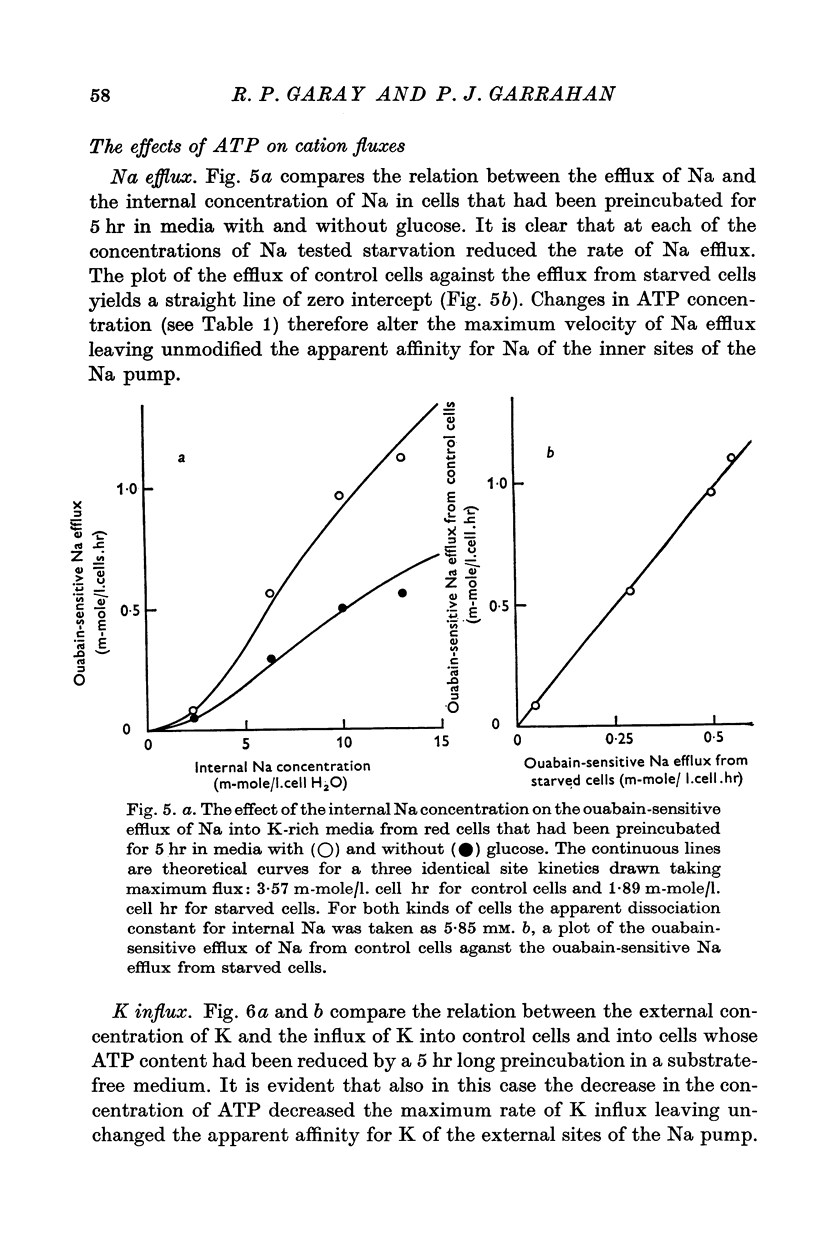
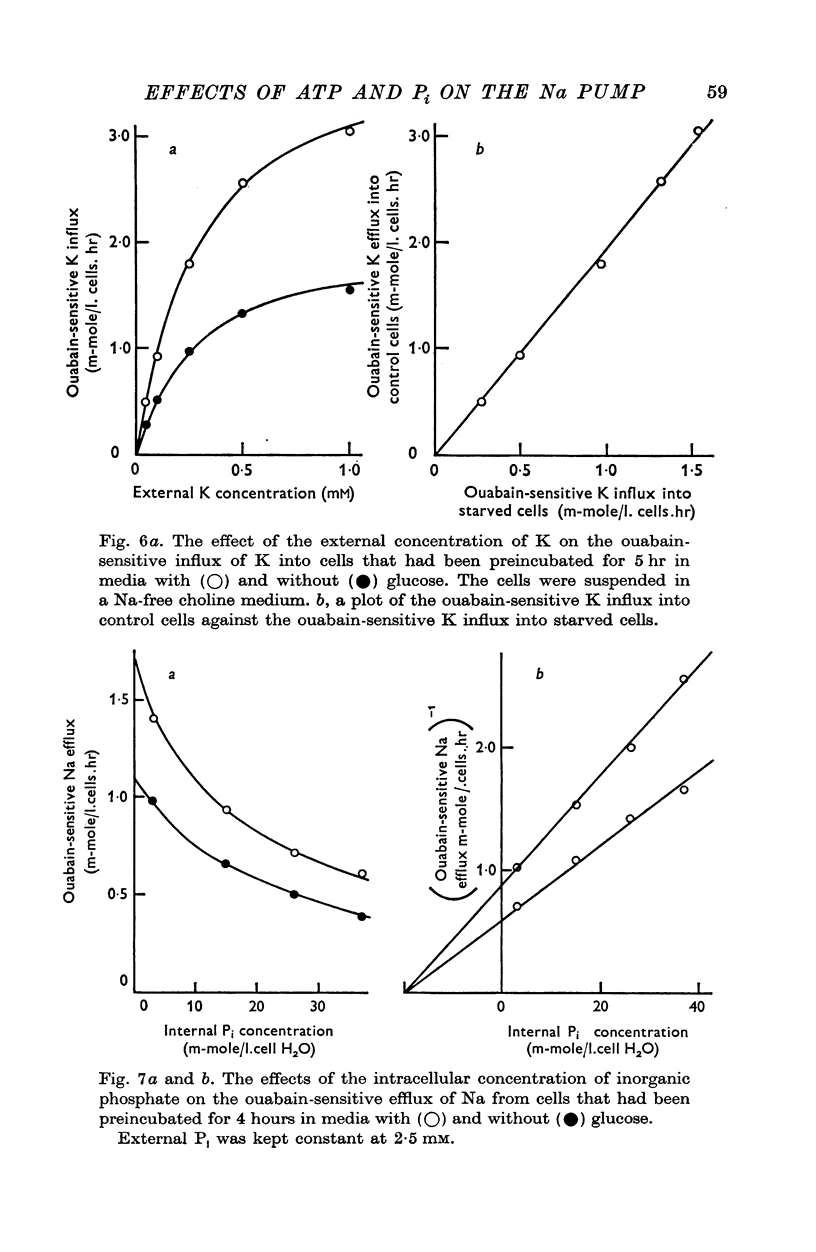
Abstract
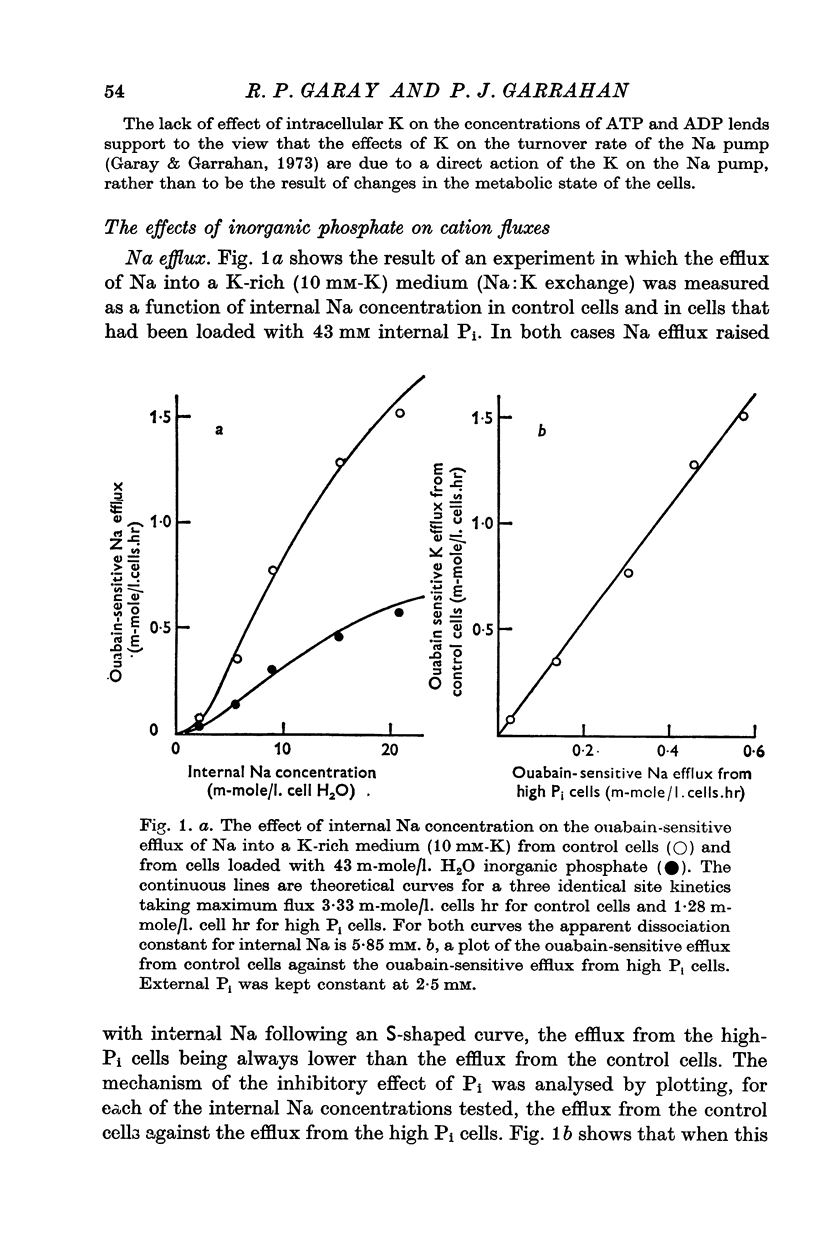
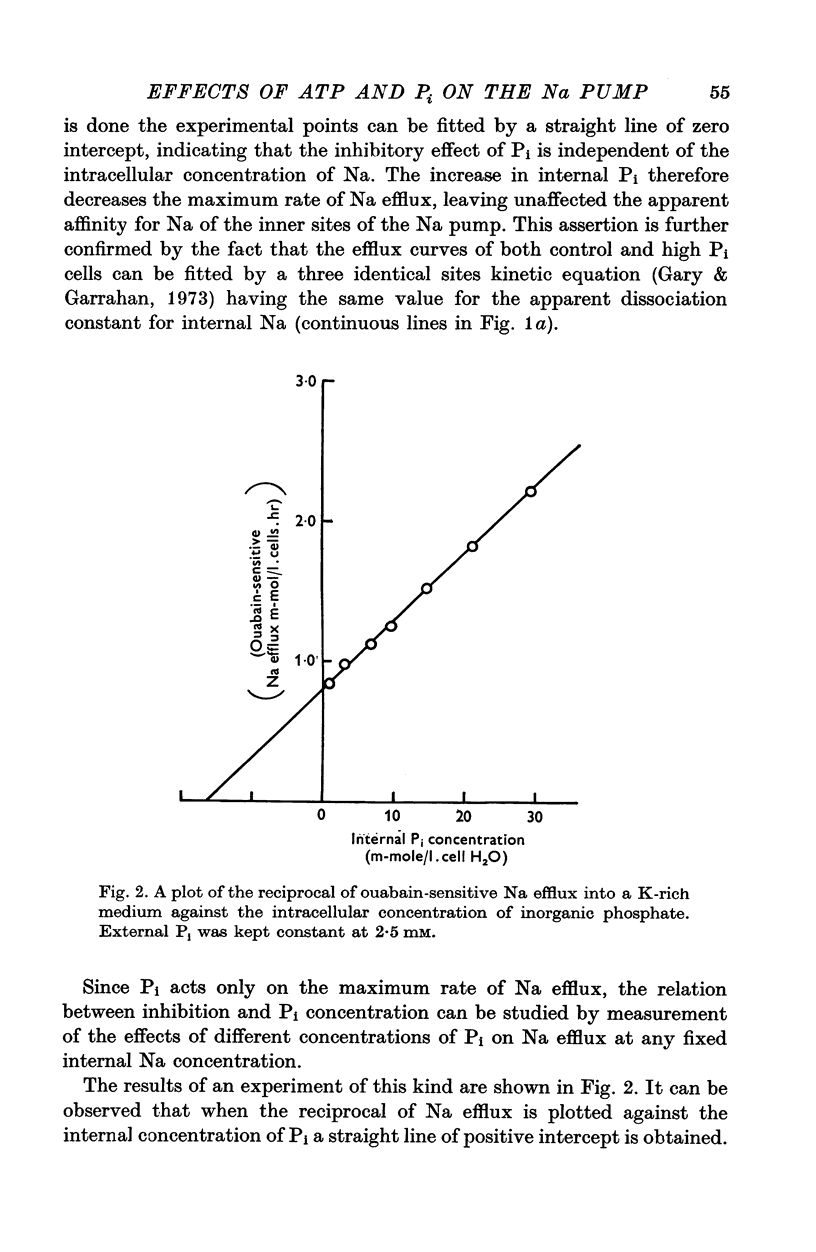
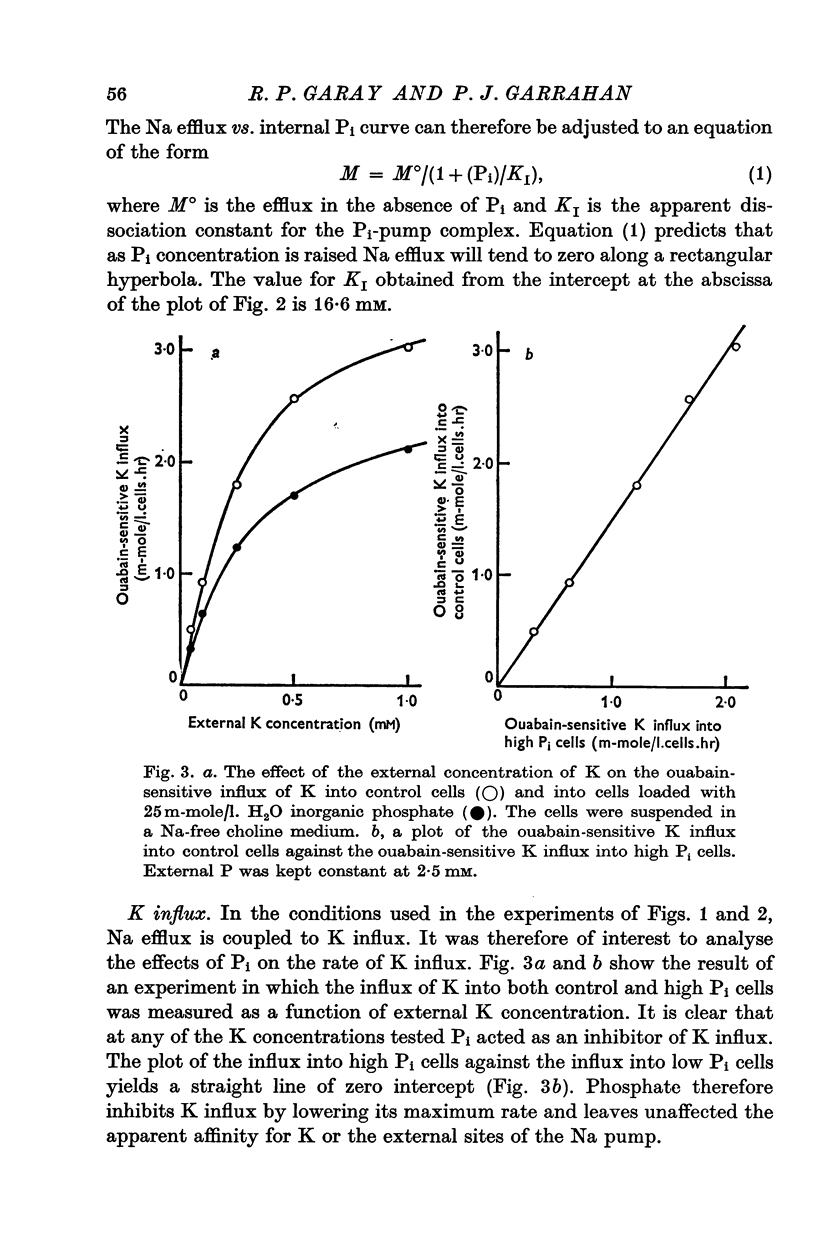
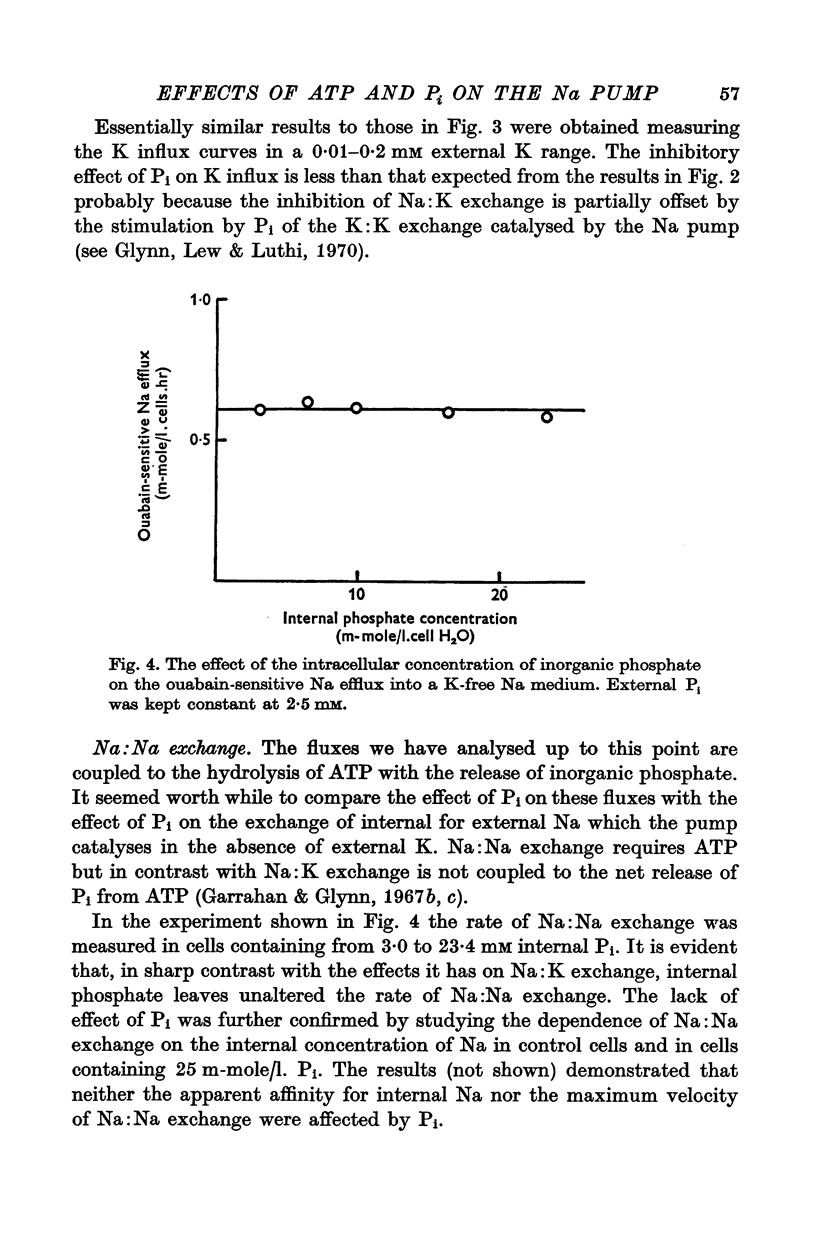
1. An increase in the intracellular concentration of inorganic phosphate (Pi) reduces the rate of the Na:K exchange catalysed by the Na pump in red cells. The inhibitory effect of Pi is exerted on the maximum rate of flux, Pi having no appreciable effect on the apparent affinity of the Na pump for either internal Na or external K. The effect of Pi is exerted along a rectangular hyperbola which tends to zero as Pi tends to infinity and is half-maximal at about 17 mM internal Pi. 2. Pi does not modify the rate of Na:Na exchange catalysed by the Na pump. 3. A reduction in the intracellular concentration of ATP reduces the maximum rate of Na:K exchange having no effect on the apparent affinity for either internal Na or external K. 4. The effects of ATP and Pi are mutually independent. 5 The lack of effect of ATP and Pi on the apparent affinity for internal Na is compatible with the idea that the affinity of the inner sites of the Na pump remains constant during a pump cycle. 6. The lack of effect of ATP on the apparent affinity for external K and the independence between the effects of ATP and Pi are difficult to explain if the only effect of ATP were its combination at a phosphorylating site. 7. The apparent affinities for K and phosphate become independent of the concentration of ATP, if it is assumed that in our experimental range the phosphorylating site is fully saturated with ATP, the rate of pumping being controlled by the state of occupation of a second non-phosphorylating site whose affinity for ATP is much lower. 8. The lack of effect of Pi on the apparent affinity for external K seems to indicate that during Na:K exchange the conformations of the pump that predominate are endowed with a reactivity towards inorganic phosphate and have the same high affinity for K in both their phospho and their dephospho states. 9. The kinetic behaviour of the Na pump in regard to its interactions with inner and outer cations, ATP and Pi seems to indicate that, in contrast with what happens with soluble allosteric proteins, in the active transport system ligand-induced changes in the reactivity are more important than ligand-induced changes in affinity. In this respect therefore the Na pump behaves as an allosteric 'V system'.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. Partial inhibition of the active transport of cations in the giant axons of Loligo. J Physiol. 1960 Jul;152:591–600. doi: 10.1113/jphysiol.1960.sp006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. V. Phosphorylation by adenosine triphosphate-32P. J Biol Chem. 1968 Apr 25;243(8):1993–2002. [PubMed] [Google Scholar]
- GIBBS R., RODDY P. M., TITUS E. PREPARATION, ASSAY, AND PROPERTIES OF AN NA+- AND K+-REQUIRING ADENOSINE TRIPHOSPHATASE FROM BEEF BRAIN. J Biol Chem. 1965 May;240:2181–2187. [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Garay R. P. A kinetic study of the Na pump in red cells: its relevance to the mechanism of active transport. Ann N Y Acad Sci. 1974;242(0):445–458. doi: 10.1111/j.1749-6632.1974.tb19108.x. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J Physiol. 1967 Sep;192(1):237–256. doi: 10.1113/jphysiol.1967.sp008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J Physiol. 1967 Sep;192(1):237–256. doi: 10.1113/jphysiol.1967.sp008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The stoicheiometry of the sodium pump. J Physiol. 1967 Sep;192(1):217–235. doi: 10.1113/jphysiol.1967.sp008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L. Synthesis of adenosine triphosphate at the expense of downhill cation movements in intact human red cells. J Physiol. 1970 Apr;207(2):393–402. doi: 10.1113/jphysiol.1970.sp009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyvary C., Post R. L. Binding of adenosine triphosphate to sodium and potassium ion-stimulated adenosine triphosphatase. J Biol Chem. 1971 Sep 10;246(17):5234–5240. [PubMed] [Google Scholar]
- Kanazawa T., Yamada A., Yamamoto T., Tonomura Y. Reaction mechanism of the Ca2 plus-dependent ATPase of sarcoplasmic reticulum from skeletal mus le. V. Vectorial requirements for calcium and magnesium ions of three partial reactions of ATPase: formation and decomposition of a phosphorylated intermediate and ATP-formation from ADP and the intermediate. J Biochem. 1971 Jul;70(1):95–123. doi: 10.1093/oxfordjournals.jbchem.a129631. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Quantitative predictions of a noncarrier model for glucose transport across the human red cell membrane. Biophys J. 1970 Jul;10(7):585–609. doi: 10.1016/s0006-3495(70)86322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Meissner G. ATP and Ca2+ binding by the Ca2+ pump protein of sarcoplasmic reticulum. Biochim Biophys Acta. 1973 Apr 16;298(4):906–926. doi: 10.1016/0005-2736(73)90395-7. [DOI] [PubMed] [Google Scholar]
- Neufeld A. H., Levy H. M. A second ouabain-sensitive sodium-dependent adenosine triphosphate in brain microsomes. J Biol Chem. 1969 Dec 10;244(23):6493–6497. [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Robinson J. D. Affinity of the (Na+ plus K+)-dependent ATPase for Na+ measured by Na+-modified enzyme inactivation. FEBS Lett. 1974 Jan 15;38(3):325–328. doi: 10.1016/0014-5793(74)80083-9. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Variable affinity of the (Na+ + K+)-dependent adenosine triphosphatase for potassium. Studies using beryllium inactivation. Arch Biochem Biophys. 1973 May;156(1):232–243. doi: 10.1016/0003-9861(73)90361-5. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Potassium efflux associated with partial reversal of the sodium pump in human red cell ghosts. J Physiol. 1972 Dec;227(2):19P–20P. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Tonomura Y. Reaction mechanism of the Ca2+-dependent ATPase of sarcoplasmic reticulum from skeletal muscle. IX. Kinetic studies on the conversion of osmotic energy to chemical energy in the sarcoplasmic reticulum. J Biochem. 1973 Dec;74(6):1091–1096. doi: 10.1093/oxfordjournals.jbchem.a130336. [DOI] [PubMed] [Google Scholar]


