Abstract
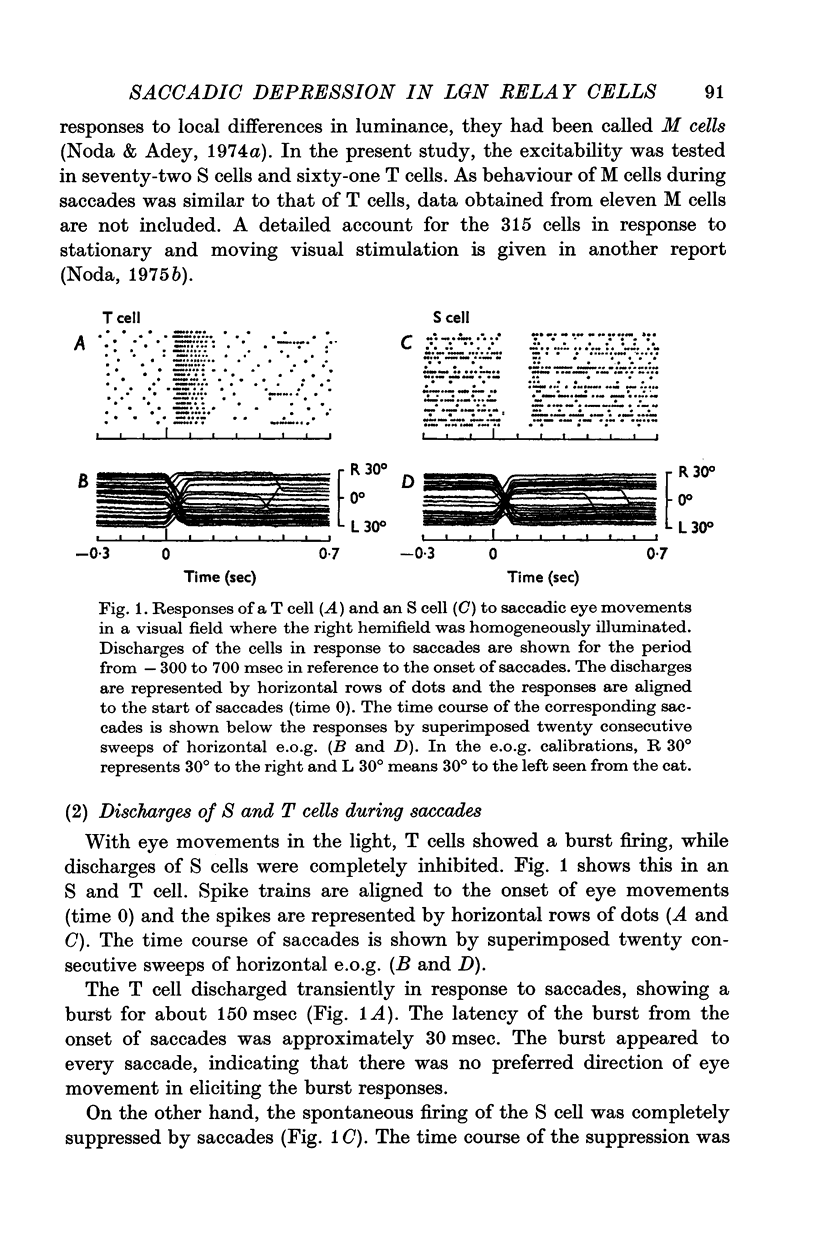
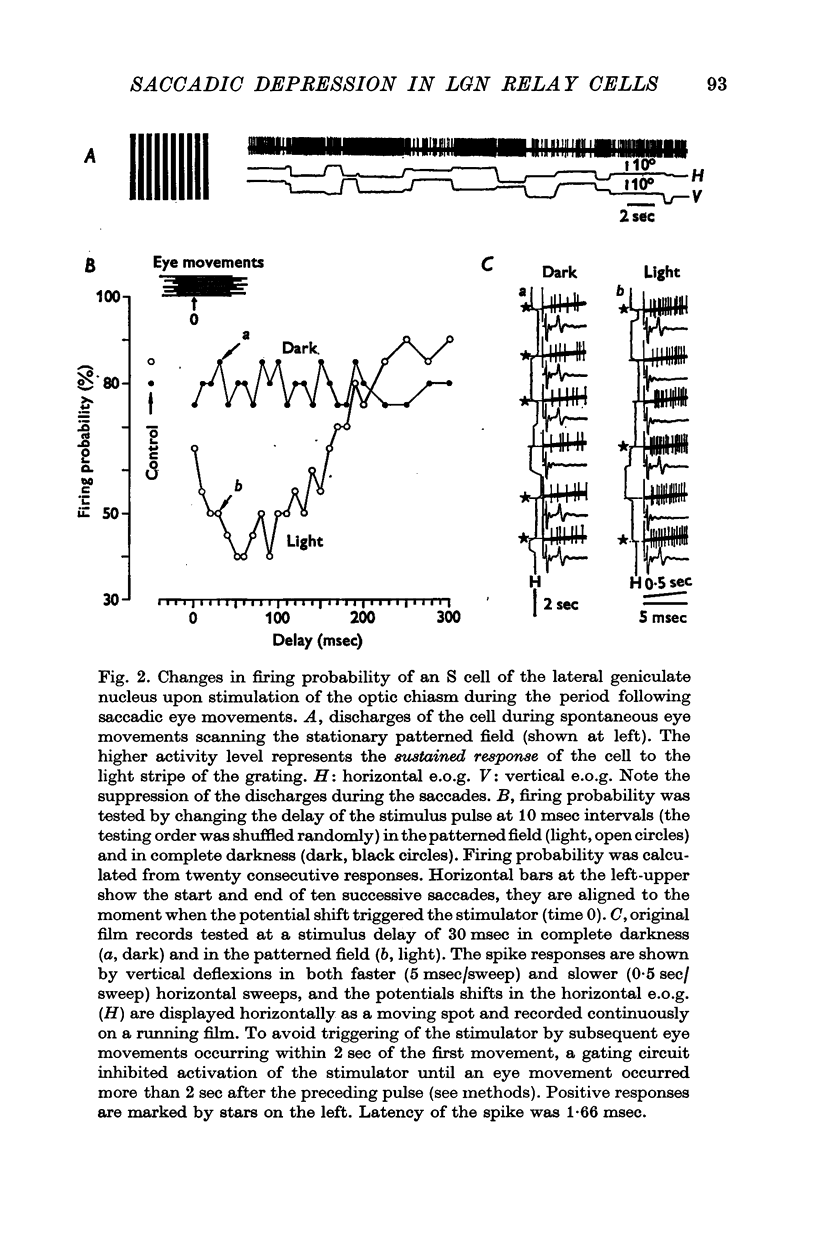
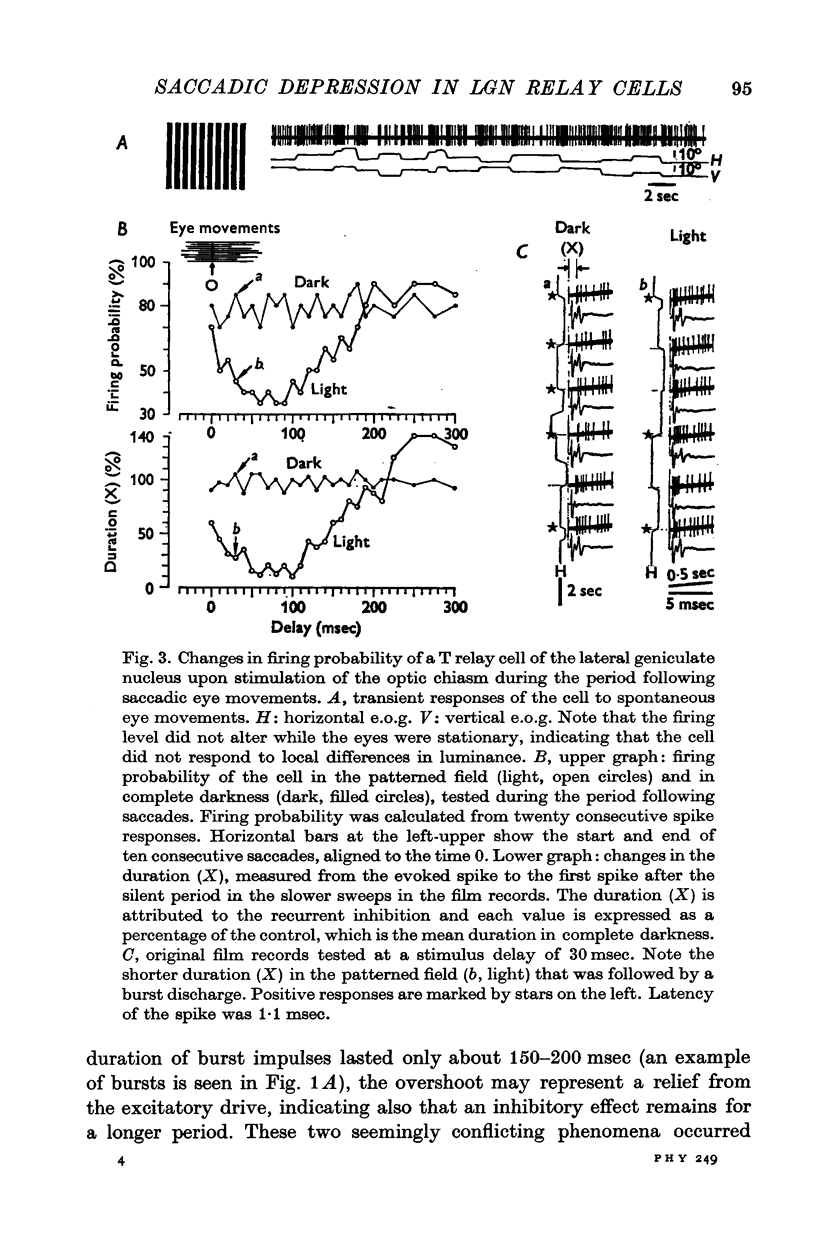
1. The excitability of relay cells of the lateral geniculate nucleus during a saccadic eye movement was studied in alert cats. Excitability was assessed by the firing probability of the cells in response to electrical stimulation of the optic chiasm. Modifications in the excitability were evaluated during the period following eye movements, by triggering a stimulator from potential shifts in electro-oculogram and altering delays in the stimulus pulse. 2. The cells were classified into S and T cells, based on their response properties and the latencies to chiasmatic stimulation. With a saccade in a stationary patterned field, T cells showed a burst discharge, while the discharges of S cells were completely suppressed. 3. The excitability was depressed in both S and T cells for 150-200 msec after a saccade, when the eye movement occurred in light. However, the depression did not occur in complete darkness. 4. The depression occurred also in the absence of eye movement, when the patterned visual field was moved in a saccadic fashion. 5. The depression in S cells occurred during an inhibitory period. Since S cells do not receive signals on image movement directly from the retina, the depression was due to a recurrent inhibition by signals transferred through the T ganglion-relay cell channel. 6. The depression in T cells occurred concomitantly with the burst discharge. Since the recurrent inhibition was operating less effectively during the period, the depression may be due to a phasic occlusion of the test impulse by coincident high-rate firings in the same cell. 7. The impairment in transmission of visual information through the lateral geniculate nucleus during the period following eye movements has been discussed in connexion with a neurophysiological basis for saccadic suppression.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adey W. R., Noda H. Influence of eye movements on geniculo-striate excitability in the cat. J Physiol. 1973 Dec;235(3):805–821. doi: 10.1113/jphysiol.1973.sp010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., McLEOD J. G. Nature of potentials associated with synaptic transmission in lateral geniculate of cat. J Neurophysiol. 1954 Jul;17(4):387–414. doi: 10.1152/jn.1954.17.4.387. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr Visual threshold changes resulting from spontaneous saccadic eye movements. Vision Res. 1967 Sep;7(9):769–775. doi: 10.1016/0042-6989(67)90039-9. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Davis R. Synaptic potentials, after-potentials, and slow rhythms of lateral geniculate neurones. J Physiol. 1960 Dec;154(3):514–546. doi: 10.1113/jphysiol.1960.sp006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E. Changes in the orthodromic and antidromic response of optic tract during the eye movements of sleep. J Neurophysiol. 1966 Sep;29(5):861–870. doi: 10.1152/jn.1966.29.5.861. [DOI] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Recovery of responsiveness of cells of lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):213–229. doi: 10.1113/jphysiol.1966.sp008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase R., Kalil R. E. Suppression of visual evoked responses to flashes and pattern shifts during voluntary saccades. Vision Res. 1972 Feb;12(2):215–220. doi: 10.1016/0042-6989(72)90112-5. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Feldman M., Diamond S. P. Effects of eye movement, brain-stem stimulation, and alertness on transmission through lateral geniculate body of monkey. J Neurophysiol. 1969 Jul;32(4):583–594. doi: 10.1152/jn.1969.32.4.583. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Changes in visual evoked responses during the fast phase of optokinetic nystagmus in the rabbit. Vision Res. 1969 Jul;9(7):803–814. doi: 10.1016/0042-6989(69)90016-9. [DOI] [PubMed] [Google Scholar]
- Dreher B., Sanderson K. J. Receptive field analysis: responses to moving visual contours by single lateral geniculate neurones in the cat. J Physiol. 1973 Oct;234(1):95–118. doi: 10.1113/jphysiol.1973.sp010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy F. H., Lombroso C. T. Electrophysiological evidence for visual suppression prior to the onset of a voluntary saccadic eye movement. Nature. 1968 Jun 15;218(5146):1074–1075. doi: 10.1038/2181074a0. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Saito H. The relationship between response characteristics to flicker stimulation and receptive field organization in the cat's optic nerve fibers. Vision Res. 1971 Mar;11(3):227–240. doi: 10.1016/0042-6989(71)90187-8. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Iwama K. A relation between latencies of initial and late spike discharges of rat lateral geniculate cells to optic tract stimulation. Brain Res. 1972 Feb 25;37(2):322–325. doi: 10.1016/0006-8993(72)90678-6. [DOI] [PubMed] [Google Scholar]
- Gross E. G., Vaughan H. G., Jr, Valenstein E. Inhibition of visual evoked responses to patterned stimuli during voluntary eye movements. Electroencephalogr Clin Neurophysiol. 1967 Mar;22(3):204–209. doi: 10.1016/0013-4694(67)90222-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Receptive field organization of 'sustained' and 'transient' retinal ganglion cells which subserve different function roles. J Physiol. 1972 Dec;227(3):769–800. doi: 10.1113/jphysiol.1972.sp010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Yamamoto M., Nakahama H. Intracellular recordings from the lateral geniculate neurons of cats. Jpn J Physiol. 1971 Jun;21(3):307–323. doi: 10.2170/jjphysiol.21.307. [DOI] [PubMed] [Google Scholar]
- Mackay D. M. Elevation of visual threshold by displacement of retinal image. Nature. 1970 Jan 3;225(5227):90–92. doi: 10.1038/225090a0. [DOI] [PubMed] [Google Scholar]
- Michael J. A., Stark L. Electrophysiological correlates of saccadic suppression. Exp Neurol. 1967 Feb;17(2):233–246. doi: 10.1016/0014-4886(67)90148-3. [DOI] [PubMed] [Google Scholar]
- Michael J. A., Stark L. Interactions between eye movements and the visually evoked response in the cat. Electroencephalogr Clin Neurophysiol. 1966 Nov;21(5):478–488. doi: 10.1016/0013-4694(66)90196-9. [DOI] [PubMed] [Google Scholar]
- Noda H., Adey W. R. Excitability changes in cat lateral geniculate cells during saccadic eye movements. Science. 1974 Feb 8;183(4124):543–545. doi: 10.1126/science.183.4124.543. [DOI] [PubMed] [Google Scholar]
- Noda H., Adey W. R. Retinal ganglion cells of the cat transfer information on saccadic eye movement and quick target motion. Brain Res. 1974 Apr 19;70(2):340–345. doi: 10.1016/0006-8993(74)90323-0. [DOI] [PubMed] [Google Scholar]
- Noda H., Freeman R. B., Jr, Gies B., Creutzfeldt O. D. Neuronal responses in the visual cortex of awake cats to stationary and moving targets. Exp Brain Res. 1971 May 26;112(4):389–405. doi: 10.1007/BF00234494. [DOI] [PubMed] [Google Scholar]
- Noda H. Sustained and transient discharges of retinal ganglion cells during spontaneous eye movements of cat. Brain Res. 1975 Feb 14;84(3):515–529. doi: 10.1016/0006-8993(75)90769-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T. Suppression of trans-geniculate transmission during the rapid phase of caloric nystagmus in the alert squirrel monkey. Brain Res. 1972 Mar 10;38(1):211–216. doi: 10.1016/0006-8993(72)90606-3. [DOI] [PubMed] [Google Scholar]
- Richards W. Visual suppression during passive eye movement. J Opt Soc Am. 1968 Aug;58(8):1159–1160. doi: 10.1364/josa.58.001159. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1971 Sep;34(5):920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- VOLKMANN F. C. Vision during voluntary saccadic eye movements. J Opt Soc Am. 1962 May;52:571–578. doi: 10.1364/josa.52.000571. [DOI] [PubMed] [Google Scholar]
- Volkmann F. C., Schick A. M., Riggs L. A. Time course of visual inhibition during voluntary saccades. J Opt Soc Am. 1968 Apr;58(4):562–569. doi: 10.1364/josa.58.000562. [DOI] [PubMed] [Google Scholar]
- Zuber B. L., Stark L. Saccadic suppression: elevation of visual threshold associated with saccadic eye movements. Exp Neurol. 1966 Sep;16(1):65–79. doi: 10.1016/0014-4886(66)90087-2. [DOI] [PubMed] [Google Scholar]


