Abstract
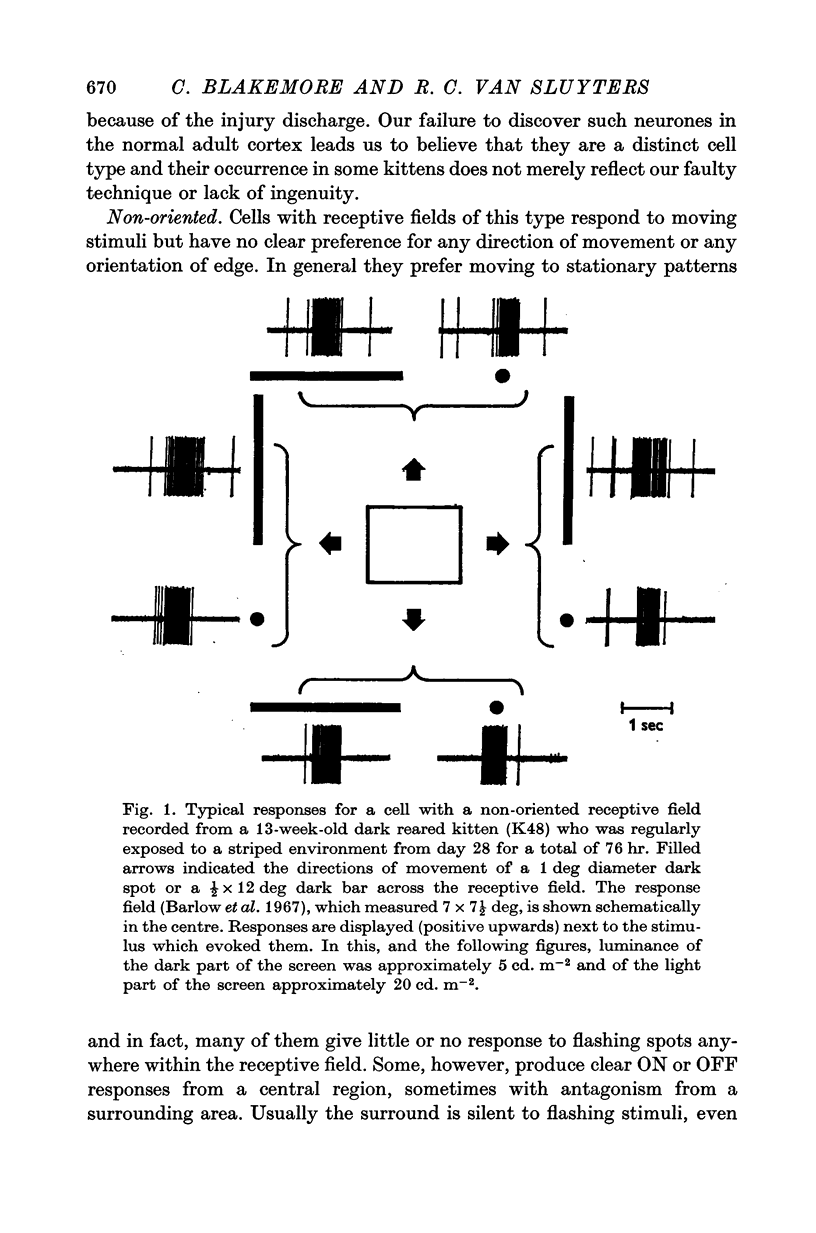
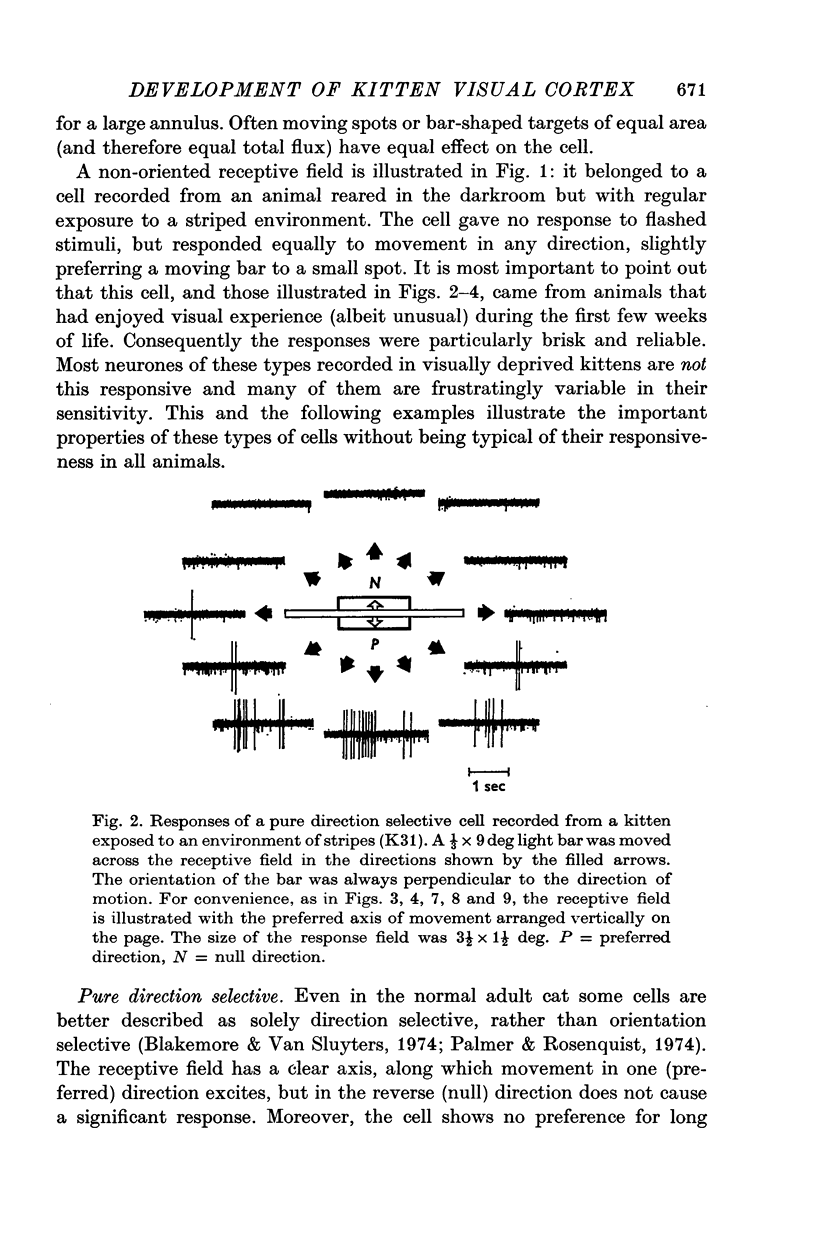
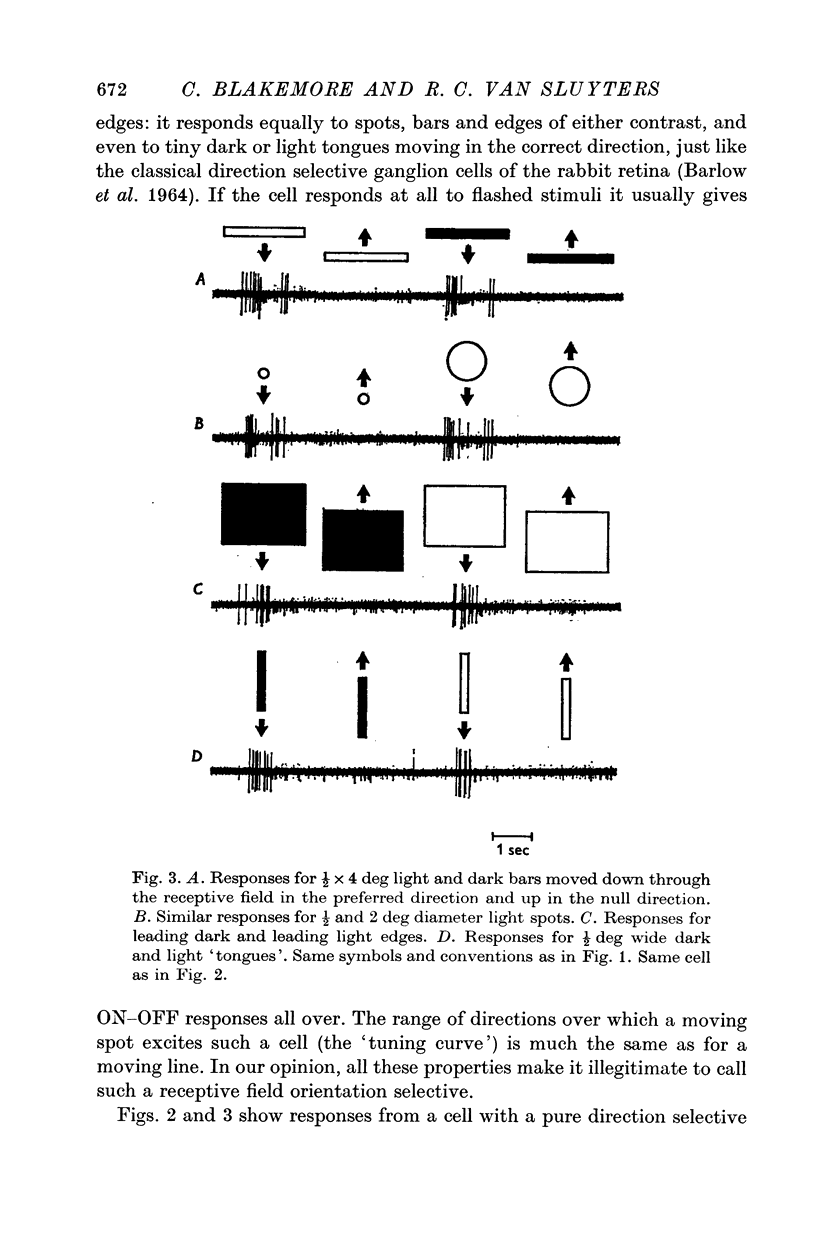
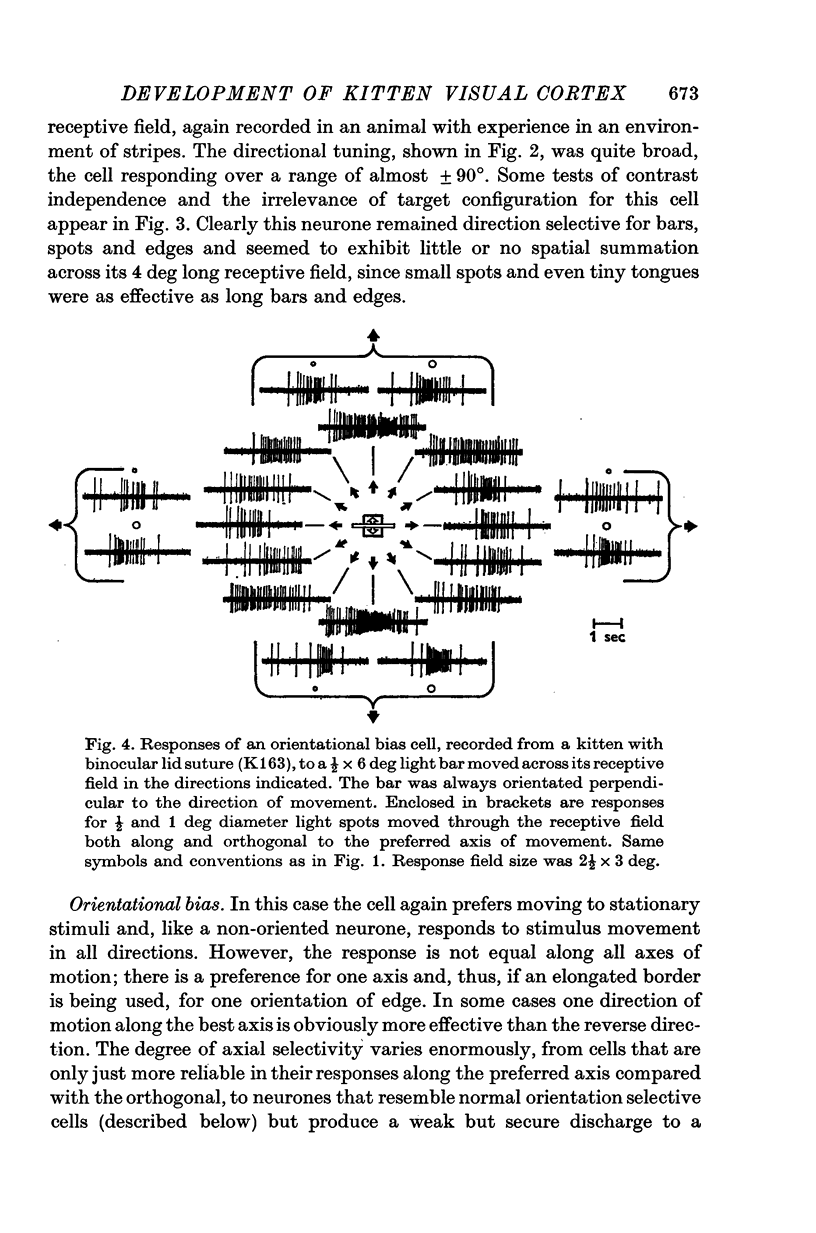
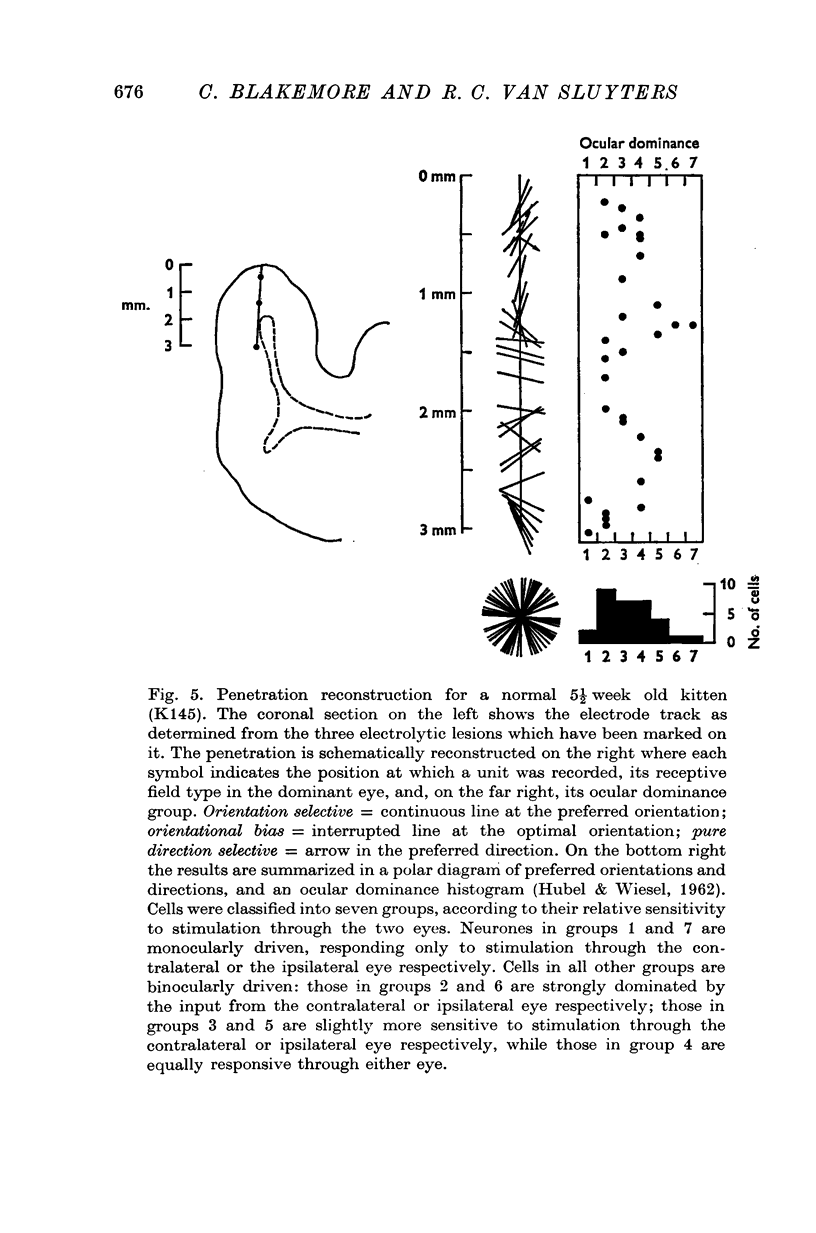
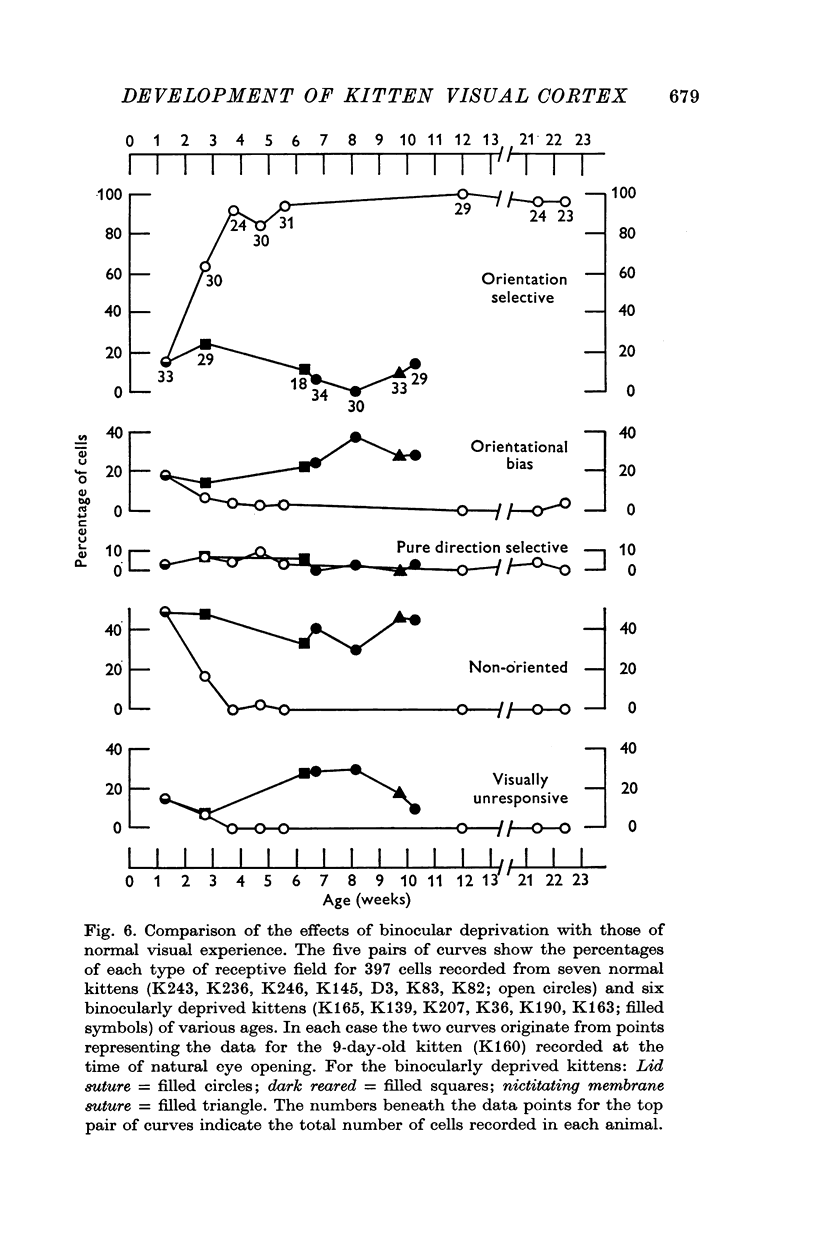
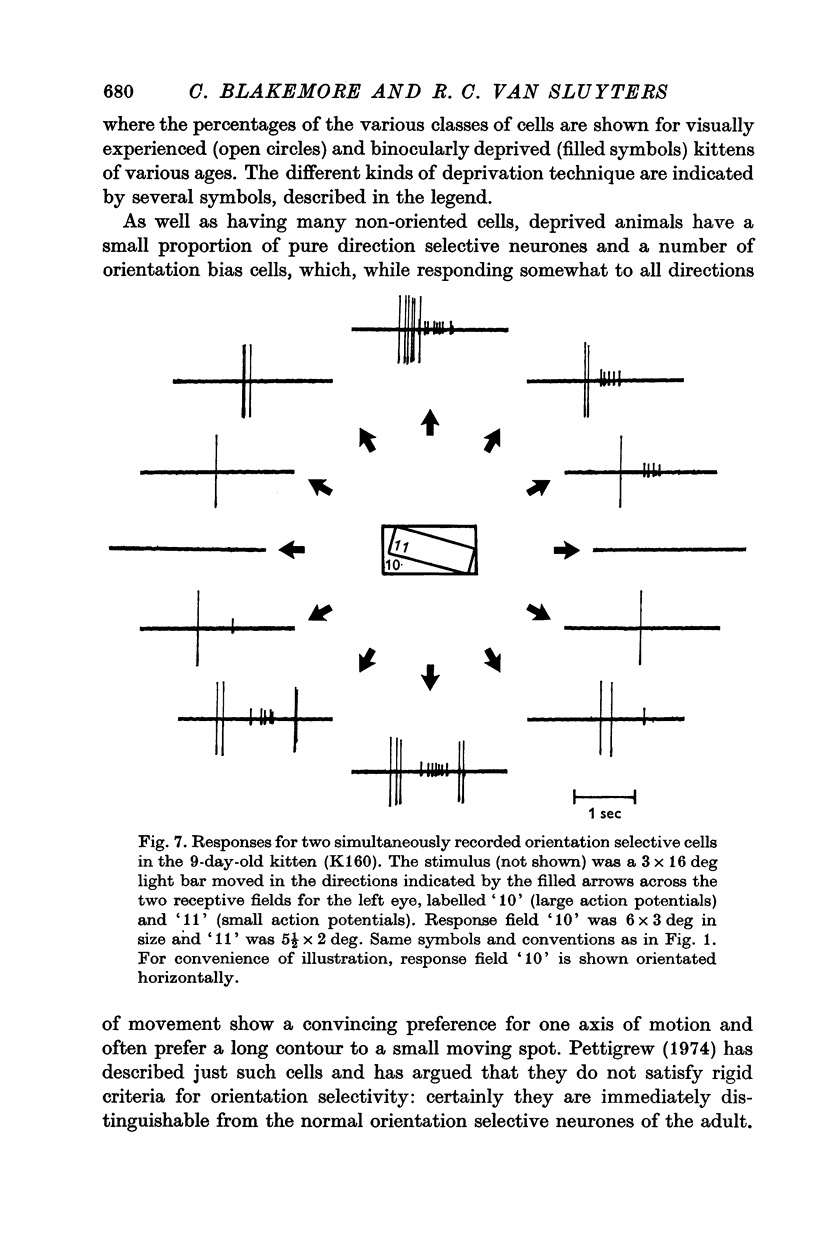
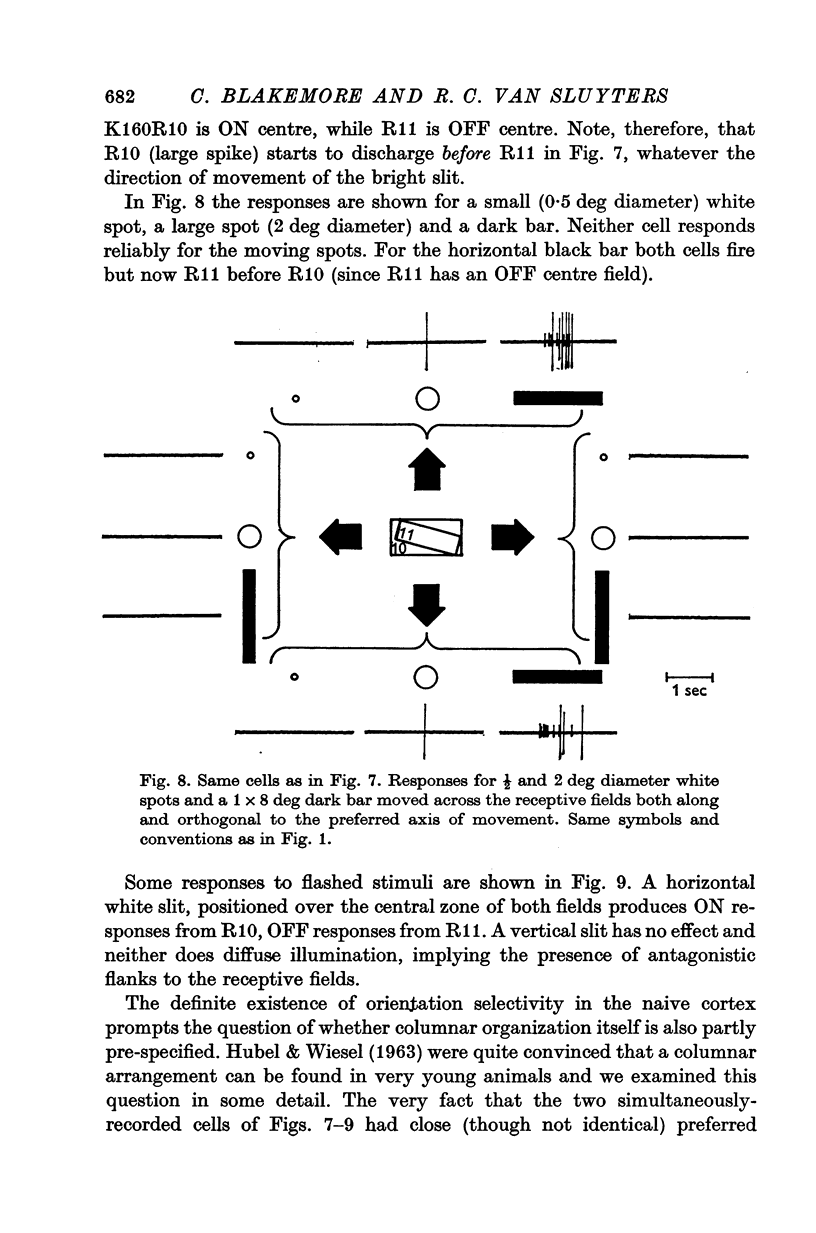
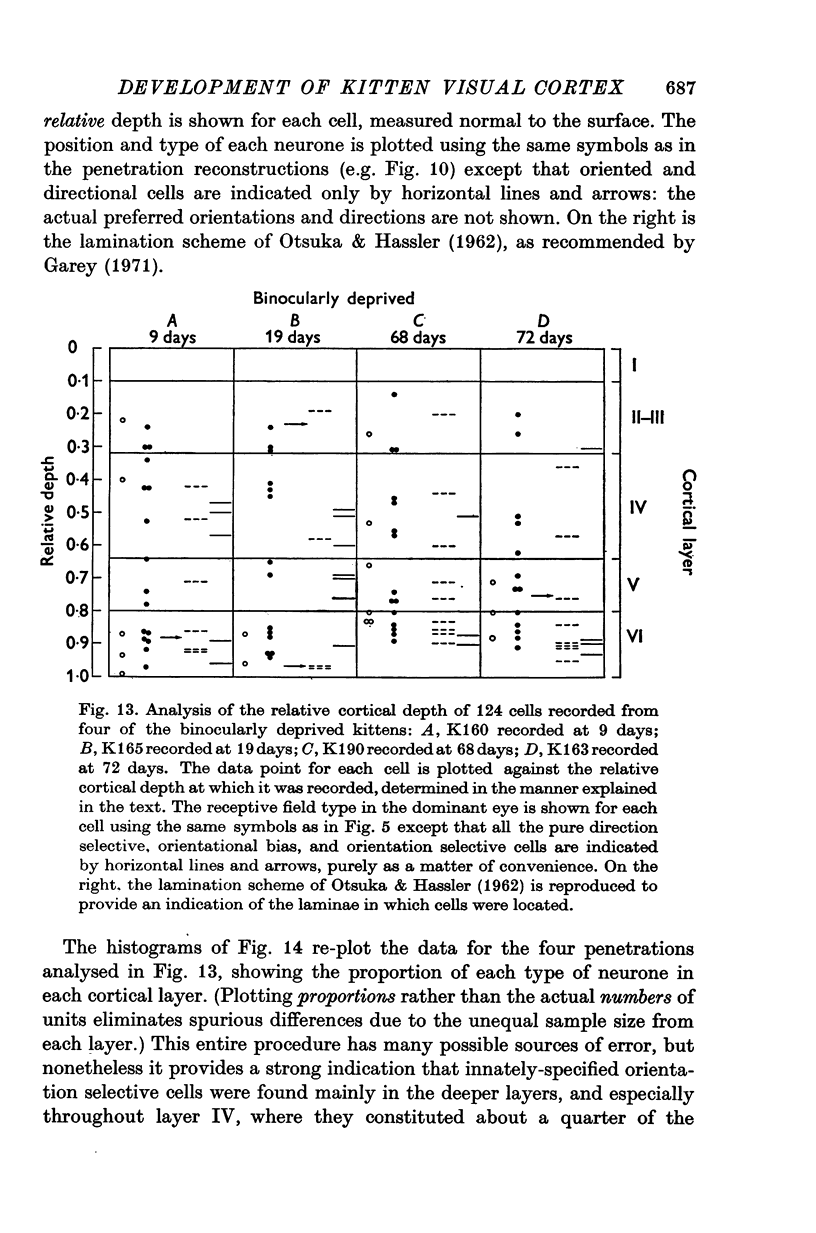
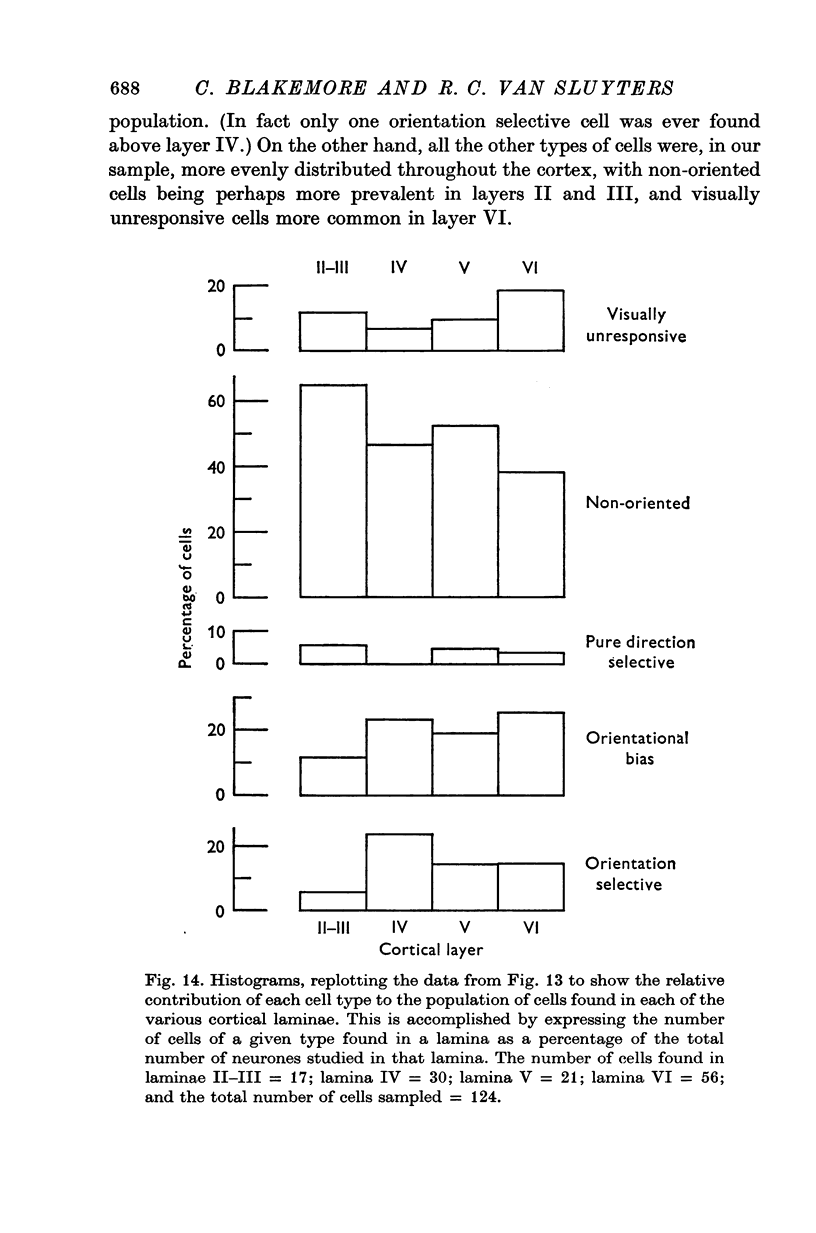
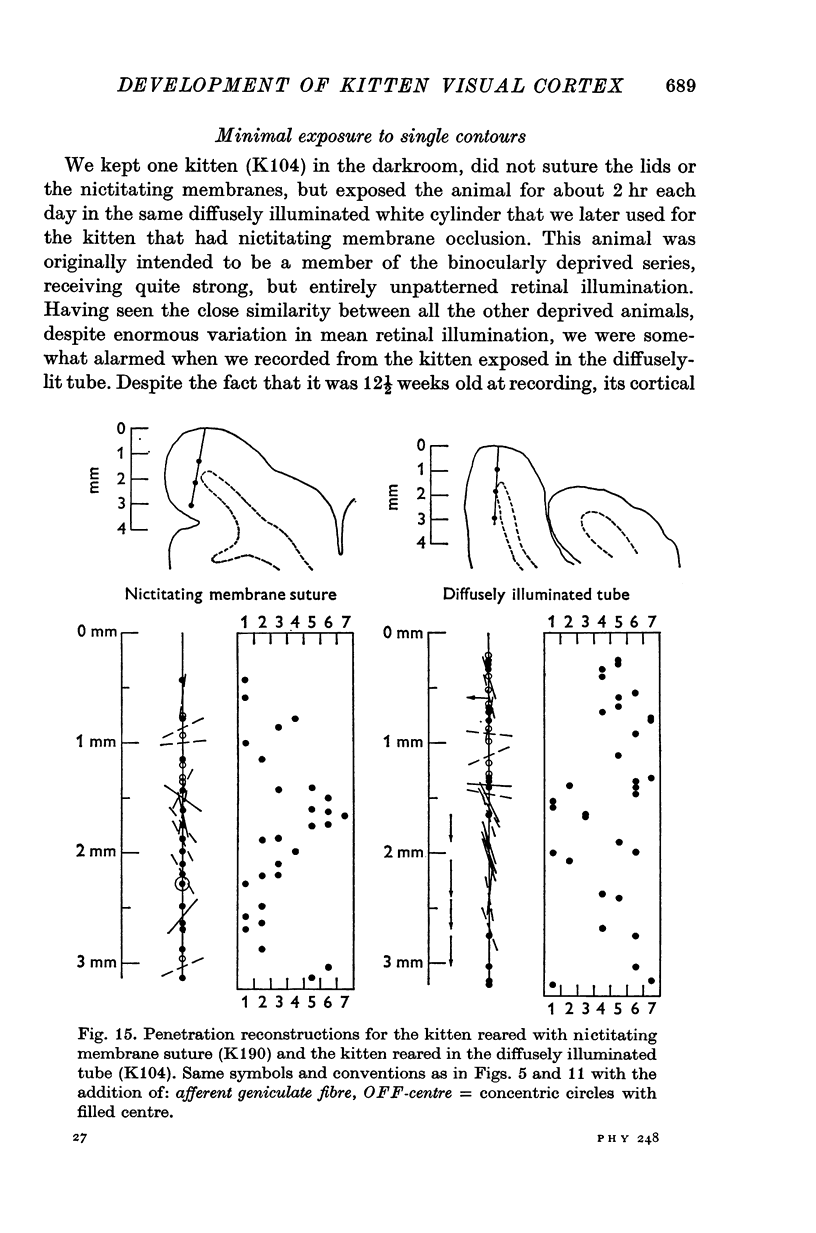
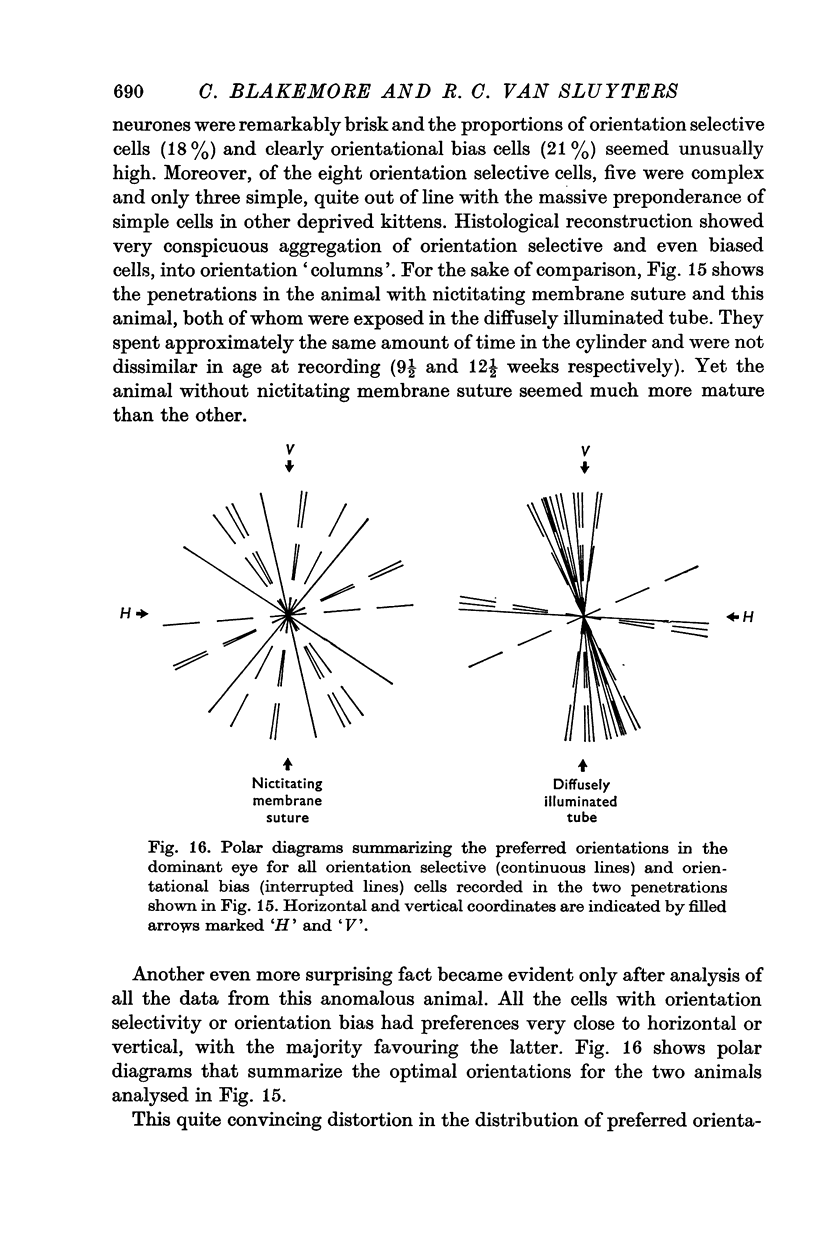
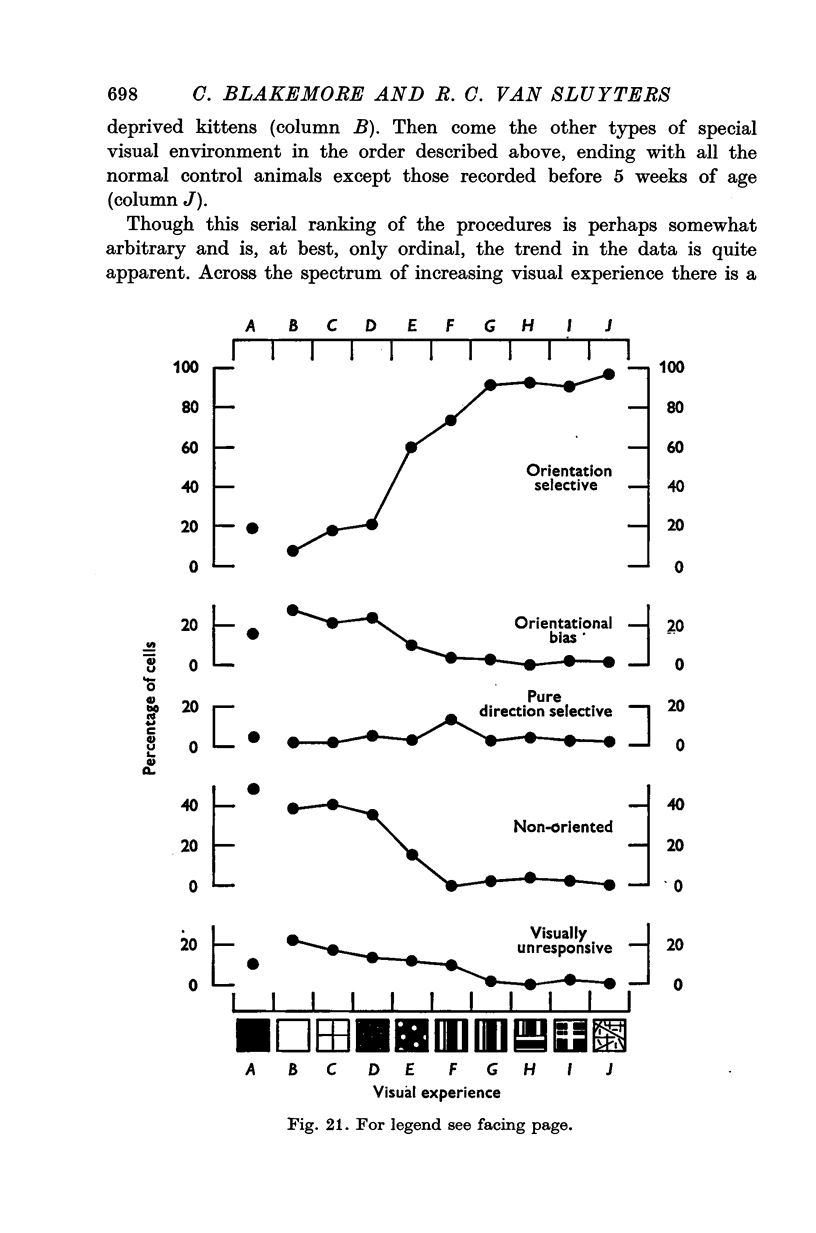
1. This is a study of the receptive fields of 771 cells recorded in the visual cortex of twenty-five kittens reared normally or subjected to various kinds of visual deprivation or environmental manipulation. 2. Kittens deprived of patterned visual experience, by dark rearing or diffuse occlusion of the eyes, have a majority of cirtical neurones with little or no specificity for the orientation or axis of movement of visual stimuli. However, in such deprived animals, especially those younger than 3 weeks, there are a number of genuinely orientation selective cells. They are broadly "turned" (by adult standards), they are almost always of the simple type, are heavily dominated by one eye, and are found mainly in the deeper layers of the cortex, especially layer IV. 3...
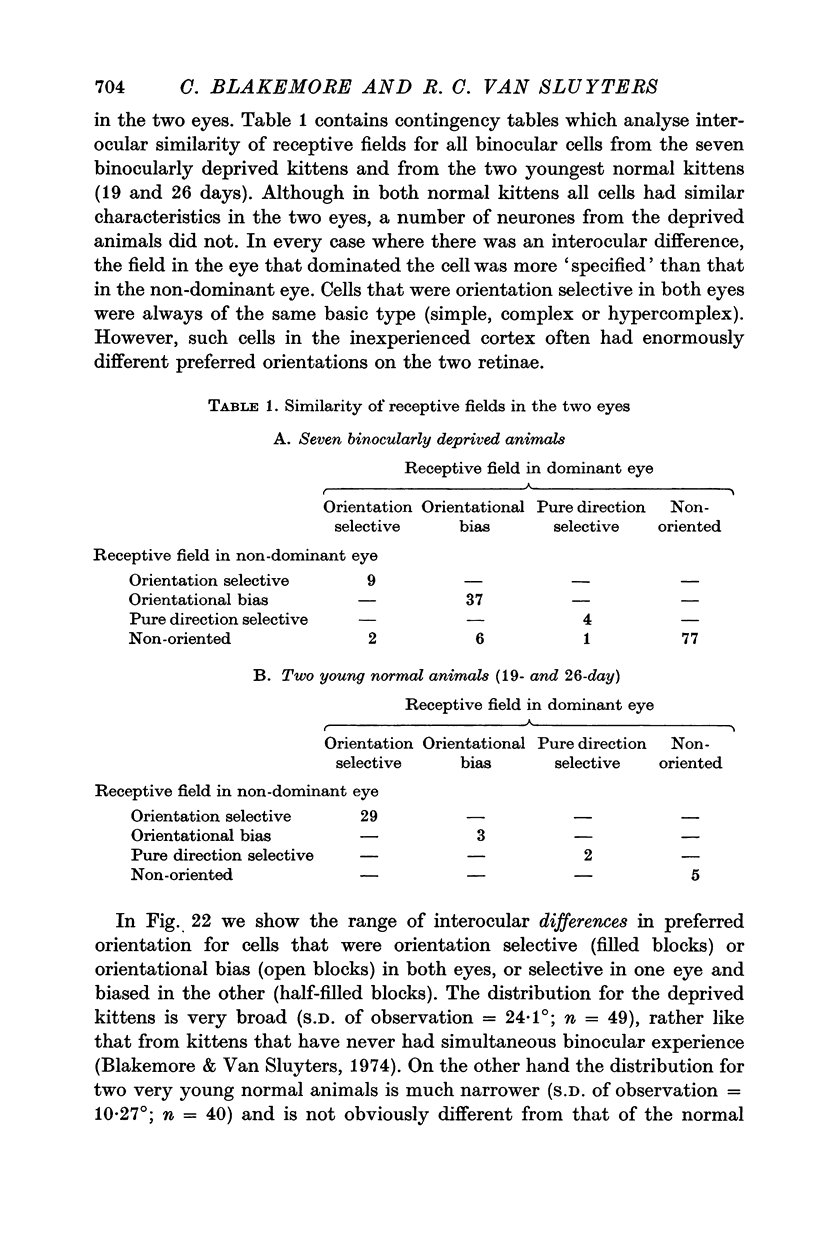
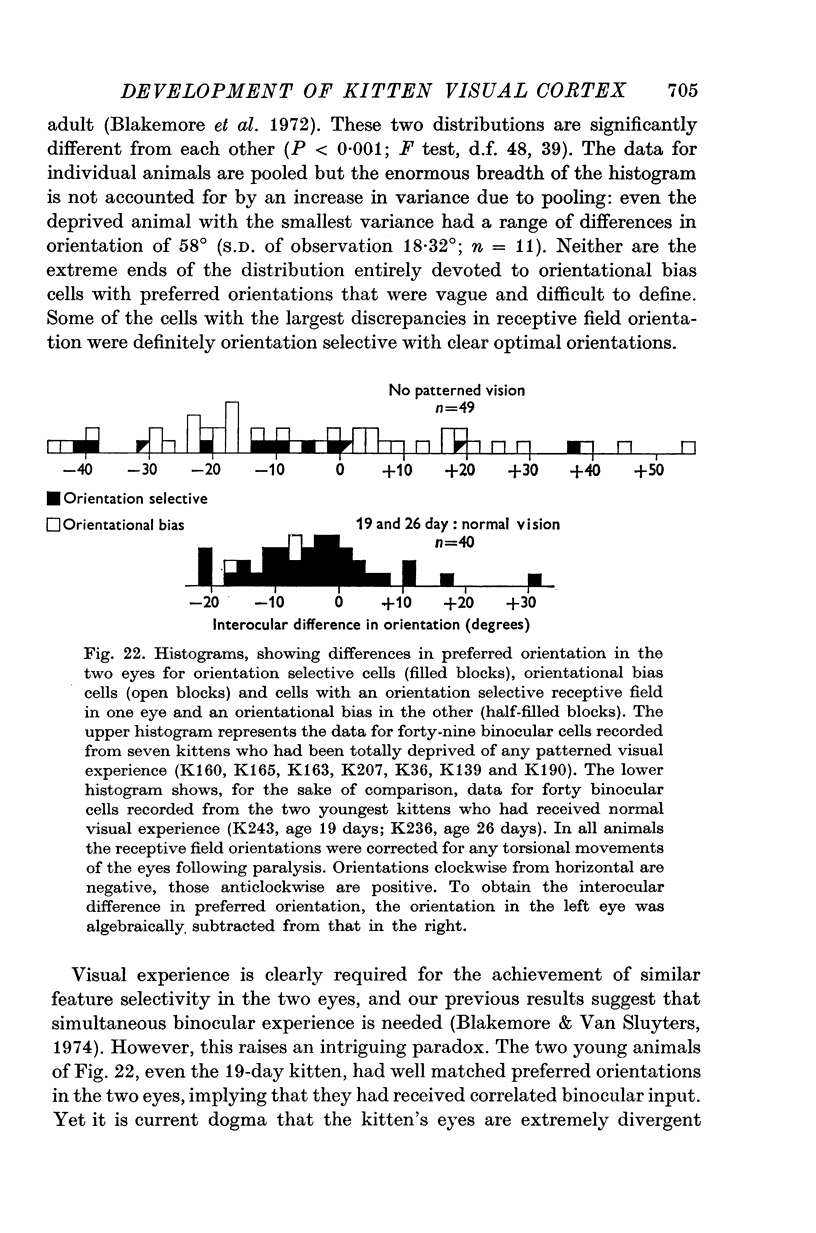
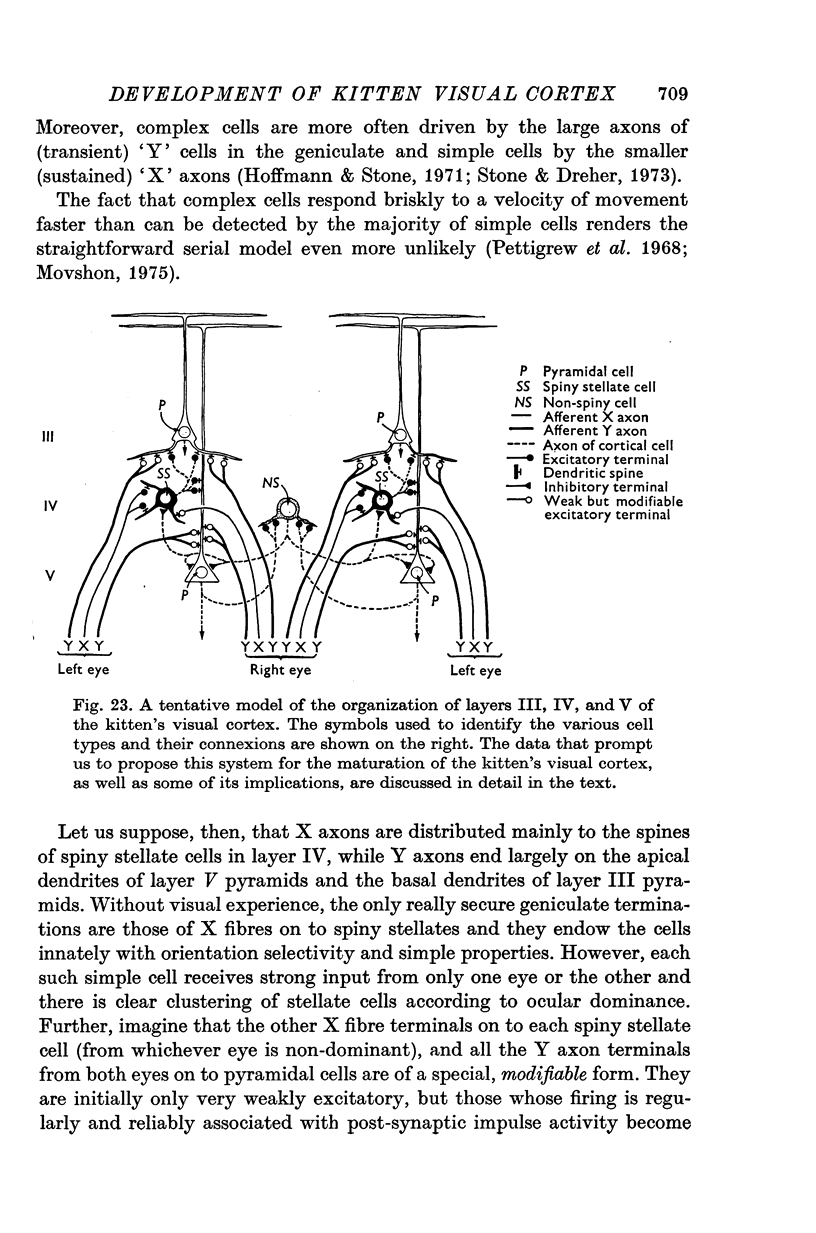
Full text
PDF





























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker R. L., Cragg B. G. Development of the extrinsic connections of the visual cortex in the cat. J Comp Neurol. 1974 Mar 1;154(1):29–41. doi: 10.1002/cne.901540103. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Pettigrew J. D. Lack of specificity of neurones in the visual cortex of young kittens. J Physiol. 1971 Oct;218 (Suppl):98P–100P. [PubMed] [Google Scholar]
- Barlow H. B. Single units and sensation: a neuron doctrine for perceptual psychology? Perception. 1972;1(4):371–394. doi: 10.1068/p010371. [DOI] [PubMed] [Google Scholar]
- Benevento L. A., Creutzfeldt O. D., Kuhnt U. Significance of intracortical inhibition in the visual cortex. Nat New Biol. 1972 Jul 26;238(82):124–126. doi: 10.1038/newbio238124a0. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Cooper G. F. Development of the brain depends on the visual environment. Nature. 1970 Oct 31;228(5270):477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Fiorentini A., Maffei L. A second neural mechanism of binocular depth discrimination. J Physiol. 1972 Nov;226(3):725–749. doi: 10.1113/jphysiol.1972.sp010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Mitchell D. E. Environmental modification of the visual cortex and the neural basis of learning and memory. Nature. 1973 Feb 16;241(5390):467–468. doi: 10.1038/241467a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Tobin E. A. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15(4):439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Responses of neurones in the striate cortex observed in normal and dark-reared kittens during post-natal life. J Physiol. 1975 Mar;246(2):98P–99P. [PubMed] [Google Scholar]
- Chow K. L., Spear P. D. Morphological and functional effects of visual deprivation on the rabbit visual system. Exp Neurol. 1974 Feb;42(2):429–447. doi: 10.1016/0014-4886(74)90036-3. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968 Jul;9(2):268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in cat visual cortex. Invest Ophthalmol. 1972 May;11(5):377–385. [PubMed] [Google Scholar]
- Creutzfeldt O., Ito M. Functional synaptic organization of primary visual cortex neurones in the cat. Exp Brain Res. 1968;6(4):324–352. doi: 10.1007/BF00233183. [DOI] [PubMed] [Google Scholar]
- Cynader M., Berman N., Hein A. Cats reared in stroboscopic illumination: effects on receptive fields in visual cortex. Proc Natl Acad Sci U S A. 1973 May;70(5):1353–1354. doi: 10.1073/pnas.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Wyatt H. J. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. J Physiol. 1974 Jul;240(2):309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B. Hypercomplex cells in the cat's striate cortex. Invest Ophthalmol. 1972 May;11(5):355–356. [PubMed] [Google Scholar]
- Fisken R. A., Garey L. J., Powell T. P. Patterns of degeneration after intrinsic lesions of the visual cortex (area 17) of the monkey. Brain Res. 1973 Apr 13;53(1):208–213. doi: 10.1016/0006-8993(73)90782-8. [DOI] [PubMed] [Google Scholar]
- Garey L. J. A light and electron microscopic study of the visual cortex of the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):21–40. doi: 10.1098/rspb.1971.0079. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science. 1970 May 15;168(3933):869–871. doi: 10.1126/science.168.3933.869. [DOI] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert M., Buisseret P. Receptive field characteristics and plastic properties of visual cortical cells in kittens reared with or without visual experience. Exp Brain Res. 1975;22(1):25–36. doi: 10.1007/BF00235409. [DOI] [PubMed] [Google Scholar]
- Joshua D. E., Bishop P. O. Binocular single vision and depth discrimination. Receptive field disparities for central and peripheral vision and binocular interaction on peripheral single units in cat striate cortex. Exp Brain Res. 1970;10(4):389–416. doi: 10.1007/BF02324766. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S. Synaptic patterns in the visual cortex of the cat and monkey. Electron microscopy of Golgi preparations. J Comp Neurol. 1973 Jul 1;150(1):53–85. doi: 10.1002/cne.901500104. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G. Selectivity of microelectrodes in recordings from cat retinal ganglion cells. J Neurophysiol. 1974 Nov;37(6):1387–1393. doi: 10.1152/jn.1974.37.6.1387. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers L. H., Chow K. L., Spear P. D., Grobstein P. Ontogenesis of receptive fields in the rabbit striate cortex. Exp Brain Res. 1974 Jan 22;19(1):20–35. doi: 10.1007/BF00233393. [DOI] [PubMed] [Google Scholar]
- Mize R. R., Murphy E. H. Selective visual experience fails to modify receptive field properties of rabbit striate cortex neurons. Science. 1973 Apr 20;180(4083):320–323. doi: 10.1126/science.180.4083.320. [DOI] [PubMed] [Google Scholar]
- Nikara T., Bishop P. O., Pettigrew J. D. Analysis of retinal correspondence by studying receptive fields of binocular single units in cat striate cortex. Exp Brain Res. 1968;6(4):353–372. doi: 10.1007/BF00233184. [DOI] [PubMed] [Google Scholar]
- Noda H., Creutzfeldt O. D., Freeman R. B., Jr Binocular interaction in the visual cortex of awake cats. Exp Brain Res. 1971 May 26;112(4):406–421. doi: 10.1007/BF00234495. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Pettigrew J. D. Single units in visual cortex of kittens reared in stroboscopic illumination. Brain Res. 1974 Apr 19;70(2):189–204. doi: 10.1016/0006-8993(74)90312-6. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Peck C. K., Blakemore C. Modification of single neurons in the kitten's visual cortex after brief periods of monocular visual experience. Exp Brain Res. 1975;22(1):57–68. doi: 10.1007/BF00235411. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Freeman R. D. Visual experience without lines: effect on developing cortical neurons. Science. 1973 Nov 9;182(4112):599–601. doi: 10.1126/science.182.4112.599. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J., Olson C., Barlow H. B. Kitten visual cortex: short-term, stimulus-induced changes in connectivity. Science. 1973 Jun 15;180(4091):1202–1203. doi: 10.1126/science.180.4091.1202. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. An analysis of orientation selectivity in the cat's visual cortex. Exp Brain Res. 1974 Apr 30;20(1):1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- Sherman S. M. Development of interocular alignment in cats. Brain Res. 1972 Feb 25;37(2):187–203. doi: 10.1016/0006-8993(72)90666-x. [DOI] [PubMed] [Google Scholar]
- Spinelli D. N., Hirsch H. V., Phelps R. W., Metzler J. Visual experience as a determinant of the response characteristics of cortical receptive fields in cats. Exp Brain Res. 1972;15(3):289–304. doi: 10.1007/BF00235913. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Stone J. Sampling properties of microelectrodes assessed in the cat's retina. J Neurophysiol. 1973 Nov;36(6):1071–1079. doi: 10.1152/jn.1973.36.6.1071. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K., Ono T., Tokashiki S. An intracellular study of neuronal organization in the visual cortex. Exp Brain Res. 1974;21(1):45–66. doi: 10.1007/BF00234257. [DOI] [PubMed] [Google Scholar]
- Tretter F., Cynader M., Singer W. Modification of direction selectivity of neurons in the visual cortex of kittens. Brain Res. 1975 Jan 24;84(1):143–149. doi: 10.1016/0006-8993(75)90808-2. [DOI] [PubMed] [Google Scholar]
- Van Sluyters R. C., Blakemore C. Experimental creation of unusual neuronal properties in visual cortex of kitten. Nature. 1973 Dec 21;246(5434):506–508. doi: 10.1038/246506a0. [DOI] [PubMed] [Google Scholar]
- Van Sluyters R. C., Stewart D. L. Binocular neurons of the rabbit's visual cortex: effects of monocular sensory deprivation. Exp Brain Res. 1974 Jan 31;19(2):196–204. doi: 10.1007/BF00238534. [DOI] [PubMed] [Google Scholar]
- Van Sluyters R. C., Stewart D. L. Binocular neurons of the rabbit's visual cortex: receptive field characteristics. Exp Brain Res. 1974 Jan 31;19(2):166–195. doi: 10.1007/BF00238533. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Konishi M., Creutzfeldt O. D. Postsynaptic potentials in the cat's visual cortex following electrical stimulation of afferent pathways. Exp Brain Res. 1966;1(3):272–283. doi: 10.1007/BF00234347. [DOI] [PubMed] [Google Scholar]
- Watkins D. W., Berkley M. A. The orientation selectivity of single neurons in cat striate cortex. Exp Brain Res. 1974 Feb 28;19(4):433–446. doi: 10.1007/BF00234465. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H., Lam D. M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974 Oct 18;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]


