4):614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- FLOOD S., JANSEN J. On the cerebellar nuclei in the cat. Acta Anat (Basel) 1961;46:52–72. doi: 10.1159/000141769. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M., Rubia F. J., Strata P. The effect of pentothal on the activity evoked in the cerebellar cortex. Exp Brain Res. 1973 Mar 29;17(1):50–62. doi: 10.1007/BF00234563. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- KRUGER L., ALBE-FESSARD D. Distribution of responses to somatic afferent stimuli in the diencephalon of the cat under chloralose anesthesia. Exp Neurol. 1960 Oct;2:442–467. doi: 10.1016/0014-4886(60)90027-3. [DOI] [PubMed] [Google Scholar]
- MASSION J., ALBE-FESSARD D. DUALIT'E DES VOIES SENSORIELLES AFF'ERENTES CONTR OLANT L'ACTIVIT'E DU NOYAU ROUGE. Electroencephalogr Clin Neurophysiol. 1963 Jun;15:435–454. doi: 10.1016/0013-4694(63)90065-8. [DOI] [PubMed] [Google Scholar]
- Massion J. The mammalian red nucleus. Physiol Rev. 1967 Jul;47(3):383–436. doi: 10.1152/physrev.1967.47.3.383. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Ikeda M. Olivary projections to the cerebellar nuclei in the cat. Exp Brain Res. 1970 Jun 25;10(5):488–500. doi: 10.1007/BF00234265. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Ikeda M. Spinal projections to the cerebellar nuclei in the cat. Exp Brain Res. 1970 Jun 25;10(5):501–511. doi: 10.1007/BF00234266. [DOI] [PubMed] [Google Scholar]
- McCance I. The role of acetylcholine in the intracerebellar nuclei of the cat. Brain Res. 1972 Dec 24;48:265–279. doi: 10.1016/0006-8993(72)90183-7. [DOI] [PubMed] [Google Scholar]
- Mortimer J. A. Temporal sequence of cerebellar Purkinje and nuclear activity in relation to the acoustic startle response. Brain Res. 1973 Feb 28;50(2):457–462. doi: 10.1016/0006-8993(73)90751-8. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Sabah N. H. Cerebellar Purkinje cell responses to afferent inputs. I. Climbing fiber activation. Brain Res. 1971 Feb 5;25(3):449–467. doi: 10.1016/0006-8993(71)90454-9. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Sabah N. H. Spontaneous firing of cerebellar Purkinje cells in decerebrate and barbiturate anesthetized cats. Brain Res. 1970 Feb 3;17(3):515–519. doi: 10.1016/0006-8993(70)90259-3. [DOI] [PubMed] [Google Scholar]
- Murphy M. G., O'Leary J. L., Cornblath D. Axoplasmic flow in cerebellar mossy and climbing fibers. Arch Neurol. 1973 Feb;28(2):118–123. doi: 10.1001/archneur.1973.00490200066009. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of rubrospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Rosén I., Scheid P. Cerebellar surface cooling influencing evoked activity in cortex and in interpositus nucleus. Brain Res. 1972 Oct 27;45(2):580–584. doi: 10.1016/0006-8993(72)90486-6. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Strata P. Responses evoked in the cerebellar cortex by stimulating mossy fibre pathways to the cerebellum. Exp Brain Res. 1967;3(2):95–110. doi: 10.1007/BF00233255. [DOI] [PubMed] [Google Scholar]
- Stenhouse D., Eccles R. M. Activity in descending tract fibres in cats anaesthetized with chloralose. Brain Res. 1971 Dec 10;35(1):127–130. doi: 10.1016/0006-8993(71)90599-3. [DOI] [PubMed] [Google Scholar]
- Talbott R. E., Towe A. L., Kennedy T. T. Physiological and histological classification of cerebellar neurons in chloralose-anesthetized cats. Exp Neurol. 1967 Sep;19(1):46–64. doi: 10.1016/0014-4886(67)90006-4. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970 Jul;33(4):527–536. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Toyama K., Kosaka K. Electrical activity of red nucleus neurones investigated with intracellular microelectrodes. Exp Brain Res. 1967;4(1):18–33. doi: 10.1007/BF00235214. [DOI] [PubMed] [Google Scholar]
- Yu J., Tarnecki R., Chambers W. W., Liu C. N., Konorski J. Mechanisms mediating ipsilateral limb hyperflexion after cerebellar paravermal cortical ablation or cooling. Exp Neurol. 1973 Jan;38(1):144–156. doi: 10.1016/0014-4886(73)90015-0. [DOI] [PubMed] [Google Scholar]


