Abstract
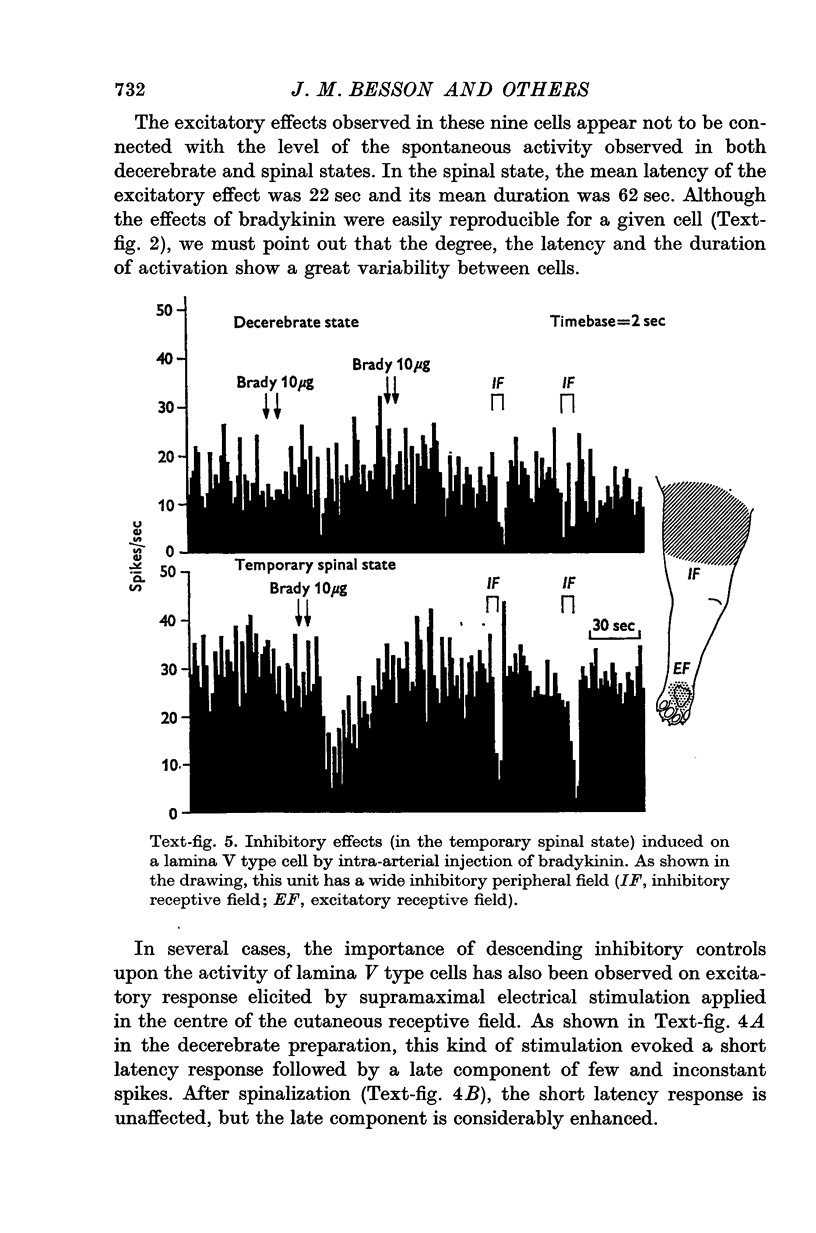
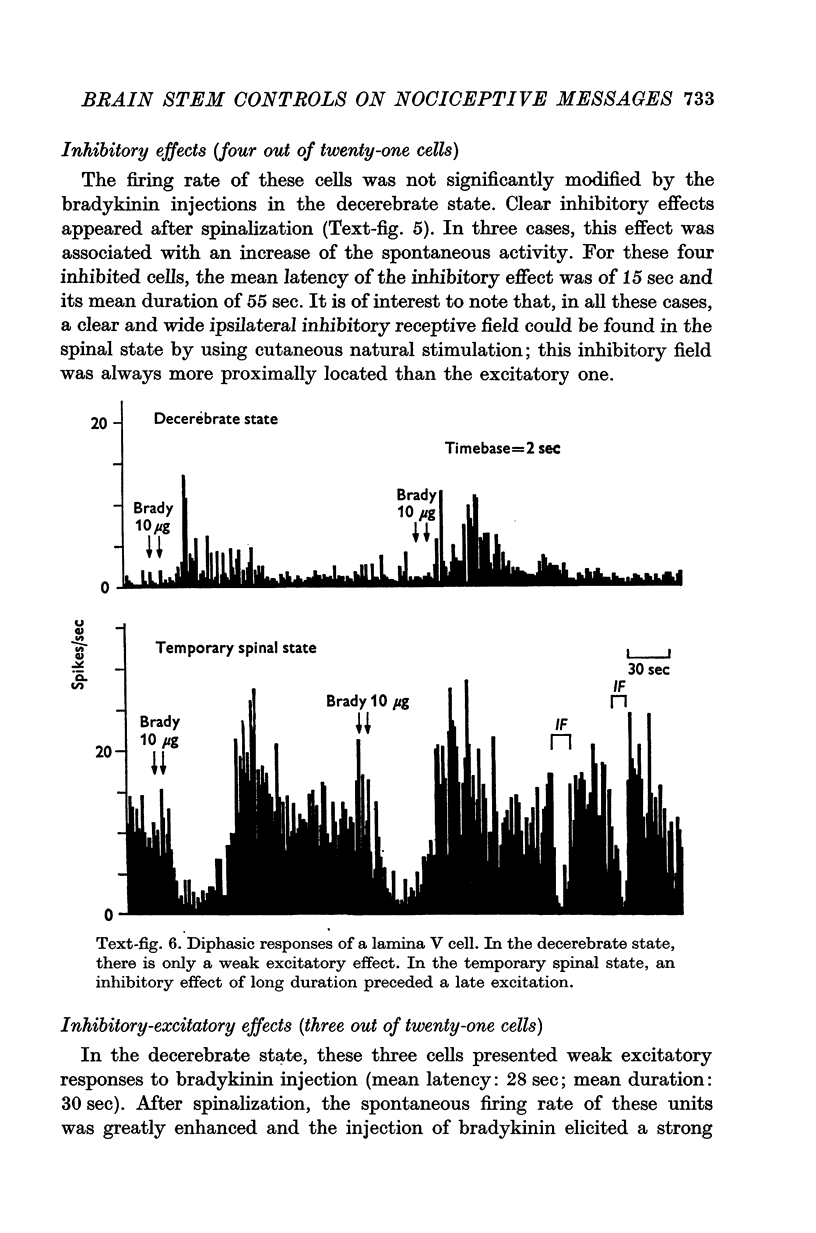
1. In order to study descending influences of the brain stem upon the transmission of nociceptive messages at the spinal level, the activities of lumbar lamina V dorsal horn cells, induced by intra-arterial injection of brandykinin into the limbs, were recorded in unanaesthetized cats in both decerebrate and temporary spinal states (reversible cold block applied at the thoracic level). 2. In the decerebrate state, the intra-arterial injection of bradykinin had little or no effect. 3. During the reversible spinalization, the effects of bradykinin were revealed or considerably enhanced. As described in a previous study, in the C1-transected cat, three types of effects were encountered: excitatory, inhibiitory and mixed (inhibitory-excitatory). 4. These modifications observed after spinalization were generally associated with a large increase of the spontaneous firing rate. 5. These results emphasize, in the decerebrate cat, the importance of descending inhibitory controls exerted by the brain stem upon the transmission of nonciceptive messages at the spinal cord level.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D., DRY R. M. L., KEELE C. A., MARKHAM J. W. Observations on chemical excitants of cutaneous pain in man. J Physiol. 1953 May 28;120(3):326–351. doi: 10.1113/jphysiol.1953.sp004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albe-Fessard D., Levante A., Lamour Y. Origin of spino-thalamic tract in monkeys. Brain Res. 1974 Jan 18;65(3):503–509. doi: 10.1016/0006-8993(74)90238-8. [DOI] [PubMed] [Google Scholar]
- BURCH G. E., DEPASQUALE N. P. Bradykinin, digital blood flow, and the arteriovenous anastomoses. Circ Res. 1962 Jan;10:105–115. doi: 10.1161/01.res.10.1.105. [DOI] [PubMed] [Google Scholar]
- Bars D. L., Menetrey D., Conseiller C., Besson J. M. Comparaison chez le chat spinal et le chat décérébré, des effets de la morphine sur les activités des interneurones de type V de la corne dorsale de la moelle. C R Acad Sci Hebd Seances Acad Sci D. 1974 Oct 14;279(16):1369–1371. [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974 Mar 11;347(3):209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Besson J. M., Conseiller C., Hamann K. F., Maillard M. C. Modifications of dorsal horn cell activities in the spinal cord, after intra-arterial injection of bradykinin. J Physiol. 1972 Feb;221(1):189–205. doi: 10.1113/jphysiol.1972.sp009748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Coulter J. D., Willis W. D. Cells of origin of the spinocervical tract in the monkey. Exp Neurol. 1974 Mar;42(3):574–586. doi: 10.1016/0014-4886(74)90080-6. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Trevino D. L., Coulter J. D., Willis W. D. Location and somatotopic organization of the cells of origin of the spino-cervical tract. Exp Brain Res. 1973 Apr 30;17(2):177–189. doi: 10.1007/BF00235027. [DOI] [PubMed] [Google Scholar]
- CHAPMAN L. F., RAMOS A. O., GOODELL H., WOLFF H. G. Evidence for kinin formation resulting from neural activity evoked by noxious stimulation. Ann N Y Acad Sci. 1963 Feb 4;104:258–274. doi: 10.1111/j.1749-6632.1963.tb17669.x. [DOI] [PubMed] [Google Scholar]
- Dilly P. N., Wall P. D., Webster K. E. Cells of origin of the spinothalamic tract in the cat and rat. Exp Neurol. 1968 Aug;21(4):550–562. doi: 10.1016/0014-4886(68)90072-1. [DOI] [PubMed] [Google Scholar]
- FJALLBRANT N., IGGO A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961 May;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E. Pyramidal tract effects on interneurons in the cat lumbar dorsal horn. J Neurophysiol. 1968 Jan;31(1):69–80. doi: 10.1152/jn.1968.31.1.69. [DOI] [PubMed] [Google Scholar]
- GUZMAN F., BRAUN C., LIM R. K. Visceral pain and the pseudaffective response to intra-arterial injection of bradykinin and other algesic agents. Arch Int Pharmacodyn Ther. 1962 Apr 1;136:353–384. [PubMed] [Google Scholar]
- Godfraind J. M. Localisation de l'extrémité de microélectrodes de verre dans le système nerveux central par électrophorèse de pontamine. J Physiol (Paris) 1969;61 (Suppl 2):436–437. [PubMed] [Google Scholar]
- Guilbaud G., Besson J. M., Oliveras J. L., Wyon-Maillard M. C. Modifications of the firing rate of bulbar reticular units (nucleus gigantocellularis) after intra-arterial injection of bradykinin into the limbs. Brain Res. 1973 Dec 7;63:131–140. doi: 10.1016/0006-8993(73)90082-6. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. K., Miller D. G., Guzman F., Rodgers D. W., Rogers R. W., Wang S. K., Chao P. Y., Shih T. Y. Pain and analgesia evaluated by the intraperitoneal bradykinin-evoked pain method in man. Clin Pharmacol Ther. 1967 Jul-Aug;8(4):521–542. doi: 10.1002/cpt196784521. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Liebeskind J. C. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res. 1974 Mar 15;68(1):73–93. doi: 10.1016/0006-8993(74)90534-4. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Wolfle T. L., Akil H., Carder B., Liebeskind J. C. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971 Dec 24;174(4016):1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Melzack R., Wall P. D. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mense S., Schmidt R. F. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974 Jun 7;72(2):305–310. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- Oliveras J. L., Besson J. M., Guilbaud G., Liebeskind J. C. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp Brain Res. 1974 Apr 30;20(1):32–44. doi: 10.1007/BF00239016. [DOI] [PubMed] [Google Scholar]
- Pomeranz B., Wall P. D., Weber W. V. Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol. 1968 Dec;199(3):511–532. doi: 10.1113/jphysiol.1968.sp008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. D., Wagman I. H. Physiological roles of A and C fiber inputs to the spinal dorsal horn of Macaca mulatta. Exp Neurol. 1970 Dec;29(3):383–399. doi: 10.1016/0014-4886(70)90066-x. [DOI] [PubMed] [Google Scholar]
- Reynolds D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969 Apr 25;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Sato M., Takagi H. Enhancement by morphine of the central descending inhibitory influence on spinal sensory transmission. Eur J Pharmacol. 1971;14(1):60–65. doi: 10.1016/0014-2999(71)90122-1. [DOI] [PubMed] [Google Scholar]
- Selzer M., Spencer W. A. Convergence of visceral and cutaneous afferent pathways in the lumbar spinal cord. Brain Res. 1969 Jul;14(2):331–348. doi: 10.1016/0006-8993(69)90114-0. [DOI] [PubMed] [Google Scholar]
- TAKAGI H., MATSURMURA M., YANAI A., OGIU K. The effect of analgesics on the spinal reflex activity of the cat. Jpn J Pharmacol. 1955 Mar;4(2):176–187. doi: 10.1254/jjp.4.176. [DOI] [PubMed] [Google Scholar]
- TAUB A., BISHOP P. O. THE SPINOCERVICAL TRACT: DORSAL COLUMN LINKAGE, CONDUCTION VELOCITY, PRIMARY AFFERENT SPECTRUM. Exp Neurol. 1965 Sep;13:1–21. doi: 10.1016/0014-4886(65)90002-6. [DOI] [PubMed] [Google Scholar]
- TAUB A. LOCAL, SEGMENTAL AND SUPRASPINAL INTERACTION WITH A DORSOLATERAL SPINAL CUTANEOUS AFFERENT SYSTEM. Exp Neurol. 1964 Oct;10:357–374. doi: 10.1016/0014-4886(64)90006-8. [DOI] [PubMed] [Google Scholar]
- Trevino D. L., Coulter J. D., Willis W. D. Location of cells of origin of spinothalamic tract in lumbar enlargement of the monkey. J Neurophysiol. 1973 Jul;36(4):750–761. doi: 10.1152/jn.1973.36.4.750. [DOI] [PubMed] [Google Scholar]
- Vogt M. The effect of lowering the 5-hydroxytryptamine content of the rat spinal cord on analgesia produced by morphine. J Physiol. 1974 Jan;236(2):483–498. doi: 10.1113/jphysiol.1974.sp010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagman I. H., Price D. D. Responses of dorsal horn cells of M. mulatta to cutaneous and sural nerve A and C fiber stimuli. J Neurophysiol. 1969 Nov;32(6):803–817. doi: 10.1152/jn.1969.32.6.803. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis W. D., Trevino D. L., Coulter J. D., Maunz R. A. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol. 1974 Mar;37(2):358–372. doi: 10.1152/jn.1974.37.2.358. [DOI] [PubMed] [Google Scholar]