Abstract
Nodules are formed on legume roots as a result of signaling between symbiotic partners and in response to the activities of numerous genes. We cloned fragments of differentially expressed genes in spot-inoculated soybean (Glycine max) roots. Many of the induced clones were similar to known genes related to oxidative stress, such as thioredoxin and β-carotene hydroxylase. The deduced amino acid sequences of full-length soybean cDNAs for thioredoxin and β-carotene hydroxylase were similar to those in other species. In situ RNA hybridization revealed that the thioredoxin gene is expressed on the pericycle of 2-d-old nodules and in the infected cells of mature nodules, suggesting that thioredoxin is involved in nodule development. The thioredoxin promoter was found to contain a sequence resembling an antioxidant responsive element. When a thioredoxin mutant of yeast was transformed with the soybean thioredoxin gene it became hydrogen peroxide tolerant. These observations prompted us to measure reactive oxygen species levels. These were decreased by 3- to 5-fold in 7-d-old and 27-d-old nodules, coincident with increases in the expression of thioredoxin and β-carotene hydroxylase genes. Hydrogen peroxide-producing regions identified with cerium chloride were found in uninoculated roots and 2-d-old nodules, but not in 7-d-old and 27-d-old nodules. RNA interference-mediated repression of the thioredoxin gene severely impaired nodule development. These data indicate that antioxidants such as thioredoxin are essential to lower reactive oxygen species levels during nodule development.
The association between a legume and Rhizobium generates a specialized organ, the root nodule, in which symbiotic-nitrogen fixation occurs (Stougaard, 2001; Mirabella et al., 2002; Oldroyd et al., 2005). The initiation of nodule formation involves diverse events such as Nod factor signaling and the expression of early nodulins. Numerous genes are induced in either the early or late stages of nodule development. Large numbers of expressed sequenced tags (ESTs) are now available for several legumes (Györgyey et al., 2000; VandenBosch and Stacey, 2003; Weidner et al., 2003; Lee et al., 2004). Many of them have been shown to function in signaling, metabolism, and defense during symbiosis, though many others remain to be characterized. For successful symbiotic association, plant defense responses need to be properly regulated (Mithöfer, 2002), but the bacterial signals and their plant counterparts needed to suppress defense responses are not yet known.
Reactive oxygen species (ROS) affect many biological processes, including differentiation, transformation, aging, and programmed cell death (Finkel, 2003; Overmyer et al., 2003; Buchanan and Balmer, 2005). They are produced in several subcellular compartments in response to environmental stresses and affect many important signaling pathways such as those involving mitogen-activated protein kinases. Nitrogen fixation is one of the most significant ROS-producing processes (Becana et al., 2000; Hérouart et al., 2002; Matamoros et al., 2003). An oxidative burst was observed in alfalfa (Medicago sativa) when inoculated with rhizobia (Santos et al., 2001), and cerium labeling of hydrogen peroxide (H2O2) was found in the infection threads as well as in regions around the symbiosomes of senescent infected cells. The antioxidants identified in nodules are ascorbate, glutathione, and homoglutathione (Matamoros et al., 2003; Frendo et al., 2005). Antioxidizing proteins are thought to play important roles in nodule development since antioxidant enzymes such as ascorbate peroxidase, superoxide dismutase (SOD), and catalase are abundant in nodules (Dalton et al., 1998; Moran et al., 2003; Rubio et al., 2004). Ascorbate peroxidase has been localized to the peripheral cell layers of nodules, and when nitrogen fixation was inhibited by exogenous nitrogen, its activity decreased, implying that the enzyme is essential for nitrogen fixation. The amount of FeSOD was found to increase in cowpea (Vigna unguiculata) during nodule development.
Thioredoxins are proteins of approximately 12 kD that function in many regulatory processes, such as responses to oxidative stress, transcription, and translation. (Schurmann and Jacquot, 2000; Buchanan and Balmer, 2005). In plants, there are many types of thioredoxins that vary in their cellular location. The thioredoxin isomers, f and m, are present in chloroplasts, where they regulate chloroplast enzymes, such as several enzymes of the Calvin cycle. Thioredoxin o, a new type of thioredoxin, exists in mitochondria. Recently, a proteomic analysis identified several thioredoxin o-linked proteins as potential substrates of thioredoxin (Balmer et al., 2004). Thioredoxin h, a cytosolic form, is active in oxidative stress, self-compatibility reactions, seed germination, and early seedling development (Besse et al., 1996; Cabrillac et al., 2001; Laloi et al., 2004). All thioredoxins are characterized by a highly conserved motif, -WCGPC-, at the redox active site. Carotenoids are natural pigments that act as antioxidants in biological systems (Niyogi, 1999). They play critical roles in photoprotection, and the seeds of T-DNA insertional mutants of xanthophyll-synthesizing enzymes have reduced levels of β-carotene-derived xanthophylls (Tian et al., 2003). When β-carotene hydroxylase, an enzyme that synthesizes zeaxanthin from β-carotene, was overexpressed in Arabidopsis (Arabidopsis thaliana), stress tolerance was enhanced (Davison et al., 2002).
In this work we isolated genes differentially expressed during nodulation in response to rhizobial inoculation of soybean (Glycine max) roots. Several of the isolated clones were similar to genes related to oxidative stress. Two of them, thioredoxin and β-carotene hydroxylase, were studied further. Their full-length cDNAs were isolated, and the deduced amino acid sequences were homologous to those of other organisms. In situ RNA hybridization with the thioredoxin gene showed that it was expressed in infected cells. The thioredoxin gene had an antioxidant responsive element (ARE)-like site in its 5′ upstream region, and its cDNA conferred tolerance to H2O2 in a yeast thioredoxin mutant. ROS levels were found to decrease as the nodules formed. RNA interference (RNAi)-mediated repression of the thioreoxin gene resulted in impaired development of nodules. These data imply that proteins with antioxidant activity, such as thioredoxin, contribute to nodule development and maintenance of the symbiotic state.
RESULTS
The Expression of Antioxidants during Nodulation
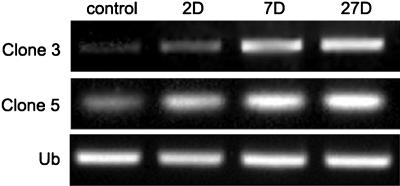
Nodule formation requires the expression of several sets of genes. Although many such genes have been isolated, we thought that novel genes might be identified by a different approach. Soybean roots were axenically grown for 3 d and inoculated with rhizobia (Caetano-Anollés et al., 1992). After 2 d, root segments were cut, their RNA extracted, and fragments of the DNA of differentially expressed genes (DEGs) were obtained with a GeneFishing DEG kit (Seegene). We noted that several clones whose expression was induced were similar to genes related to oxidative stress: thioredoxin, ascorbate peroxidase, β-carotene hydroxylase, and catalase (Table I). We chose to examine the expression of the many clones homologous to the thioredoxin and β-carotene hydroxylase genes by semiquantitative reverse transcription (RT)-PCR, as the corresponding enzymes had not been well characterized in nodules, unlike ascorbate peroxidase and catalase (Matamoros et al., 2003). We used RNA from both 7-d-old and 27-d-old nodules since nodules began to appear 7 d postinoculation (dpi), and 27-d-old nodules seemed to be active in nitrogen fixation (Fig. 1). Both genes were induced 2 dpi and their expression was elevated at both 7 and 27 dpi. This behavior indicates that both play a role in nodulation.
Table I.
Genes induced in soybean roots 2 dpi
| Clone No. | Putative Identity | Strongest BLASTX Hit | Functional Category |
|---|---|---|---|
| 1, 27 | Putative ATP citrate lyase | Rice NP_914078 | Primary metabolism |
| 3, 21 | Thioredoxin | Nicotiana alata AAY42864 | Defense and stress response |
| 4, 5 | β-Carotene hydroxylase | Vitis vinifera AAM77007 | Defense and stress response |
| 7 | Cytosolic ascorbate peroxidase | Vigna unguiculata AAB03844 | Defense and stress response |
| 11 | Putative poly(A)-binding protein (PABP1) | Arabidopsis AAL47336 | Gene expression |
| 12 | Ubiquitin-conjugating enzyme E2 | Cicer arietinum CAR299066 | Protein processing |
| 15 | Ser/Thr kinase | Arabidopsis AAG50566 | Signal transduction |
| 17 | Cold-regulated protein | Triticum aestivum BAC41494 | Defense and stress response |
| 22 | NADH dehydrogenase subunit 4 | Mus musculus NP_904337 | Primary metabolism |
| 35, 42 | Catalase | Campylobacter jejuni CAA59444 | Defense and stress response |
| 55 | Putative 40S ribosomol protein S19 | Cicer arietinum CAA10125 | Protein synthesis |
Figure 1.
Expression of clones homologous to thioredoxin and β-carotene hydroxylase genes during nodulation. Clone 3 is the putative thioredoxin gene and clone 5 the putative β-carotene hydroxylase gene. RNA was extracted from 2-, 7-, and 27-d-old nodules and used as template for semiquantitative RT-PCR. Uninoculated roots served as controls. Ubiquitin primers were used for PCR to ensure use of uniform amounts of templates.
Isolation of Full-Length cDNAs of Soybean Thioredoxin and β-Carotene Hydroxylase
Although the two DNA fragments were very similar to thioredoxin and β-carotene hydroxylase, respectively, their identities needed to be confirmed by cloning full-length cDNAs. The DNA sequence of the thioredoxin fragment contained most of the open reading frame (ORF) but lacked the N-terminal region including the start codon. An EST sequence (GenBank accession no. BE821192) for the N-terminal region of soybean thioredoxin (GmTRX) was obtained from GenBank, and we made the full-length cDNA by RT-PCR with primers based on the EST sequence. The ORF contains 408 nucleotides encoding a protein of 135 amino acids. The conserved motif of the redox active site, WCGPC, is present in GmTRX (indicated by asterisks in Fig. 2A; Buchanan and Balmer, 2005). The putative amino acid sequence for GmTRX, a thioredoxin h, was aligned with those from other species. It has 67% identity to Arabidopsis thioredoxins, 44% to tobacco (Nicotiana tabacum) thioredoxin, and 37% to human and yeast thioredoxins.
Figure 2.
A, Comparison of amino acid sequences of thioredoxins. The asterisks denote the conserved redox active site, and dashes represent gaps introduced to optimize alignment. GenBank accession numbers of the thioredoxins are as follows (in parentheses): Arabidopsis (AraTrx 3, CAA84611), tobacco (tobacco h1, PIR-S16590), human (TRX, PIR-JH0568), and yeast (TRX1p, NP_013144). B, Comparison of amino acid sequences of β-carotene hydroxylases. The asterisks identify conserved motif 1. GenBank accession numbers of the β-carotene hydroxylases are as follows (in parentheses): citrus (AAG33636), Arabidopsis (NP_194300), and rice (CAE03252). The nucleotide sequences have been submitted to GenBank with accession numbers AY575954 (thioredoxin) and AY575953 (β-carotene hydroxylase).
We cloned the full-length cDNA of soybean β-carotene hydroxylase using a CapFishing kit (Seegene). First, cDNAs were synthesized with the oligo(dT)-annealing control primer (ACP) included in the kit. The full-length cDNA was generated with a 5′-RACE primer and a primer specific to the β-carotene hydroxylase gene, according to the manufacturer's instructions. The ORF contains 1,002 nucleotides, and the deduced protein has 334 amino acids with 64% identity to CHX2 (citrus [Citrus unshiu] β-carotene hydroxylase), 57% to OSJNBa (rice [Oryza sativa] β-carotene hydroxylase), and 55% to Arabidopsis β-carotene hydroxylase (Sun et al., 1996). Several regions were found to be conserved, including a termed Motif1 (asterisks; Sun et al., 1996).
Expression of Thioredoxin in Nodules
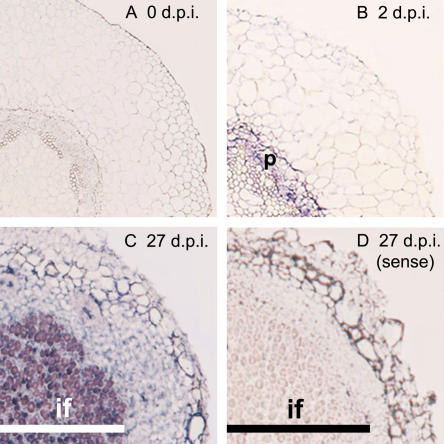
The increased expression of the GmTRX gene during nodulation (Fig. 1) implies that it plays a role in symbiosis. To examine its relevance to symbiosis, we performed in situ RNA hybridization with root nodule sections from different developmental stages (Fig. 3). As shown in Figure 3B, GmTRX was expressed in the pericycle of root segments 2 dpi, and nodule sections at later stages showed intense staining in the central infection zone. However, there was no hybridization with sense probe in 7-d-old (data not shown) or 27-d-old nodules (Fig. 3C). This expression pattern is consistent with the data in Figure 1 and indicates that nodule development may require thioredoxin from the start of nodulation to the nitrogen-fixing stage.
Figure 3.
In situ hybridization of GmTRX during nodulation. A and B, Sections from roots 0 and 2 dpi were hybridized with an antisense thioredoxin riboprobe. C and D, Sections from nodules 27 dpi were hybridized with antisense (C) and sense (D) thioredoxin riboprobes. p, Pericycle; if, infection zone. Bars in C and D indicate the zone of infection of the mature nodules. Data are representative of results obtained in three independent experiments.
Isolation of the GmTRX Promoter
Since expression of GmTRX was observed in the infection zone and it increased during nodule development, we isolated the 5′ upstream region of the gene with the GenomeWalker kit (CLONTECH). Four different sets of GenomeWalker soybean DNA libraries were made and used for two rounds of PCR. Only the PvuII library produced a significant PCR product (0.6 kb) and this was shown to contain part of the GmTRX cDNA sequence. We then made a new primer based on the 5′ sequence from the PCR, to clone a region further upstream. The DraI library then yielded a 1.0-kb PCR band that was sequenced and found to contain an ARE-like site (Rushmore et al., 1991) 490 nucleotides upstream from the translation start site (Fig. 4). This indicates that the GmTRX gene may be induced in response to stress caused by ROS.
Figure 4.
Promoter sequences of GmTRX. The ARE-like site is underlined.
H2O2 Tolerance Analysis with GmTRX
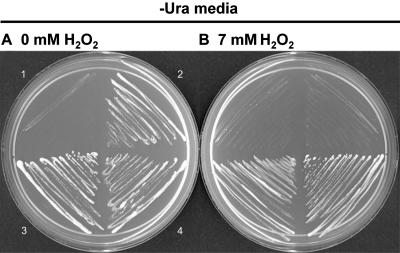
Arabidopsis has at least eight thioredoxin isoforms of the h-type, not all of which are able to complement the yeast thioredoxin mutant (Mouaheb et al., 1998; Bréhélin et al., 2000). As the 5′-upstream sequence of GmTRX contains an ARE-like element, it is highly likely that GmTRX, a thioredoxin h, confers tolerance to H2O2 stress. To test this, the full-length cDNA was introduced into the yeast trx1, trx2 double mutant (strain EMY63). The mutant transformed with the control YCpIF5 plasmid could not grow in the presence of 0.7 mm H2O2, whereas transformants expressing GmTRX under the control of the GAL1 promoter could grow as well as wild-type cells (strain EMY60; Fig. 5).
Figure 5.
H2O2 tolerance of the yeast trx1, trx2 mutant expressing GmTRX. Transformed cells were streaked on SD/Gal/−Ura agar medium containing 0 mm H2O2 (A) and 0.7 mm H2O2 (B). 1, Saccharomyces cerevisae strain EMY63 (thioredoxin double mutant); 2, S. cerevisae strain EMY60 (parent) transformed with YCpIF5; 3, S. cerevisae strain EMY63 transformed with YCpIF5 control; 4, S. cerevisae strain EMY63 transformed with YCpIF5 expressing GmTRX.
ROS Levels during Nodule Development
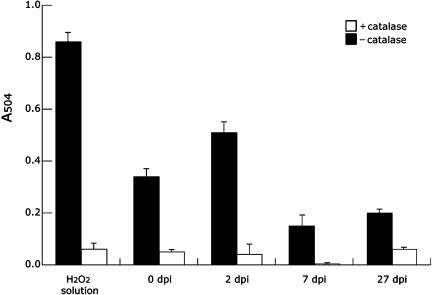
The results so far led us to suppose that antioxidants such as thioredoxin are induced in response to ROS generated during nodulation and confer tolerance to ROS. We therefore harvested root nodules at various developmental times and measured ROS levels. Two-day-old nodules had higher levels of ROS than uninoculated root segments (Fig. 6). Levels of ROS then fell 3- to 5-fold in 7-d-old and 27-d-old nodules, respectively, coincident with the increase in expression of the antioxidants. This suggests that the antioxidants are essential for lowering the level of ROS during nodule development.
Figure 6.
ROS production in nodules 0, 2, 7, and 27 dpi. Data are means and sds of the means of three independent experiments. Black bars show ROS levels in the root/root nodules at different stages of nodule development, whereas the white bars refer to catalase-treated samples as negative controls. The H2O2 sample with and without catalase treatment also served as control. A504, A504.
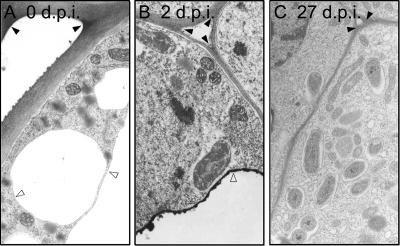
To investigate where ROS were produced, fresh root/nodule tissue was treated with cerium chloride and processed for electron microscopy. We found cerium perhydroxide precipitates at sites of H2O2 production in the cell wall of uninoculated roots (black arrowhead in Fig. 7A), while in 2-d-old nodules some tonoplasts contained precipitates whereas the cell wall did not (white and black arrowheads, respectively, in Fig. 7B). In addition, infection thread matrix and cell wall showed precipitates, as observed in alfalfa (Rubio et al., 2004; data not shown). Nodules 27 dpi did not stain significantly with cerium staining (Fig. 7C), suggesting that levels of ROS are low at that stage. Similarly, the N2-fixing zone (III) of pea (Pisum sativum) nodules did not show much precipitates of cerium perhydroxide, whereas the meristem (I) and invasion (II) zones exhibited large amounts of H2O2 (Rubio et al., 2004). These are in agreement with the ROS measurements in Figure 6.
Figure 7.
Detection of H2O2-producing regions by the formation of cerium perhydroxides in uninoculated roots (A), nodules at 2 dpi (B), and nodules at 27 dpi (C). The black arrowhead indicates the cell wall matrix, and the white arrowhead indicates tonoplast.
Nodule Development on Hairy Roots Containing RNAi Construct of GmTRX
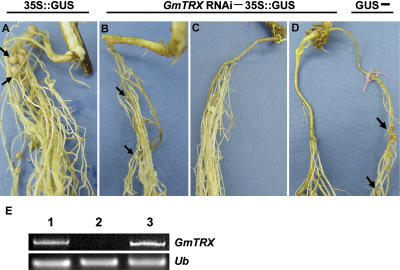
The decline of ROS during nodule development and the concomitant increase of antioxidants (thioredoxin and carotenoids synthesized by β-carotene hydroxylase) led us to consider the possibility that lowering the level of ROS by the antioxidants is essential for nodule development. To explore this possibility, we used RNAi. A DNA fragment containing a GmTRX construct suitable for RNAi was cloned into the β-glucuronidase (GUS) vector, pCAMBIA3301, under the control of the leghemoglobin (Lbc3) promoter (see “Materials and Methods”), and the resulting plasmid was introduced into Agrobacterium rhizogenes. Hairy roots were formed on the soybean using agrobacteria containing the GmTRX RNAi construct, and only transgenic hairy roots (identified by assaying GUS) were inoculated with rhizobia (Cheon et al., 1993). While nodules formed normally on the hairy roots harboring pCAMBIA3301 only (arrows in Fig. 8A), nodule development was severely impaired on the hairy roots containing the GmTRX RNAi construct; in fact most these exhibited poorly developed nodules (arrows in Fig. 8B), others did not form nodules (Fig. 8C), and a few formed nodules of normal size (data not shown). We examined the expression of GmTRX in these nodules by semiquantitative RT-PCR. The poorly developed nodules did not show any expression of GmTRX while the nodules of normal size had normal level of expression (lanes 2 and 3 of Fig. 8E). This shows that nodule development is severely affected when the expression of GmTRX is inhibited. Since we used the leghemoglobin promoter, which is active only in infected cells, to make the GmTRX RNAi construct, the impairment of nodule formation was very evident. In other GmTRX RNAi experiments, we placed GUS-negative (untransformed; tagged with pink thread) hairy root together with GUS-positive hairy root (transgenic; tagged with yellow thread), in order to keep plants alive in case the effect of the GmTRX RNAi construct was too damaging (Fig. 8D). In those cases, nodules formed on GUS-negative hairy root but not on the GUS-positive hairy roots, thus confirming that nodule development is prevented by inhibiting expression of GmTRX. We conclude that the production of antioxidants such as GmTRX is important for nodule development.
Figure 8.
Nodule development on hairy roots expressing GmTRX-RNAi. Hairy roots were formed on soybean roots using A. rhizogenes harboring pCAMBIA3301 only (A) or the GmTRX-RNAi construct cloned into pCAMBIA3301 (B–D). GUS-positive (transgenic) hairy roots were inoculated with Bradyrhizobium japonicum (K599). D, The hairy root tagged with a yellow thread is GUS positive and the other hairy root is GUS negative (untransformed control). E, RT-PCR products obtained from RNA from nodules as follows: lane 1, nodules containing pCAMBIA3301; lanes 2 and 3, poorly developed nodules (lane 2) and normal-sized nodules (lane 3) containing the GmTRX-RNAi construct. Arrows indicate nodules formed on hairy roots. These experiments were repeated three times and representative results are shown.
DISCUSSION
A number of genes are expressed in legumes at different stages of nodulation. In this study we cloned DEGs by spot inoculation (Caetano-Anollés et al., 1992) followed by use of a GeneFishingDEG kit (Seegene). This method is an improvement on differential display for isolating DEGs as it produces PCR products that can be detected on agarose gels (Kim et al., 2004). The identified genes began to be expressed 2 dpi or showed increased expression compared to uninoculated roots at some point (Table I). Several proteins differentially expressed in Medicago nodules (Mathesius et al., 2003) are also encoded by the genes we isolated; these include several genes involved in oxidative stress, an auxin-induced protein, etc. We adopted the method of inoculation developed by Caetano-Anollés et al. (1992), and the similarity of the results obtained may be due to using the same inoculation method and examining the same tissue stages. However, we isolated several different genes in our study, including those for thioredoxin and β-carotene hydroxylase, perhaps because a different detection method was used.
Thioredoxins carry out diverse reactions and are named according to their cellular locations: thioredoxin h for cytosol; thioredoxins f, m, x, and y for chloroplast; and thioredoxin o for mitochondria (Buchanan and Balmer, 2005). The GmTRX gene (GmTRX) isolated in this study is a thioredoxin h and is highly homologous to Arabidopsis thioredoxin h. Among the thioredoxins isolated from Arabidopsis, only AtTRX3 could confer H2O2 tolerance on the yeast thioredoxin mutant, while AtTRX2 enabled the mutant to grow in the presence of organic sulfur (Mouaheb et al., 1998; Bréhélin et al., 2000). These observations led us to test whether GmTRX is involved in H2O2 tolerance. The yeast mutant transformed with GmTRX could grow on H2O2-containing medium (Fig. 5). This indicates that GmTRX could play the role of a cofactor for the peroxiredoxin in reducing ROS, or it itself could reduce proteins oxidized under these conditions, thus permitting the yeast to survive. This idea is supported by the fact that the GmTRX gene has an ARE-like motif in its upstream region (Fig. 4).
A number of functions of thioredoxins have been identified in various organisms (Meyer et al., 1999; Watson et al., 2004). One function is to act as cofactor for the thioredoxin peroxidase that inactivates H2O2. Thioredoxins also reduce glutathione peroxidase and induce manganese SOD. They play a part in DNA repair as cofactors for ribonucleotide reductase and provide electrons for reduction of peptide Met sulfoxide reductase, which reduces Met sulfoxides in various proteins. This action is thought to be involved in protein activation/inactivation and protection of proteins from oxidative damage. Chloroplast cyclophilin, a catalyst of protein folding, is a target of thioredoxin (Motohashi et al., 2003); the peptidyl-prolyl cis-trans isomerase activity of oxidized cyclophilin was restored by reduction of the internal disulfide bond by thioredoxin. In addition, thioredoxins are involved in redox-sensitive signal transduction; many transcription factors respond to H2O2 by forming disulfide bonds through thioredoxin (Buchanan and Balmer, 2005). Ran, a small GTPase active in nuclear transport, is a target of thioredoxin in Chlamydomonas (Lemaire et al., 2004). Thioredoxin is able to bind and inhibit ASK1, a member of the mitogen-activated protein KKK family involved in apoptosis, suggesting that thioredoxin can inhibit this cell death process (Saitoh et al., 1998). Recently, a proteomic approach has been employed to isolate thioredoxin-linked proteins (Balmer et al., 2003, 2004), and identification of such target proteins could throw light on the specific functions of thioredoxins. If the target proteins of GmTRX in nodules could be identified, this might clarify the regulatory role of thioredoxin in nodule formation.
We detected expression of GmTRX in the nodule endodermis and especially in the infected zone. Ascorbate peroxidase, a key enzyme for removing H2O2, has been localized to the endodermis of alfalfa nodules and to cells peripheral to the infected region in soybean (Dalton et al., 1998). It is not clear if this difference in location reflects different antioxidant functions. The roles of other antioxidants and antioxidant enzymes (SOD, catalase, and glutathione reductase) in nodule function have been well characterized (Becana et al., 2000; Hérouart et al., 2002; Matamoros et al., 2003): They prevent ROS accumulation during nodule development, with the result that the level of ROS decreases in mature nodules (Fig. 6). We were unable to detect significant cerium chloride labeling in EM sections of mature nodules, which is consistent with the measurements of ROS, and a similar pattern of cerium chloride labeling was reported in alfalfa nodules (Santos et al., 2001). In addition, expression of a GmTRX RNAi construct severely impaired nodule development. Most hairy roots containing the GmTRX RNAi construct exhibited poorly developed nodules, whereas a few did not form nodules. Since transcripts of soybean lbc3 were detected in root nodules 8 dpi, inhibition of GmTRX expression by RNAi was able to compromise nodule development, leading to the formation of poorly developed nodules (Fig. 8B). Cvitanich et al. (2000) reported the expression of a fusion of Gmlbc3 promoter-gusA in nodule primordia, albeit at low levels. The early expression of GmTRX RNAi, in a few cases, might severely inhibit nodule formation (Fig. 8C). Taken together, our findings indicate that thioredoxin, along with the antioxidants and antioxidant enzymes already characterized, acts to prevent ROS accumulation and to permit symbiotic nodule development. It will be interesting to identify the target proteins of GmTRX in nodules and so clarify how accumulation of ROS is prevented during nodule development.
MATERIALS AND METHODS
Plant, Rhizobia, and Yeast
Soybean (Glycine max) seedlings were sterilized and grown in darkness on moist, absorbent paper at 28°C for 3 d. Three-day-old seedlings were inoculated with rhizobia (Bradyrhizobium japonicum USDA110), which were grown at 28°C for 5 d. Saccharomyces cerevisiae EMY60 is the parent strain (Mata, ade2-1, ade3-100, his3-11, lew2-3, lys2-801, trp1-1, and ura3-1), and S. cerevisiae EMY63 is an isogenic derivative with mutations at the thioredoxin loci (trx1::TRP1, trx2::LEU2; Mouaheb et al., 1998). Both strains were kindly provided by Dr. Y. Meyer (University of Perpignan, France).
Isolation of DEGs with the GeneFishing DEG Kit
RT of total RNA was performed using M-MLV Reverse Transcriptase (Promega), and synthesized cDNAs were amplified by 20 randomly selected arbitrary ACPs in the GeneFishing DEG kit (Seegene). Products that were differentially expressed were selected.
Semiquantitative RT-PCR
The gene-specific primers were designed on the basis of known sequences. Oligonucleotides used were as follows: For the thioredoxin gene, the upstream primer was 5′-TTCAACGAGCTCAAAGAAAG-3′ and the downstream primer 5′-TACAACCCCATCCATCATCA-3′; for the β-carotene hydroxylase gene, the upstream primer was 5′-ATCCTAGCTTGGCATGGTCA-3′ and the downstream primer 5′-TTTTTGGGACCAAAGGAAGT-3′. As a loading control, we used primers specific for ubiquitin (upstream primer, 5′-GGGTTTTAAGCTCGTTGT-3′; downstream primer, 5′-GGACACATTGAGTTCAAC-3′).
Cloning Full-Length cDNAs for GmTRX and β-Carotene Hydroxylase
The full-length cDNA for GmTRX was cloned by RT-PCR using total RNA extracted from 2-d-old soybean root segments after rhizobial infection: The upstream primer was 5′-CACCACCGCGGATCCATGGGCGCT-3′ (the underlined sequence is the BamHI site) and the downstream primer 5′-AAATTGGATTCTAGAGCTAAGATT-3′ (the XbaI site is underlined). The full-length cDNA for soybean β-carotene hydroxylase was cloned using the CapFishing kit (Seegene) according to the manufacturer's protocol. Full-length first-strand cDNA was synthesized with oligo(dT)-ACP, and PCR was performed with the 5′-RACE primer and the gene-specific primer. Primer sequences were as follows: 5′-RACE primer, 5′-GTCTACCGGCATTCGCTTCAT-3′; β-carotene hydroxylase gene-specific primer, 5′-CTCTTCTAGCCCTCCCACTTC-3′.
In Situ RNA Hybridization
Nodules were harvested 2 and 27 d after rhizobial inoculation. Each nodule was processed and hybridized with digoxigenin-labeled antisense and sense RNA probes (Oh et al., 2001) made using the GmTRX cDNA cloned into pSPT19.
Analysis of H2O2 Tolerance
The GmTRX gene was cloned downstream of the GAL1 promoter in YCpIF5. The resulting plasmid was introduced into yeast by the lithium acetate method (Moreschell et al., 1991). Transformed cells were first cultured overnight in YCP medium (1% yeast extract, 2% peptone, and 2% D-Glc) and then diluted into nine volumes of minimal SD medium to induce transcription of the thioredoxin gene from the GAL1 promoter for 6 h. One hundred microliters of each culture was plated on SD agar medium containing various concentrations of H2O2, and incubated for 3 d at 30°C (Mouaheb et al., 1998).
Isolation of the Promoter from Soybean Genomic DNA
Soybean genomic DNA isolated from leaves was digested with DraI, SspI, EcoRV, and PvuII to make GenomeWalker DNA Libraries (CLONTECH) and then ligated with the adaptor provided. PCR was performed with adaptor and gene-specific primers: thioredoxin primer, 5′-GACGCCGAGAAATCTATCACAACGAGCTTA-3′; secondary nested thioredoxin primer, 5′-GAAGAGTGGAAAGACTGGACCCTAGAAGC-3′. The PCR products were cloned into the TOPO vector (Invitrogen) and sequenced.
Determination of ROS
ROS were determined by measuring the conversion of dichlorofluorescin-diacetate to fluorescent dichlorofluorescein (Valkonen and Kuusi, 1997) in a spectrophotometer. After tissue homogenization in liquid nitrogen, ground sample was added to the extraction buffer (0.25 m Suc, 50 mm HEPES, 1 mm EDTA [pH 7.4]). The mixture was centrifuged at 600g for 10 min, and the supernatant was used for measuring the absorbance (OD504) with the Biochrome 4060 (Pharmacia). The OD data were normalized based on protein amount of each sample.
Transmission Electron Microscopy
Root segments or nodules were treated with CeCl3 as described by Bestwick et al. (1997). Tissues were then fixed in 2% glutaraldehyde/4% paraformaldehyde in HEPES buffer (pH 7.4) at room temperature for 2 h. After fixation, they were washed three times for 10 min in 0.065 m HEPES/0.01 m Gly buffer at room temperature and post-fixed for 95 min in 1% osmium tetroxide. Tissues were then washed three times in 0.065 m of HEPES buffer and dehydrated in a graded ethanol series: 50%, 79%, 80%, 90%, and 100% ethanol. Samples were transferred to propylene oxide for 10 min and progressively embedded in Epon. Sections were made and examined with a transmission electron microscope (JEOL 1200EX-II).
Development of Transgenic Root Nodules Containing the GmTRX RNAi Construct
To make the GmTRX RNAi construct, a 270-bp fragment of GmTRX was amplified by PCR using the full-length cDNA as template with primers as follows: TRX-sense forward primer, 5′-ggtccagtctagacactcttcg-3′; TRX-sense reverse primer, 5′-cccacaagcttgtcgacttcc-3′; TRX-antisense forward primer, 5′-tctaggctcgagtctttcc-3′; TRX-antisense reverse primer, 5′-cgcccggtaccttgtcgacttcc-3′. The amplified fragments were inserted into the KpnI/XhoI and HindIII/XbaI sites of pKANNIBAL (Wesley et al., 2001) to generate both sense and antisense orientations of GmTRX. The GmTRX RNAi construct was inserted into the binary vector pCAMBIA3301 containing the 2.7-kb Lbc3 promoter (Cheon et al., 1993) and a PCaMV 35S::GUS cassette. The resulting plasmid was introduced into Agrobacterium rhizogenes (K599) by the freeze-thaw method (Höfgen and Willmitzer, 1988). To produce transgenic soybean hairy roots, hypocotyls of seedlings were infected with A. rhizogenes carrying the GmTRX RNAi construct using 18G needles (Cheon et al., 1993). The hairy roots formed at the infected sites of the soybean roots were examined for GUS activity in order to identify the transgenic hairy roots, and only GUS-positive hairy roots were used for nodule formation.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY575954 (thioredoxin) and AY575953 (β-carotene hydroxylase).
Acknowledgments
We are grateful to Dr. Y. Meyer (University of Perpignan, France) for the yeast thioredoxin mutant, and to Drs. W.D. Bauer and T.L. Graham (Ohio State University, Columbus, Ohio) for comments during the course of this work.
This work was supported by the Technology Development Program of the Ministry of Agriculture and Forestry (grant no. 305005–4); the BioGreen 21 Program, Rural Development Administration (grant no. 20050501034831); and the Crop Functional Genomics Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of the Republic of Korea (grant no. CG1542).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Choong-Ill Cheon (ccheon@sookmyung.ac.kr).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.067884.
References
- Balmer Y, Koller A, del Val G, Manieri W, Schurmann P, Buchanan BB (2003) Proteomics gives insight into the regulatory function of chloroplast thioredoxins. Proc Natl Acad Sci USA 100: 370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer Y, Vensel WH, Tanaka CK, Hurkman WJ, Gelhaye E, Rouhier N, Jacquot J, Manieri W, Schürmann P, Droux M, et al (2004) Thioredoxin links redox to the regulation of fundamental processes of plant mitochondria. Proc Natl Acad Sci USA 101: 2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becana M, Dalton DA, Moran JF, Iturbe-Ormaetxe I, Matamoros MA, Rubio CM (2000) Reactive oxygen species and antioxidants in legume nodules. Physiol Plant 109: 372–381 [Google Scholar]
- Besse I, Wong JH, Kobrehel K, Buchanan BB (1996) Thiocalsin: a thioredoxin-linked, substrate-specific protease dependent on calcium. Proc Natl Acad Sci USA 93: 3169–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréhélin C, Mouaheb N, Verdoucq L, Lancelin J-M, Meyer Y (2000) Characterization of determinants for the specificity of Arabidopsis thioredoxins h in yeast complementation. J Biol Chem 275: 31641–31647 [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Annu Rev Plant Biol 56: 187–220 [DOI] [PubMed] [Google Scholar]
- Cabrillac D, Cock JM, Dumas C, Gaude T (2001) The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 410: 220–223 [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G, Wrobel-Boerner E, Bauer WD (1992) Growth and movement of spot inoculated Rhizobium meliloti on the root surface of alfafa. Plant Physiol 98: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon C-I, Lee N-G, Siddique A-BM, Bal AK, Verma DPS (1993) Roles of plant homologs of Rab1p and Rab7p in the biogenesis of the peribacteroid membrane, a subcellular compartment formed de novo during root nodule symbiosis. EMBO J 12: 4125–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvitanich C, Pallisgaard N, Nielsen KA, Hansen AC, Larsen K, Pihakaski-Maunsbach K, Marcker KA, Jensen EØ (2000) CPP1, a DNA-binding protein involved in the expression of a soybean leghemoglobin c3 gene. Proc Natl Acad Sci USA 97: 8163–8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Joyner SL, Becana M, Ormaetxe II, Chatfield JM (1998) Antioxidant defenses in the peripheral cell layers of legume root nodules. Plant Physiol 116: 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison PA, Hunter CN, Horton P (2002) Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418: 203–206 [DOI] [PubMed] [Google Scholar]
- Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15: 247–254 [DOI] [PubMed] [Google Scholar]
- Frendo P, Harrison J, Norman C, Hernandez Jimenez MJ, Van de Sype G, Gilabert A, Puppo A (2005) Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol Plant Microbe Interact 18: 254–259 [DOI] [PubMed] [Google Scholar]
- Györgyey J, Vaubert D, Jiménez-Zurdo JI, Charon C, Troussard L, Kondorosi A, Kondorosi E (2000) Analysis of Medicago truncatula nodule expressed sequence tags pages. Mol Plant Microbe Interact 13: 62–71 [DOI] [PubMed] [Google Scholar]
- Hérouart D, Bauouin E, Frendo P, Harrison J, Santos R, Jamet A, Van de Sype G, Touati D, Puppo A (2002) Reactive oxygen species, nitric oxide and glutathione: a key role in the establishment of the legume-Rhizobium symbiosis? Plant Physiol Biochem 40: 619–624 [Google Scholar]
- Höfgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16: 9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kwak CI, Gu YY, Hwang IT, Chun JY (2004) Annealing control primer system for identification of differentially expressed genes on agarose gels. BioTechniques 36: 424–434 [DOI] [PubMed] [Google Scholar]
- Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld J-P (2004) The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol 134: 1006–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Hur CG, Oh CJ, Kim HB, Park SY, An CS (2004) Analysis of the root nodule-enhanced transcriptome in soybean. Mol Cells 18: 53–62 [PubMed] [Google Scholar]
- Lemaire SD, Guillon B, Maréchal PL, Keryer E, Miginiac-Maslow M, Decottignies P (2004) New thioredoxin targets in the unicellular photosynthetic eukaryote Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 101: 7475–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M (2003) Biochemistry and molecular biology of antioxidants in the Rhizobia-legume symbiosis. Plant Physiol 133: 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anollés G, Rolfe BG, Bauer WD (2003) Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci USA 100: 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y, Verdoucq L, Vignols F (1999) Plant thioredoxins and glutaredoxins: identitiy and putative roles. Trends Plant Sci 4: 388–394 [DOI] [PubMed] [Google Scholar]
- Mirabella R, Franssen H, Bisseling T (2002) LCO singalling in the interaction between rhizobia and legumes. In D Scheel, C Wasternack, eds, Plant Signal Transduction. Oxford University Press, Oxford, pp 250–271
- Mithöfer A (2002) Suppression of plant defense in rhizobia-legume symbiosis. Trends Plant Sci 7: 440–444 [DOI] [PubMed] [Google Scholar]
- Moran JF, James EK, Rubio MC, Sarath G, Klucas RV, Becana M (2003) Functional characterization and expression of a cytosolic iron-superoxide dismutase from cowpea root nodules. Plant Physiol 133: 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreschell RP, Das G, Sherman F (1991) Transformation of yeast directly with synthetic oligonucleotide. Methods Enzymol 194: 362–369 [DOI] [PubMed] [Google Scholar]
- Motohashi K, Koyama F, Nakanishi Y, Ueoka-Nakanishi H, Hisabori T (2003) Chloroplast cyclophilin is a target protein of thioredoxin. J Biol Chem 278: 31848–31852 [DOI] [PubMed] [Google Scholar]
- Mouaheb N, Thomas D, Verdoucq L, Monfort P, Meyer Y (1998) In vivo functional discrimination between plant thioredoxins by heterologous expression in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 95: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Oh H-S, Son O, Chun JY, Stacey G, Lee M-S, Min K-H, Song E, Cheon C-I (2001) The Bradyrhizobium japonicum hsfA gene exhibits a unique developmental expression pattern in cowpea nodules. Mol Plant Microbe Interact 14: 1286–1292 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Harrison MJ, Udvardi M (2005) Peace talks and trade deals: keys to long-term harmony in legume-microbe symbioses. Plant Physiol 137: 1205–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Brosche M, Kangasjarvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335–342 [DOI] [PubMed] [Google Scholar]
- Rubio MC, James EK, Clemente MR, Bucciarelli B, Fedorova M, Vance CP, Becana M (2004) Localization of superoxide dismutase and hydrogen peroxide in legume root nodules. Mol Plant Microbe Interact 17: 1294–1305 [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB (1991) The antioxidant responsive element. J Biol Chem 266: 11632–11639 [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R, Hérouart D, Sigaud S, Touati D, Puppo A (2001) Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol Plant Microbe Interact 14: 86–89 [DOI] [PubMed] [Google Scholar]
- Schurmann P, Jacquot J-P (2000) Plant thioredoxin systems revisited. Annu Rev Plant Physiol Plant Mol Biol 51: 371–400 [DOI] [PubMed] [Google Scholar]
- Stougaard J (2001) Genetics and genomics of root symbiosis. Curr Opin Plant Biol 4: 328–335 [DOI] [PubMed] [Google Scholar]
- Sun Z, Gantt E, Cunningham FX (1996) Cloning and functional analysis of the beta-carotene hydroxylase of Arabidopsis thaliana. J Biol Chem 271: 24349–24352 [DOI] [PubMed] [Google Scholar]
- Tian L, Magallanes-Lundback M, Musetti V, DellaPenna D (2003) Functional analysis of β- and ɛ-ring carotenoid hydroxylases in Arabidopsis. Plant Physiol 15: 1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen M, Kuusi T (1997) Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum. J Lipid Res 38: 823–833 [PubMed] [Google Scholar]
- VandenBosch KA, Stacey G (2003) Summaries of legume genomics projects from around the globe: community resources for crops and models. Plant Physiol 131: 840–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP (2004) Thioredoxin and its role in toxicology. Toxicol Sci 78: 3–14 [DOI] [PubMed] [Google Scholar]
- Weidner S, Pühler A, Küster H (2003) Genomics insights into symbiotic nitrogen fixation. Curr Opin Biotechnol 14: 200–205 [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590 [DOI] [PubMed] [Google Scholar]