Abstract
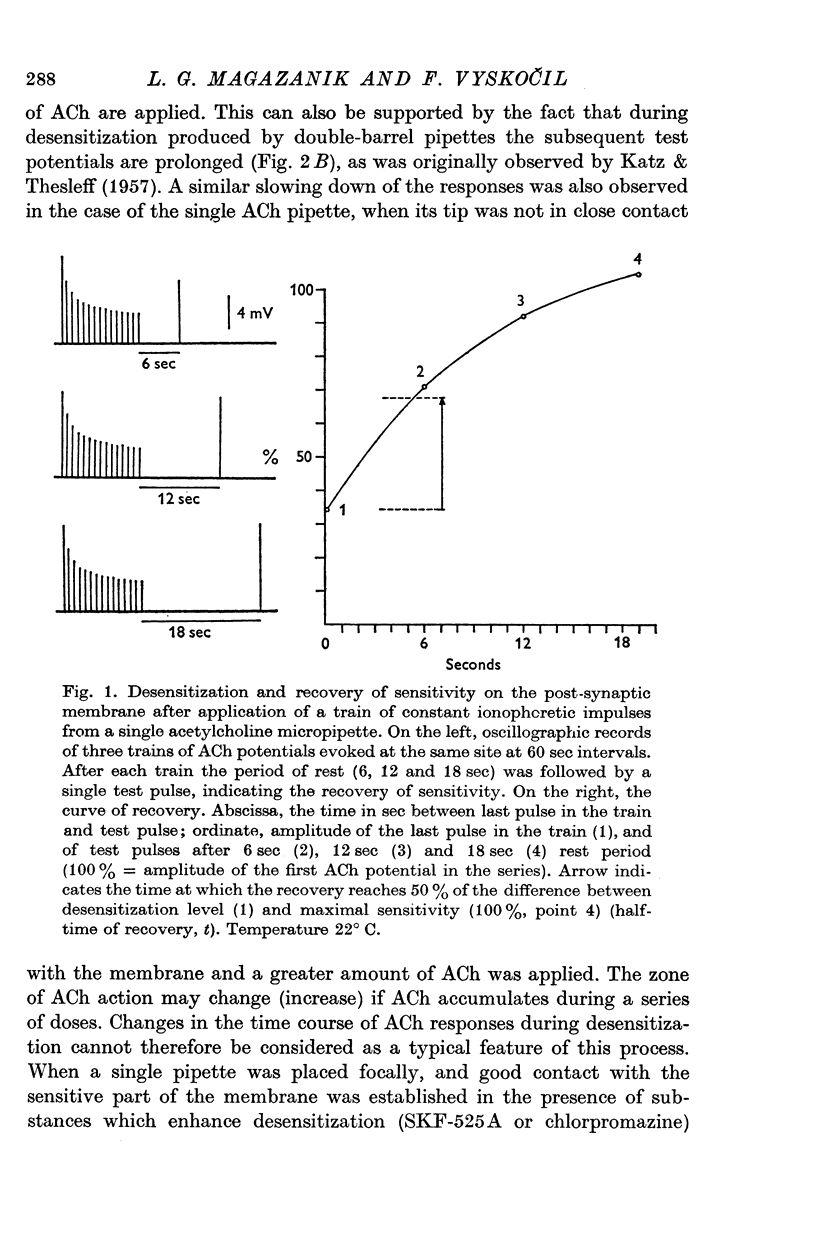
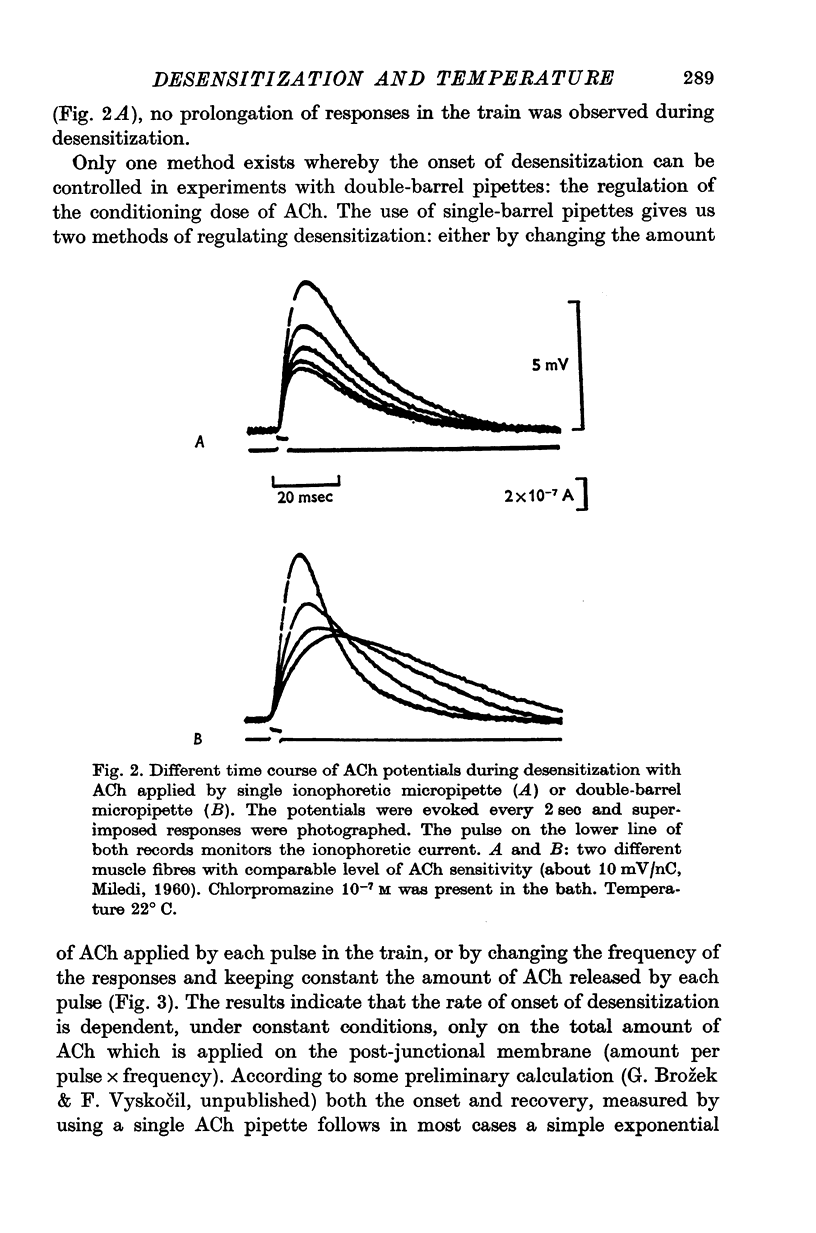
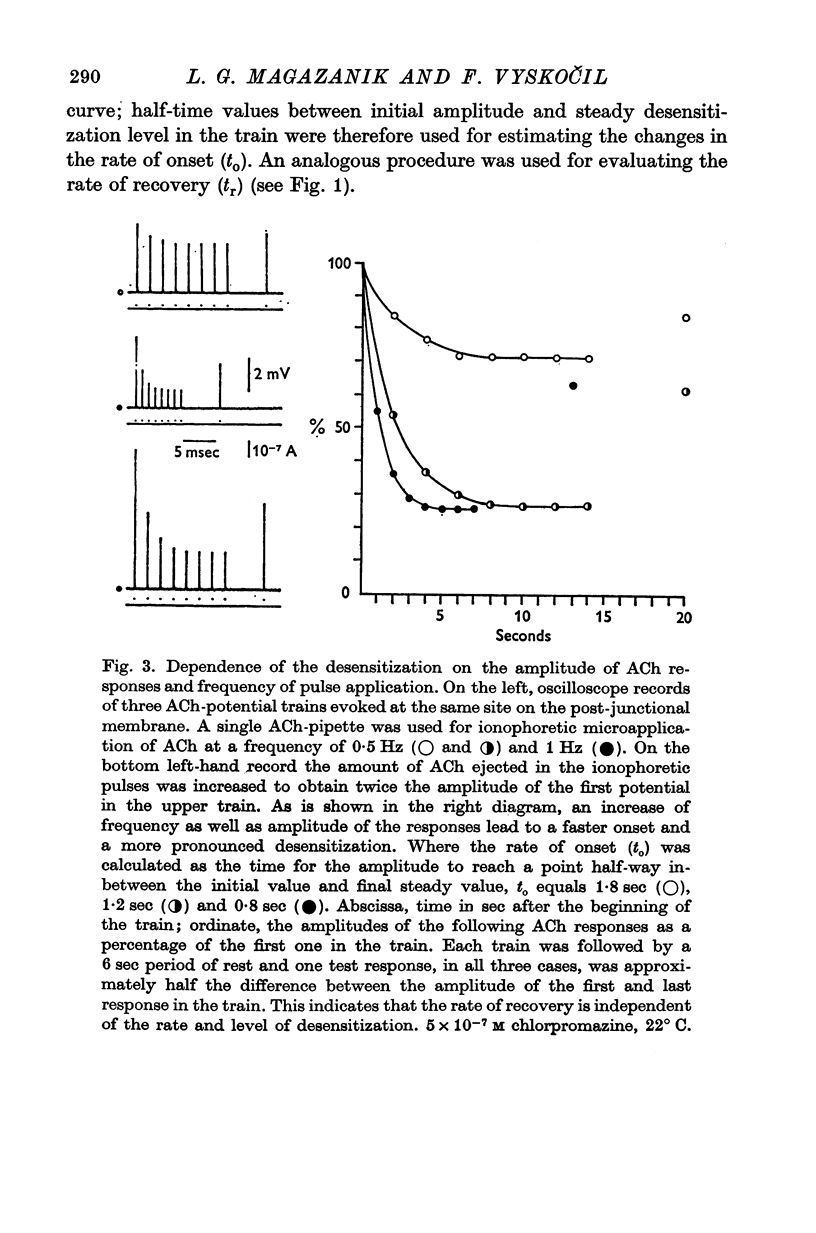
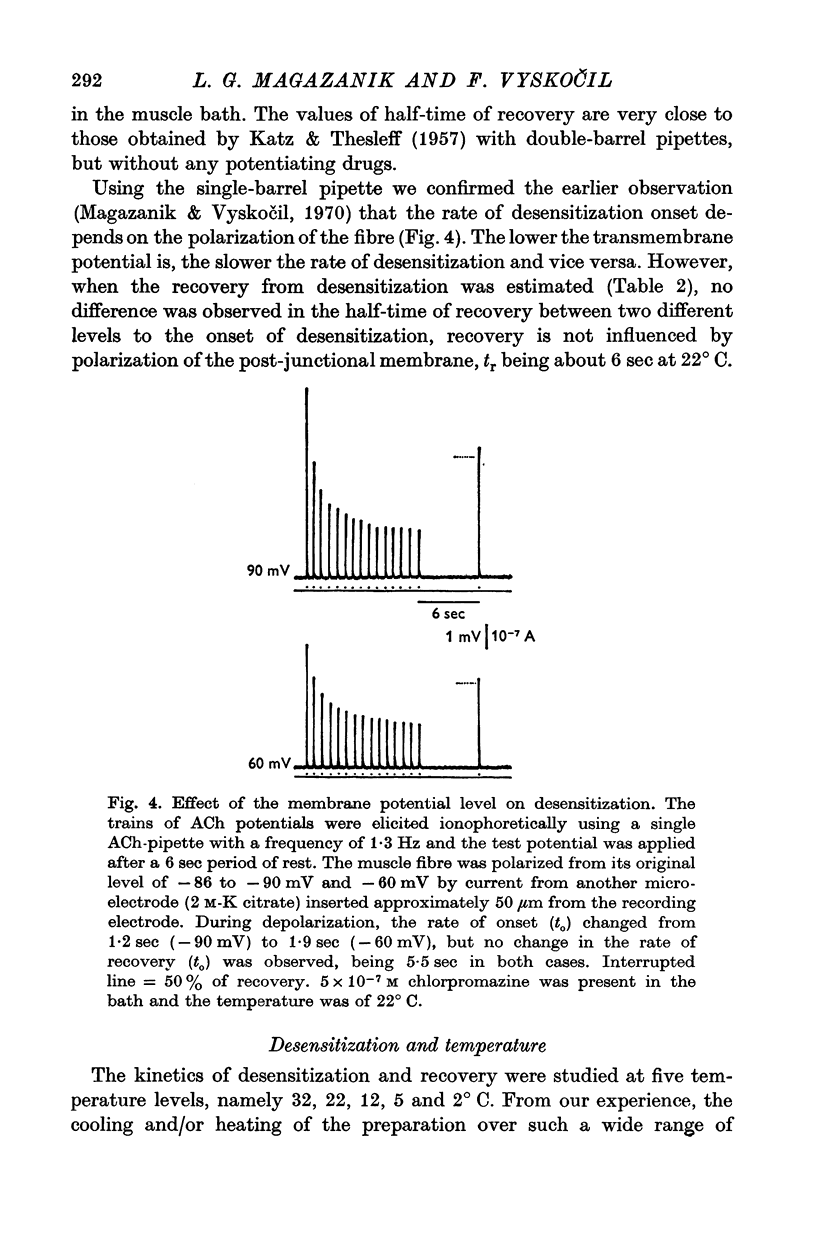
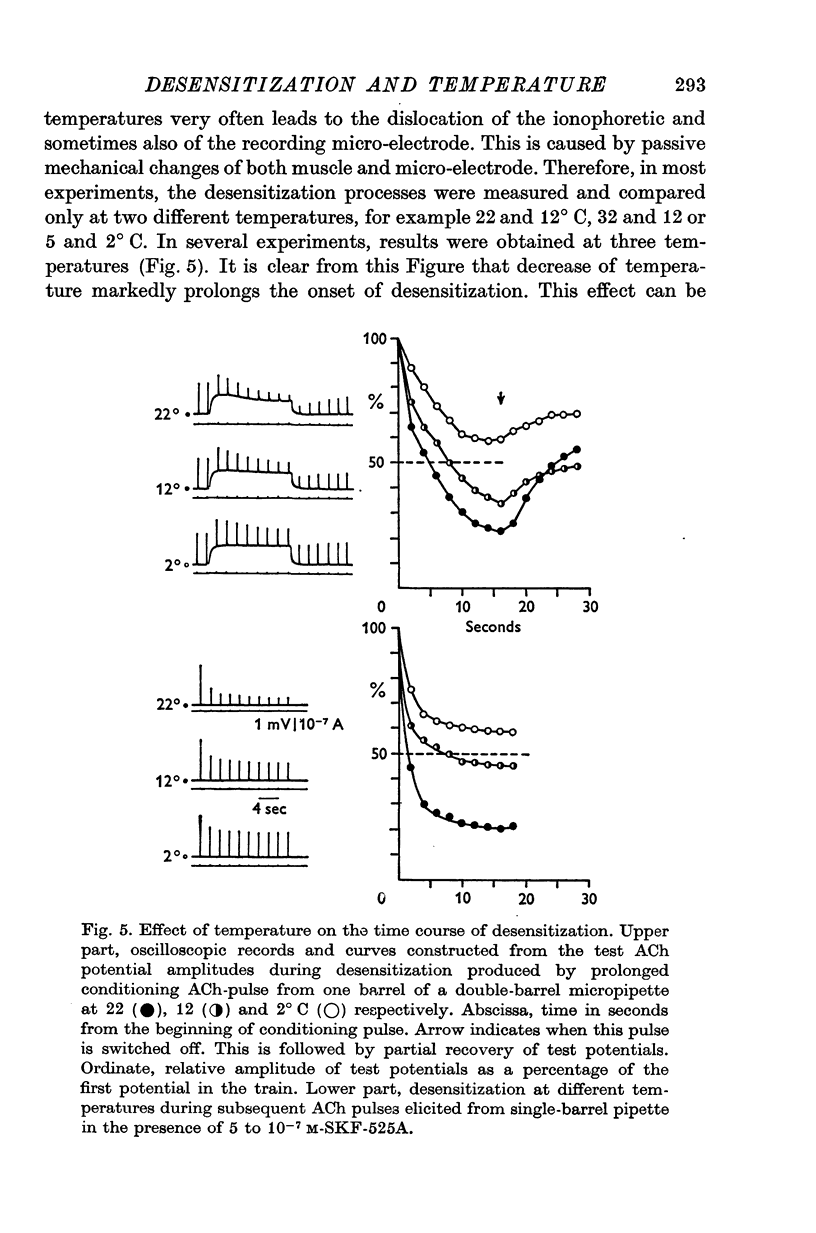
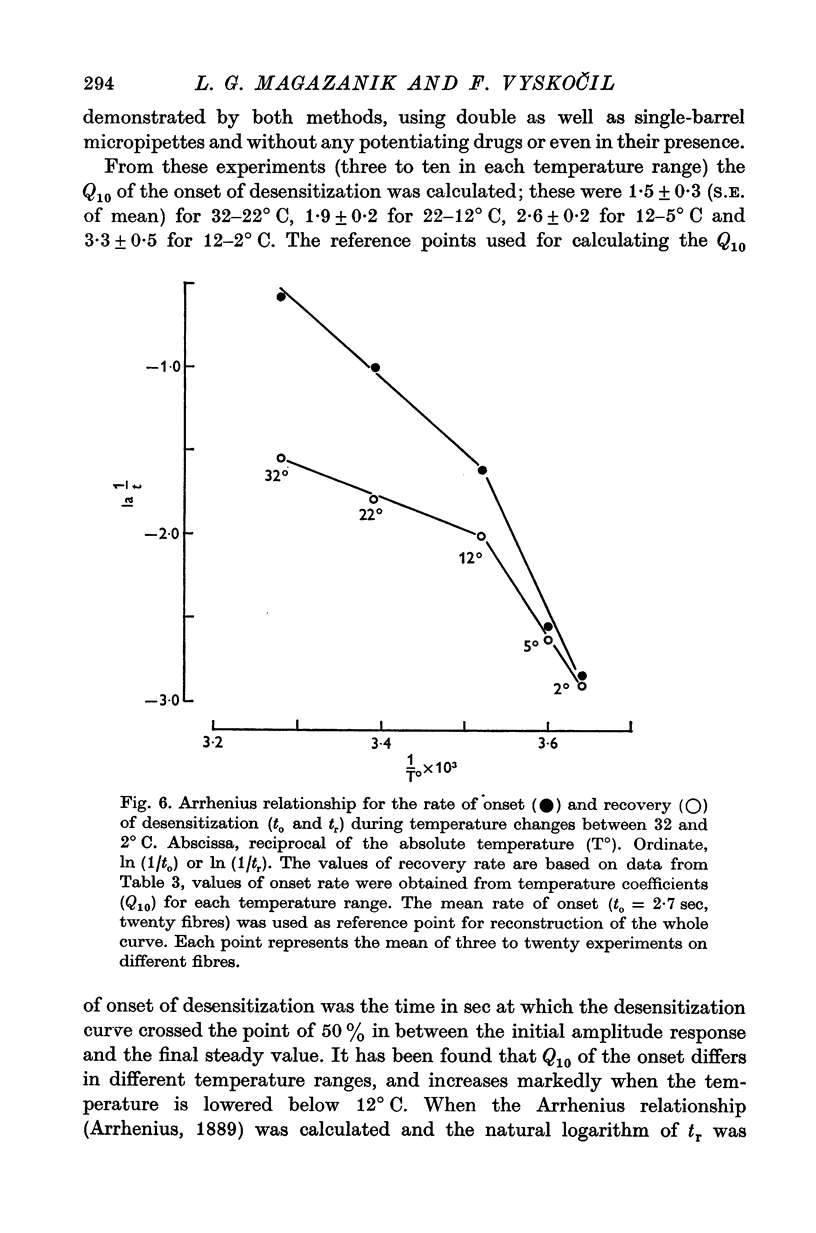
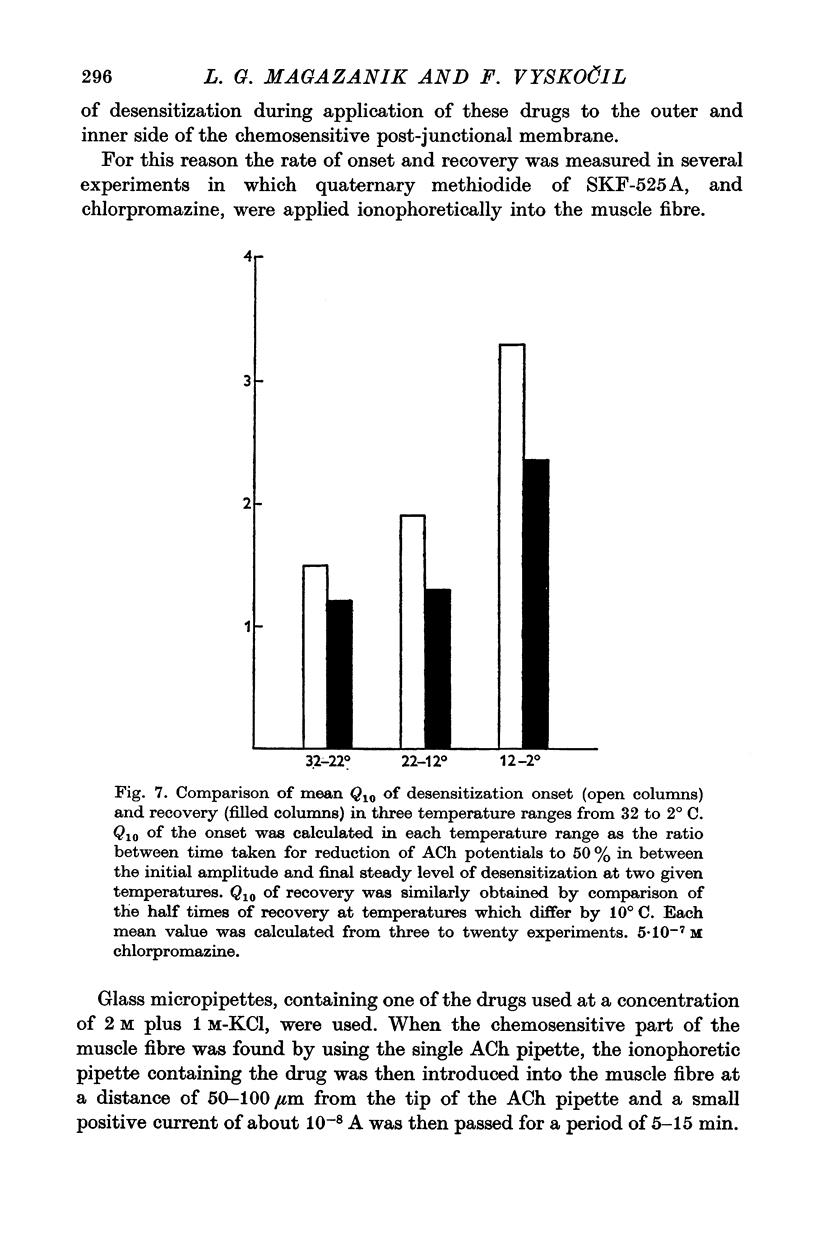
1. The time course of acetylcholine (ACh) potentials during development of desensitization was prolonged when a double-barrel ACh pipette was used for evoking desensitization. When a single-barrel ACh pipette was used, no change in the time course of ACh potential was to be seen during desensitization. 2. The recovery after desensitization did not depend on the rate of onset or on its level when a single ACh pipette was used. The half-time of recovery had a constant value of about 5.8 sec in the presence of chlorpromazine or SKF-525 A in a muscle bath at 22 degrees C. 3. Unlike the rate of onset, recovery from desensitization does not depend on the membrane potential. 4. The rate of onset of desensitization, i.e. time taken for reduction of ACh potentials to half-way between the initial amplitude and final steady value, decreased when temperature of the muscle bath was lowered. 5. Q10 of desensitization onset was found to be 1.5 for a change of temperature from 32 to 22 degrees C, 1.9 from 22 to 12 degrees C, 2.6 from 12 to 5 degrees C and 3.3 from 12 to 2 degrees C. 6. A similar temperature effect was observed in the case of desensitization recovery, the Q10 being 1.2 for temperature changes from 32 to 22 degrees C, 1.3 from 22 to 12 degrees C and 2.36 from 12 to 2 degrees C. 7. Intracellular application of quaternary methiodide of SKF-525 A or chloropromazine caused more rapid desensitization by ACh. The rate of desensitization onset depends on the ACh dose and on the frequency of application. The rate of recovery, however, has a constant value with a half-time of 5.5-5.7 sec at 22 degrees C. 8. Both the rate of onset and the rate of recovery changed with temperature in the case of intracellular potentiation of desensitization, in a similar manner to that observed after extracellular application of these drugs. 9. The onset of desensitization can thus be influenced by different substances as well as by changes in temperature. Recovery apparently has a different mechanism from the onset, because its time course can be altered only by changes in temperature of the muscle.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bregestovski P. D., Chailachjan L. M., Dunin-Barkovski V. L., Potapova T. W., Veprintsev B. N. Effect of temperature on the equilibrium endplate potential. Nature. 1972 Apr 28;236(5348):453–454. doi: 10.1038/236453a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G. O mekhanizme antiatsetilkholinovykh effektov nekotorykh monoazotistykh kholinolitikov v nervno-myshechnom sinapse. Farmakol Toksikol. 1971 May-Jun;34(3):292–297. [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. The loci of -bungarotoxin action on the muscle postjunctional membrane. Brain Res. 1972 Dec 24;48:420–423. doi: 10.1016/0006-8993(72)90203-x. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastuk W. L. Activation and inactivation of muscle postjunctional receptors. Fed Proc. 1967 Nov-Dec;26(6):1639–1646. [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. The relationship between desensitization and the metaphilic effect at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):383–390. [PubMed] [Google Scholar]
- Vyskocil F., Magazanik L. G. The desensitization of postjunctional muscle membrane after intracellular application of membrane stabilizers and snake venom polypeptides. Brain Res. 1972 Dec 24;48:417–419. doi: 10.1016/0006-8993(72)90202-8. [DOI] [PubMed] [Google Scholar]


