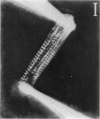
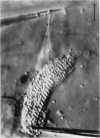
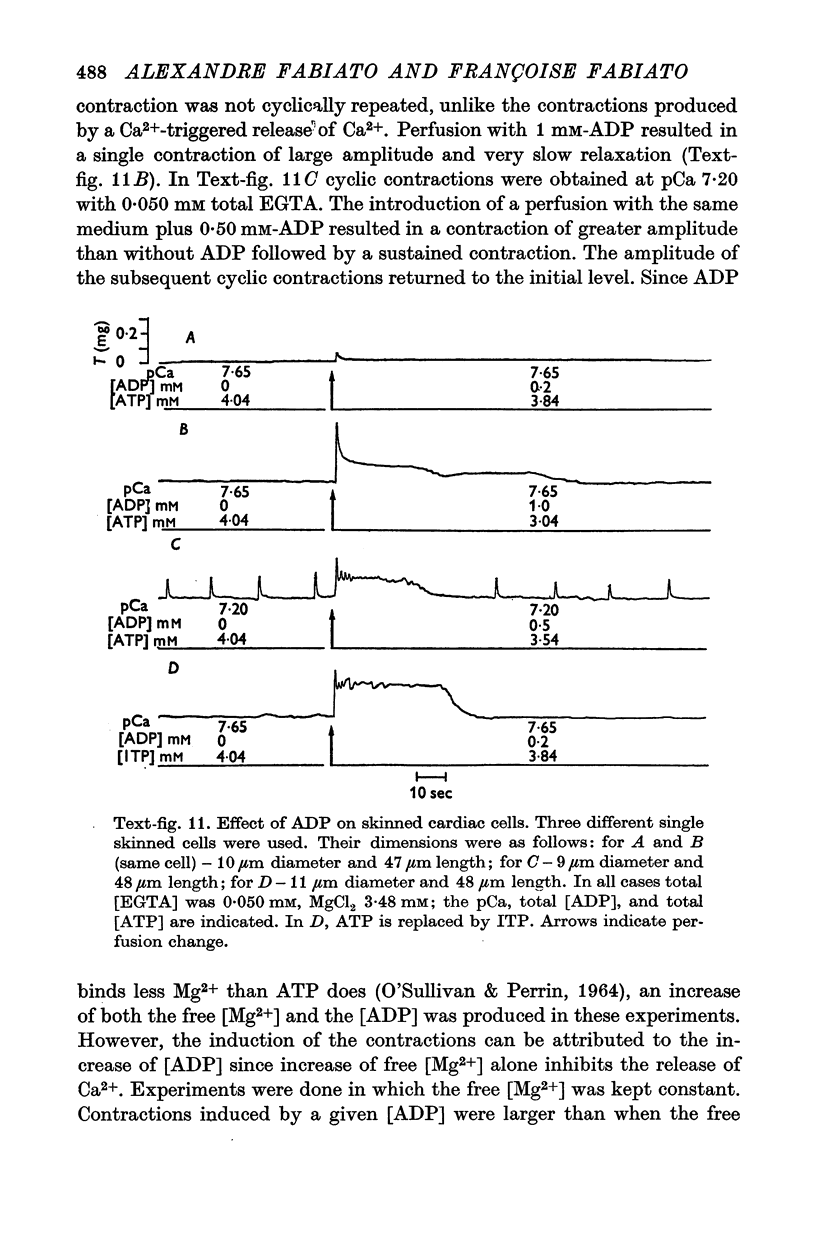
Abstract
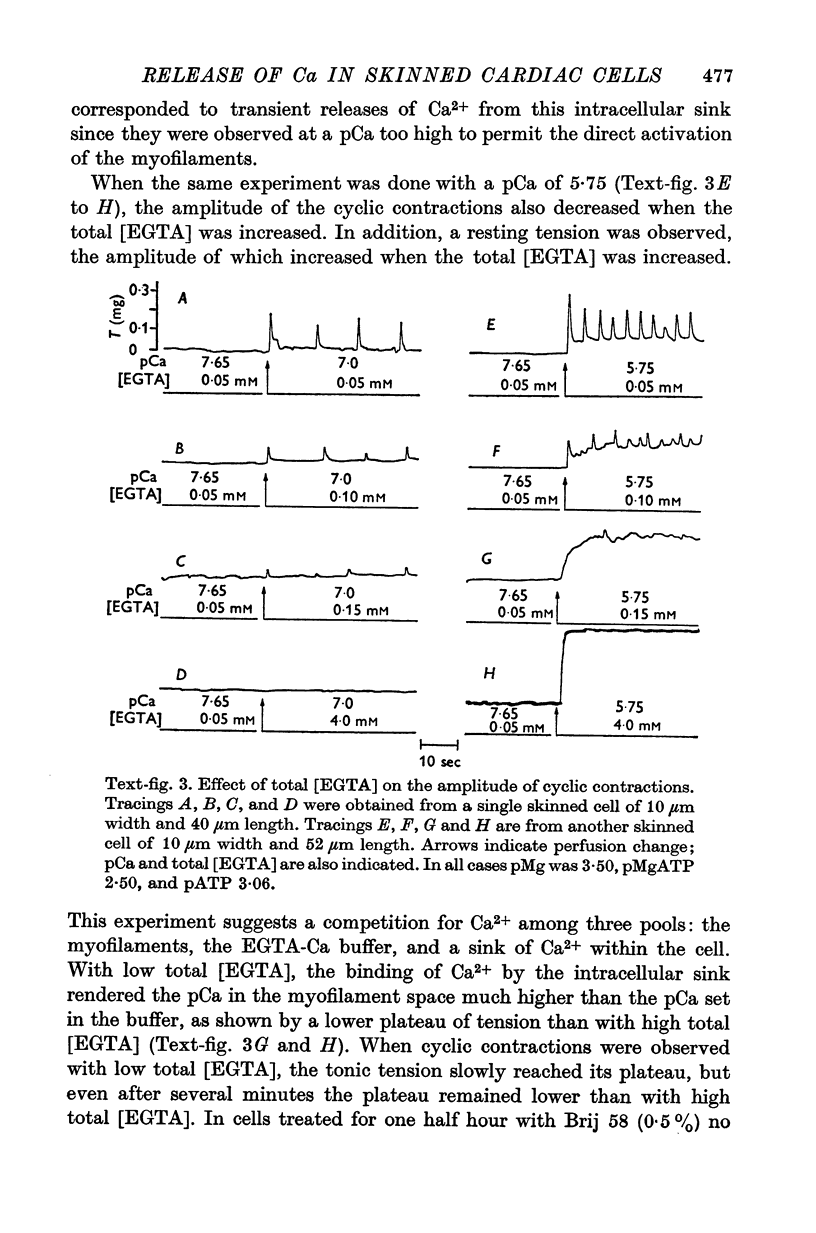
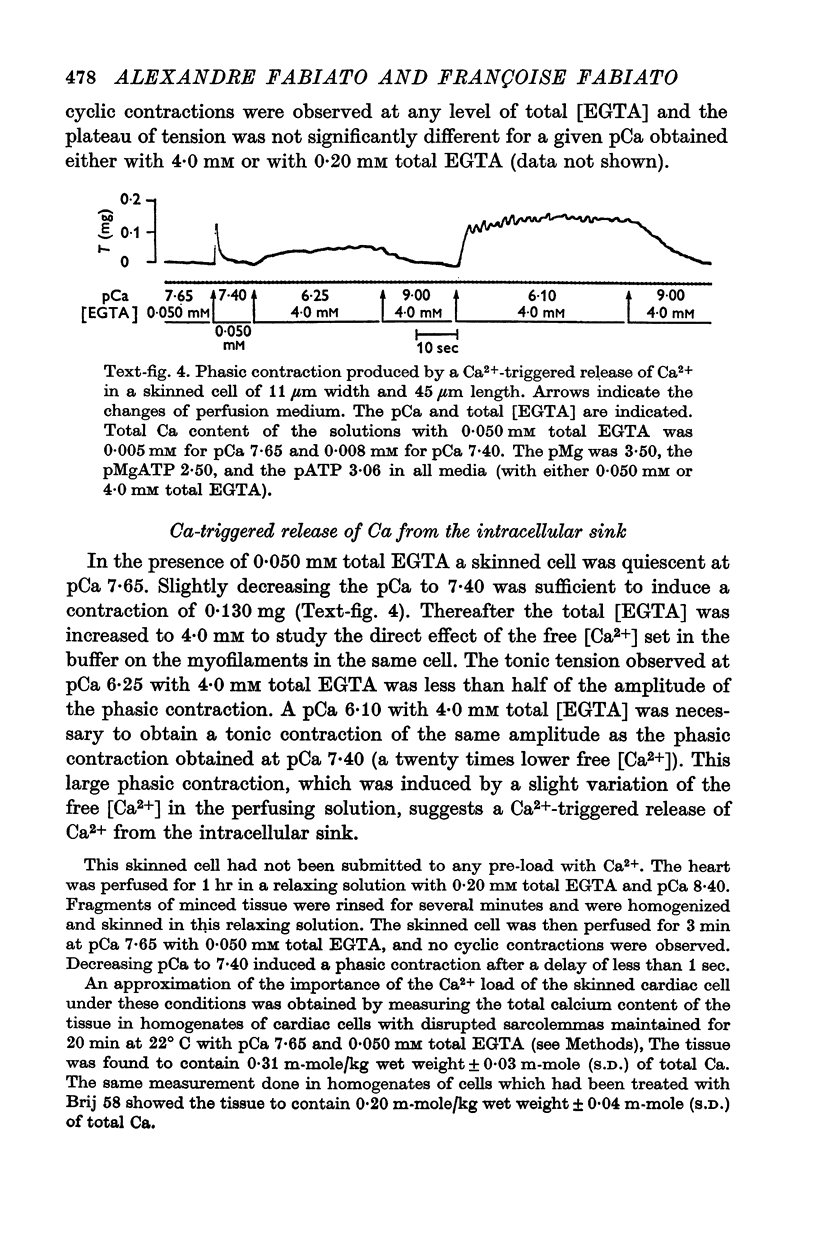
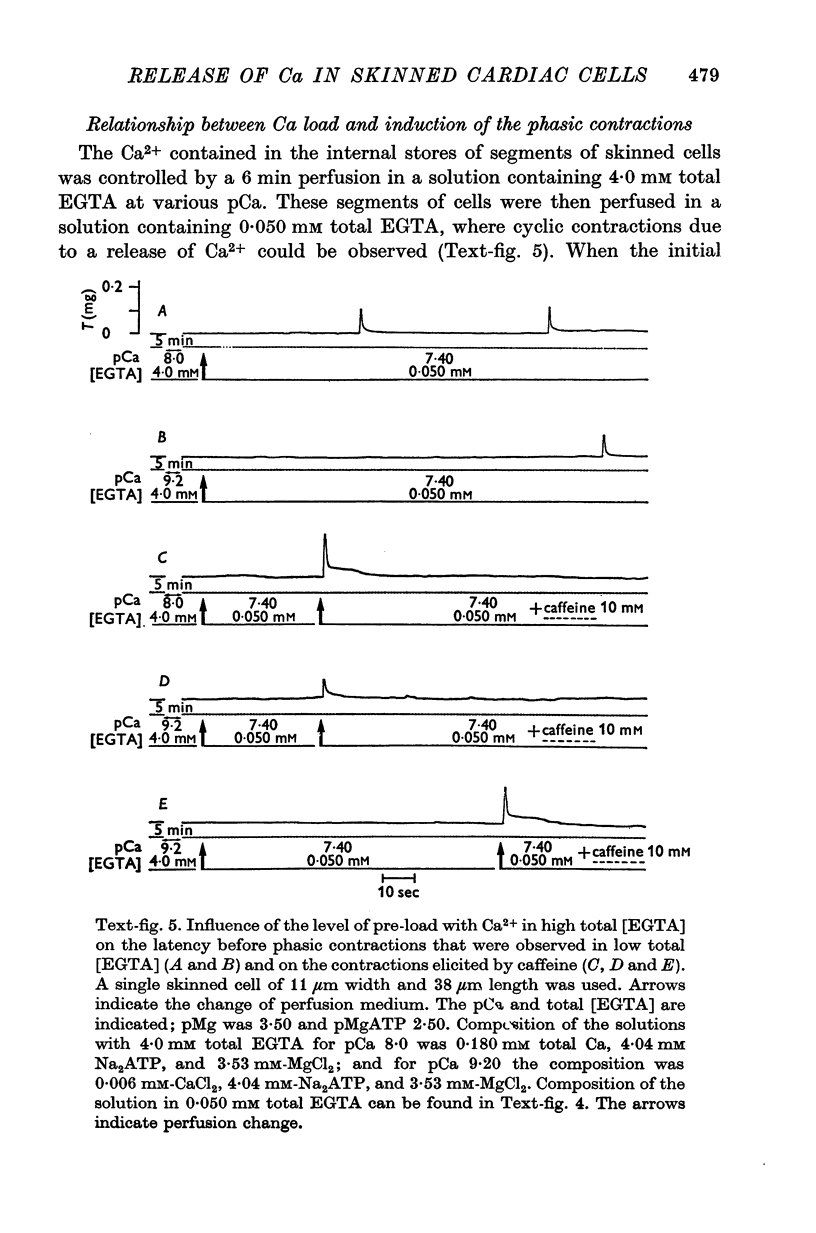
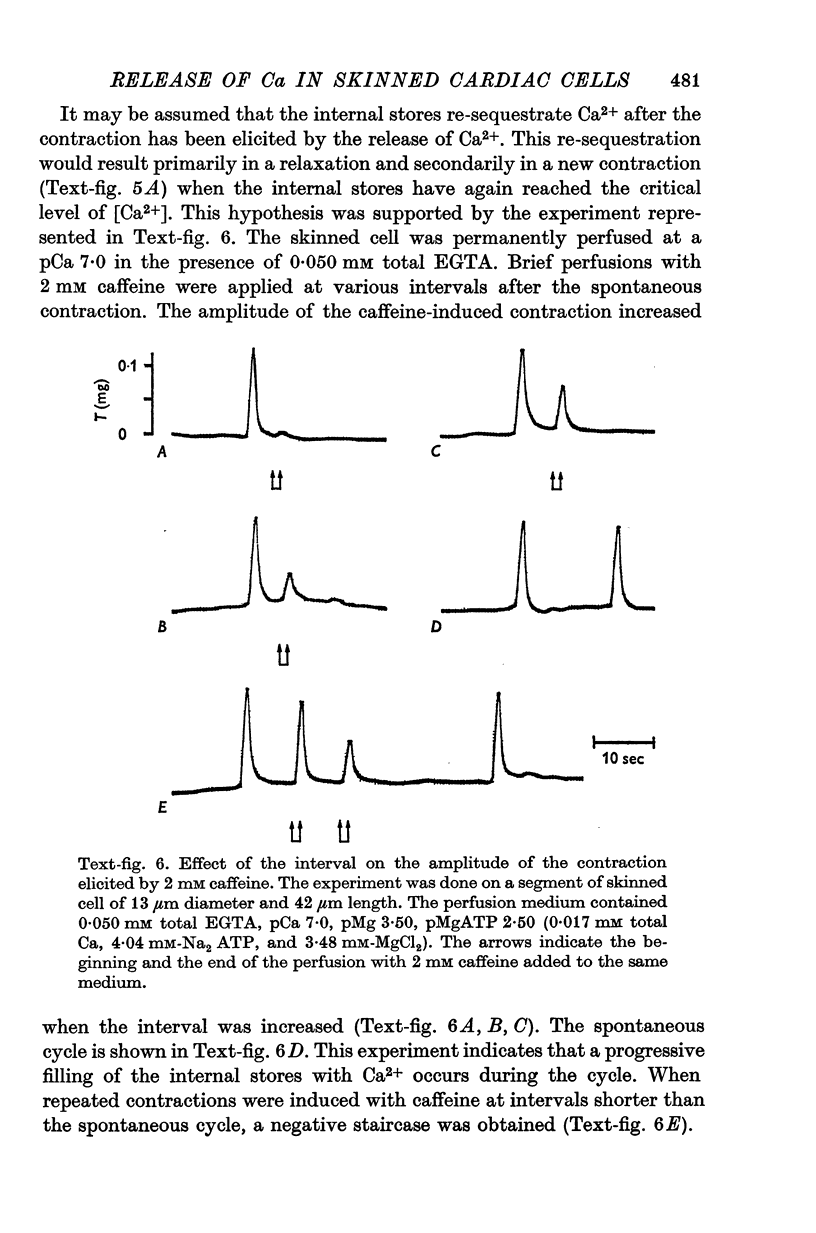
1. Fragments of single cardiac cells were obtained by homogenization of ventricular tissue from adult rats. Remaining pieces of sacrolemma were removed by micro-dissection. Tension was recorded from the ends of the skinned (sarcolemma-free) cells with a photodiode force transducer. 2. In the presence of a strong buffering of the free [Ca2+] with 4-0 mM total EGTA, a tonic tension was obtained that increased according to t sigmoid curve when the free ([Ca2+] was increased from 10(-6-75)M to 10(-5-0)M. This curve was not modified by the destruction of the sarcoplasmic reticulum (SR) by the detergent Brij 58. Therefore, the tonic tension corresponded to the direct effect of the free [Ca2+] present in the buffer on the myofilaments. 3. In the presence of a slight buffering of the free [Ca2+] with 0-050 mM total EGTA, cyclic contractions were observed that were attributed to cyclic releases and re-sequestrations of Ca2+ by the SR. The absence of effect of azide and ruthenium red on the cyclic contractions obtained at a free [Ca2+] lower than 10(-6-50)M demonstrated that the mitochondria played no role in the triggering of these contractions. 4. Cyclic contractions were induced by a slight variation of free [Ca2+] in the buffer from 10(-7-65)M to 10(-7-40)M. Their amplitude at 10(-7-40)M free Ca2+ was equal to the tonic tension developed by a free [Ca2+] 20 times higher applied to the myofilaments when the SR was destroyed by detergent or functionally inhibited by high total [EGTA]. It was concluded that these cyclic contractions corresponded to a Ca2+-triggered release of Ca2+ from the SR. 5. The cyclic contractions were induced by the filling of the SR with Ca2+ to a critical level at which it released a fraction of the Ca2+ it contained. Each contraction was followed by a re-sequestration of Ca2+, the kinetics of which conditioned the duration of the cycles. 6. The amplitude of the cyclic contractions increased when the free [Ca2+] that triggered them was increased. This gradation was deemed incompatible with a simple regenerative process, which should produce an all-or-nothing response. Additional process, such as a modulation of the Ca2+ release by free [Mg2+] and [ADP] may help to explain the gradation of the contractions. 7. It was concluded that a Ca2+-triggered release of Ca2+ from the SR of rat ventricular cells may amplify the Ca2+ flux crossing the sarcolemma during the plateau of the action potential, thereby permitting the activation of the myofilaments.
Full text
PDF



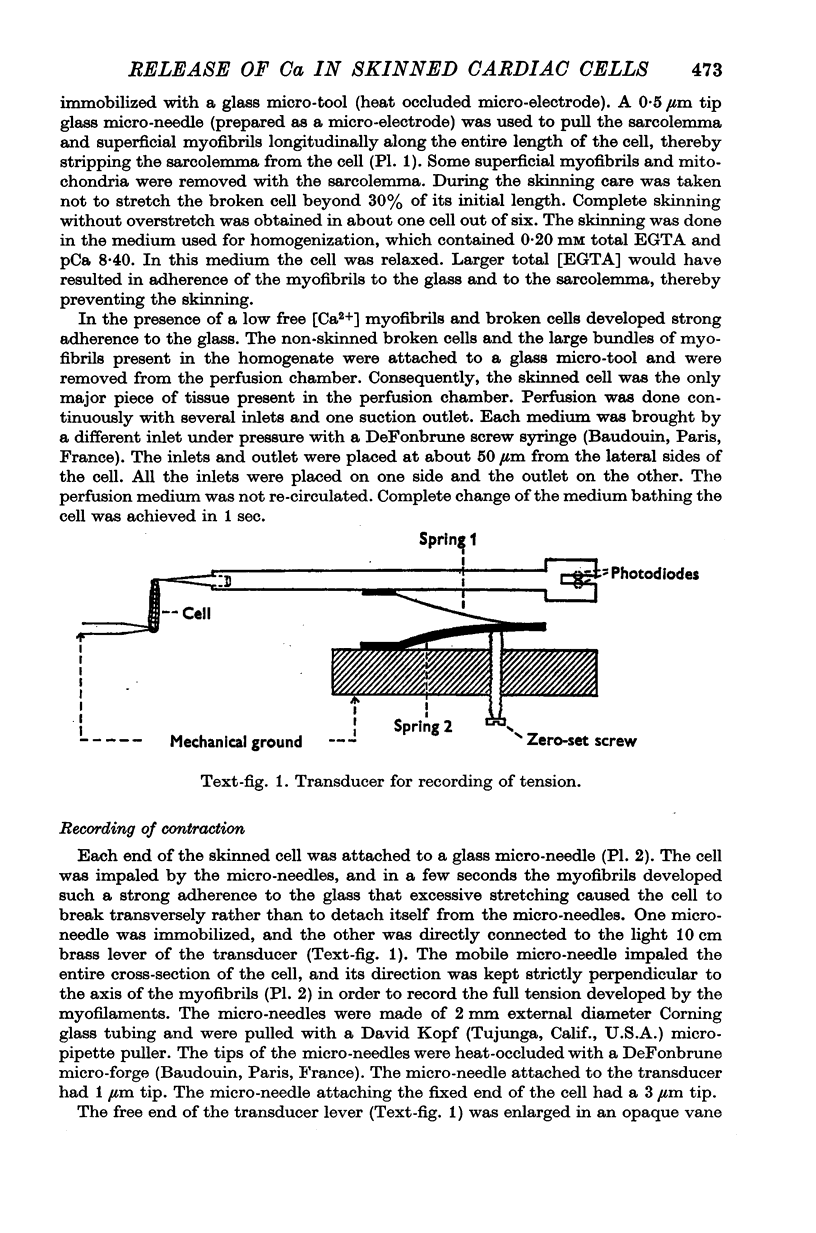


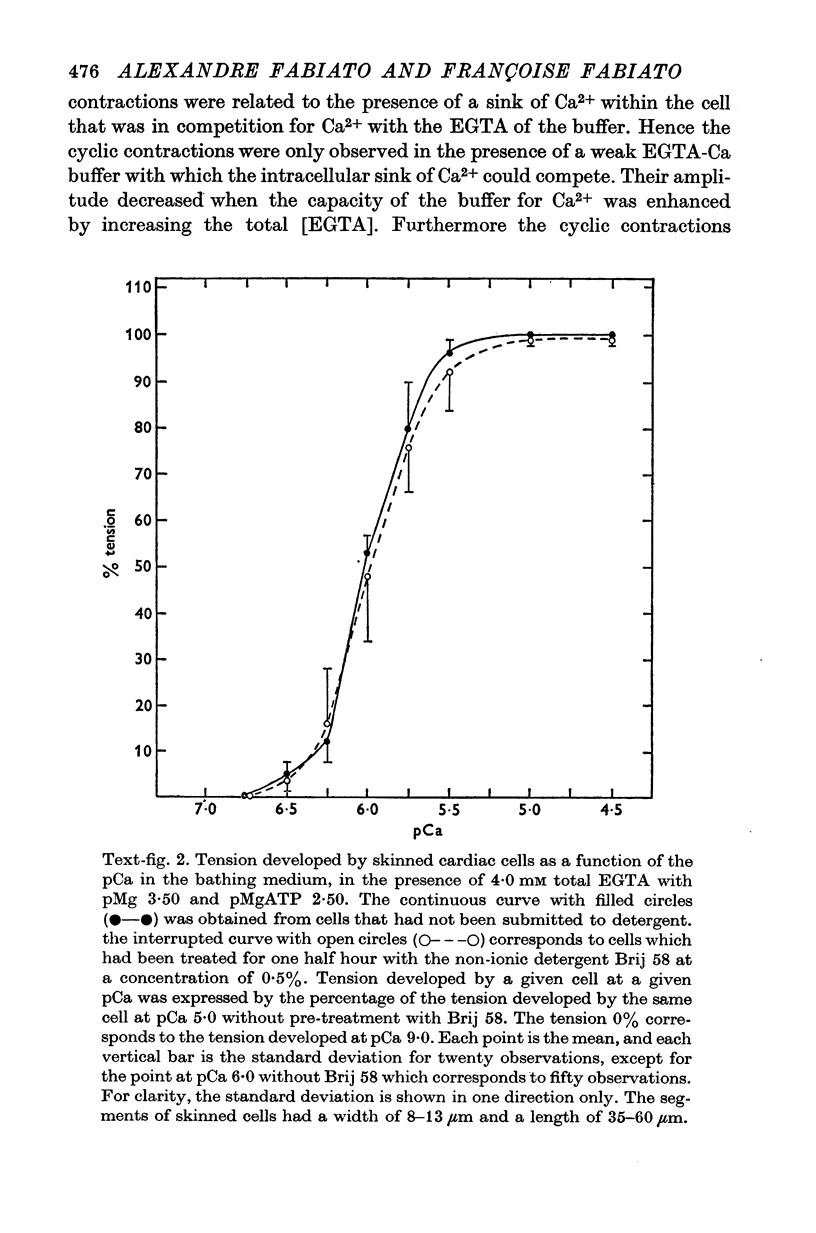












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Costantin L. L., Taylor S. R. Graded activation in frog muscle fibers. J Gen Physiol. 1973 Apr;61(4):424–443. doi: 10.1085/jgp.61.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Blinks J. R. Inconstant association of aequorin luminescence with tension during calcium release in skinned muscle fibres. Nat New Biol. 1973 Dec 19;246(155):218–221. doi: 10.1038/newbio246218a0. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Activation of skinned cardiac cells. Subcellular effects of cardioactive drugs. Eur J Cardiol. 1973 Dec;1(2):143–155. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972 Sep;31(3):293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Intracellular calcium movements in skinned muscle fibres. J Physiol. 1972 May;223(1):21–33. doi: 10.1113/jphysiol.1972.sp009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Kretzschmar K. M., Wilkie D. R., Woledge R. C. Chemical change and energy output during muscular contraction. J Physiol. 1971 Oct;218(1):163–193. doi: 10.1113/jphysiol.1971.sp009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E., Donaldson S. K., Harris C. E. Tension in skinned frog muscle fibers in solutions of varying ionic strength and neutral salt composition. J Gen Physiol. 1973 Nov;62(5):550–574. doi: 10.1085/jgp.62.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. H., Brutsaert D. L., Forman R., Sonnenblick E. H. Influence of caffeine on force development and force-frequency relations in cat and rat heart muscle. Cardiovasc Res. 1974 Mar;8(2):162–172. doi: 10.1093/cvr/8.2.162. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1973;35:55–86. doi: 10.1146/annurev.ph.35.030173.000415. [DOI] [PubMed] [Google Scholar]
- NANNINGA L. B. The association constant of the complexes of adenosine triphosphate with magnesium, calcium, strontium, and barium ions. Biochim Biophys Acta. 1961 Dec 9;54:330–338. doi: 10.1016/0006-3002(61)90373-0. [DOI] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. The apparent binding constant of glycoletherdiaminetetraacetic acid for calcium at neutral pH. J Biochem. 1968 Aug;64(2):255–257. doi: 10.1093/oxfordjournals.jbchem.a128887. [DOI] [PubMed] [Google Scholar]
- Orentlicher M., Reuben J. P., Grundfest H., Brandt P. W. Calcium binding and tension development in detergent-treated muscle fibers. J Gen Physiol. 1974 Feb;63(2):168–186. doi: 10.1085/jgp.63.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Panet R., Selinger Z. Synthesis of ATP coupled to Ca 2+ release from sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1972 Jan 17;255(1):34–42. doi: 10.1016/0005-2736(72)90005-3. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I., Page E. Magnesium in heart muscle. Circ Res. 1973 Oct 5;33(4):367–374. doi: 10.1161/01.res.33.4.367. [DOI] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S. A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. J Physiol. 1883 Jan;4(1):29–42.3. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Graziotti P. Mechanisms for intracellular calcium regulation in heart. I. Stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J Gen Physiol. 1973 Dec;62(6):756–772. doi: 10.1085/jgp.62.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer J. Myocardial metabolism in cardiac hypoxia. Am J Cardiol. 1967 Mar;19(3):385–392. doi: 10.1016/0002-9149(67)90452-3. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Briggs F. N. Estimating the functional capabilities of sarcoplasmic reticulum in cardiac muscle. Calcium binding. Circ Res. 1974 Apr;34(4):531–540. doi: 10.1161/01.res.34.4.531. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Dhalla N. S. Excitation-contraction coupling in heart. VI. Demonstration of calcium activated ATPase in the dog heart sarcolemma. Life Sci I. 1971 Feb 15;10(4):185–191. doi: 10.1016/0024-3205(71)90247-5. [DOI] [PubMed] [Google Scholar]
- Taylor E. W., Lymn R. W., Moll G. Myosin-product complex and its effect on the steady-state rate of nucleoside triphosphate hydrolysis. Biochemistry. 1970 Jul 21;9(15):2984–2991. doi: 10.1021/bi00817a008. [DOI] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. Regulatory mechanisms of the calcium transport system of fragmented rabbit sarcoplasmic rticulum. I. The effect of accumulated calcium on transport and adenosine triphosphate hydrolysis. J Gen Physiol. 1971 Jan;57(1):50–63. doi: 10.1085/jgp.57.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. Regulatory mechanisms ofthe calcium transport system of ramented rabbit sarcoplasmic rticulum. II. Inhibition of outflux in calcium-free media. J Gen Physiol. 1971 Jan;57(1):64–70. doi: 10.1085/jgp.57.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium binding and release in frog heart. J Gen Physiol. 1973 Dec;62(6):693–706. doi: 10.1085/jgp.62.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Tonomura Y. Reaction mechanism of the Ca 2+ -dependent ATPase of sarcoplasmic reticulum from skeletal muscle. VII. Recognition and release of Ca 2+ ions. J Biochem. 1972 Aug;72(2):417–425. doi: 10.1093/oxfordjournals.jbchem.a129917. [DOI] [PubMed] [Google Scholar]