Abstract
DNA replication in higher eukaryotes requires activation of a Cdk2 kinase by Cdc25A, a labile phosphatase subject to further destabilization upon genotoxic stress. We describe a distinct, markedly stable form of Cdc25A, which plays a previously unrecognized role in mitosis. Mitotic stabilization of Cdc25A reflects its phosphorylation on Ser17 and Ser115 by cyclin B–Cdk1, modifications required to uncouple Cdc25A from its ubiquitin–proteasome-mediated turnover. Cdc25A binds and activates cyclin B–Cdk1, accelerates cell division when overexpressed, and its downregulation by RNA interference (RNAi) delays mitotic entry. DNA damage-induced G2 arrest, in contrast, is accompanied by proteasome-dependent destruction of Cdc25A, and ectopic Cdc25A abrogates the G2 checkpoint. Thus, phosphorylation-mediated switches among three differentially stable forms ensure distinct thresholds, and thereby distinct roles for Cdc25A in multiple cell cycle transitions and checkpoints.
Keywords: Cdc25A phosphatase/G2 checkpoint/mitosis/phosphorylation/protein degradation
Introduction
The molecular mechanisms underlying cell multiplication and maintenance of genomic integrity require precise timing, velocity and spatial distribution of phosphorylation events carried out by cyclin-dependent kinases (CDKs) (Nigg, 1995; Nurse et al., 1998; Zhou and Elledge, 2000). Owing to their central role in such fundamental biological processes, CDKs are subject to complex regulation in response to signals from the extracellular environment or cell cycle checkpoints (Morgan, 1995). One important mode of CDK control reflects phosphorylation of the conserved N-terminal ATP-binding region (Lew and Cornbluth, 1996). Phosphorylations of Tyr15 by the Wee1 kinase, and Thr14 by Myt1, hinder phosphate transfer to CDK-bound substrates and ATP binding to CDKs, respectively (Atherton-Fessler et al., 1993; Booher et al., 1997). Conversely, activation of CDKs requires dephosphorylation of Thr14 and Tyr15 by Cdc25 phosphatases (Nilsson and Hoffmann, 2000). The balance between the inhibiting and activating effects on CDKs, mediated by Wee1/Myt1 and Cdc25, respectively, determines key cellular decisions such as cell cycle arrest in response to DNA damage (Zhou and Elledge, 2000; Kastan, 2001).
In mammals, the Cdc25 family includes three homologs, Cdc25A, B and C (Sadhu et al., 1990; Galaktionov and Beach, 1991; Nagata et al., 1991). Cdc25B and C are regarded as mitotic regulators (Nilsson and Hoffmann, 2000) and serve as targets of checkpoint pathways that delay entry into mitosis in response to DNA damage or stalled replication (Peng et al., 1997; Kumagai et al., 1998; Bulavin et al., 2001). Cdc25B probably represents a ‘starter’ phosphatase that triggers the initial activation of mitosis-promoting CDKs including cyclin B–Cdk1, which in turn activate Cdc25C and create a positive amplification loop required for irreversible commitment to mitosis (Nilsson and Hoffmann, 2000).
Cdc25A appears to regulate earlier cell cycle transitions. Both c-Myc and E2F transcription factors stimulate Cdc25A expression in mid to late G1 (Galaktionov et al., 1996; Vigo et al., 1999) and, through activation of cyclin E(A)–Cdk2 complexes (Hoffmann et al., 1994; Blomberg and Hoffmann, 1999), Cdc25A induces S-phase entry. Microinjection of Cdc25A antibodies or transfection of Cdc25A antisense oligonucleotides arrests cells in G1 (Hoffmann et al., 1994; Jinno et al., 1994; Vigo et al., 1999; Cangi et al., 2000). Conversely, overexpressed Cdc25A shortens G1 and prematurely activates Cdk2 (Blomberg and Hoffmann, 1999; Sexl et al., 1999). The role of Cdc25A in initiation of DNA replication is also consistent with the ubiquitin–proteasome-mediated destruction of Cdc25A in G1- and intra-S-phase checkpoint responses to DNA damage and replicational stress (Mailand et al., 2000; Molinari et al., 2000; Falck et al., 2001). This requires Chk1- or Chk2-mediated phosphorylation of Cdc25A, and phosphorylation of Ser123 is a prerequisite for such accelerated ubiquitylation and degradation (Mailand et al., 2000; Falck et al., 2001). Consequently, the absence of Cdc25A in damaged cells precludes dephosphorylation of Thr14 and Tyr15 of Cdk2, and locks this essential S-phase-promoting kinase in its inactive form. In Xenopus laevis, an analogous checkpoint mechanism inhibits Cdk2 and disallows loading of Cdc45, an essential component of the pre-replicative complexes, onto replication origins, thereby preventing initiation of DNA replication (Costanzo et al., 2000).
Despite recent progress, our understanding of overlapping and unique roles for the Cdc25 phosphatases at various cell cycle transitions is incomplete. One crucial question has emerged from a finding that homozygous disruption of Cdc25C in mice yielded healthy animals whose cells suffered no mitotic defect, had no alteration of Cdk1 phosphorylation and responded normally to DNA damage (Chen et al., 2001). What then performs the function of what has been widely regarded as a ‘master regulator’ of mitosis? Cdc25B is a plausible candidate, although the fact that this phosphatase is destabilized by cyclin A–Cdk2 (Baldin et al., 1997), highly active in G2, indicates that Cdc25B may not be the complete answer. Unexpectedly, during our studies of protein turnover of human Cdc25 phosphatases, we found that Cdc25A stability undergoes dramatic changes at the G2/M transition. Thus, Cdc25A became abruptly stabilized upon entry into mitosis and contributed to the cellular phosphatase pool required to dephosphorylate Cdk1 fully. In addition, DNA damage-induced inhibition of mitotic entry was accompanied by destruction of Cdc25A, an event required for the productive G2/M arrest. Our results point to novel functions and regulatory modes of Cdc25A, and highlight a concept of phosphorylation-mediated switches among multiple, differentially stable states of a cell cycle-regulatory protein as a flexible way to re-set its activity thresholds under diverse biological conditions.
Results
Cdc25A is stabilized in mitosis
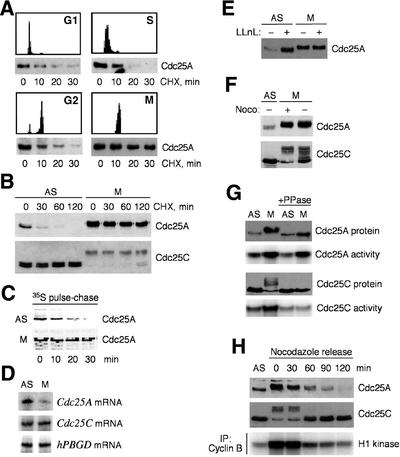
To study mechanisms that determine the abundance of Cdc25A, we measured the stability of the endogenous protein in synchronized U-2-OS cells. Short inhibition of protein synthesis by cycloheximide showed that in late G1, S and G2, Cdc25A was degraded with a half-life of ∼20 min (Figure 1A). In contrast, Cdc25A became remarkably stabilized in purified mitotic cells synchronized by the microtubule-depolymerizing drug nocodazole (Figure 1A), as judged from the extended half-life of well over 2 h (Figure 1B). Cdc25C, on the other hand, remained stable under all conditions (Figure 1B). Pulse–chase measurement of the radioactively labeled proteins confirmed a rapid turnover of Cdc25A in asynchronously growing U-2-OS cells and its marked stabilization in cells arrested in mitosis (Figure 1C). Since Cdc25A has been regarded as a G1/S regulator, its accumulation and stabilization in mitosis were surprising and inspired us to elucidate this phenomenon.
Fig. 1. Mitotic stabilization of Cdc25A. (A) Cdc25A is labile in interphase and stabilized in mitosis. U-2-OS cells were released from a double thymidine block for 4, 9 or 18 h to obtain cells in S, G2 or G1 phase, respectively, or arrested in prometaphase (M) by treatment with nocodazole. After addition of cycloheximide (CHX), cells were harvested at the indicated times and analyzed for Cdc25A levels by western blotting. (B) Differential stability of Cdc25A in asynchronous (AS) versus mitotic (M, purified by shake-off after nocodazole treatment) cells, compared with Cdc25C control, in an extended CHX experiment analogous to that in (A). (C) Pulse–chase measurement of the Cdc25A protein turnover in exponentially growing (AS) and nocodazole-arrested M U-2-OS cells labeled with [35S]methionine. (D) Abundance of Cdc25A, Cdc25C and a control hPBGD mRNA, determined by RT–PCR of poly(A)+ RNA from asynchronous or mitotic cells. (E) Interphase, but not mitotic, Cdc25A accumulates after proteasome inhibition. Asynchronous or mitotic cells were treated with LLnL (25 µM) for 6 h, lysed and analyzed for Cdc25A by western blotting. (F) Cdc25A is hyperphosphorylated in mitosis independently of nocodazole treatment. Mitotic cells were obtained by shake-off after nocodazole (+), or upon release for 14 h from a double thymidine block (–). Cdc25A was analyzed by western blotting. (G) M-phase-specific phosphorylation regulates Cdc25C, but not Cdc25A, activity. Cdc25 proteins were immunoprecipitated from asynchronous or mitotic cells, left untreated or dephosphorylated with λ phosphatase (PPase) and assayed for Cdc25 phosphatase activity. (H) Cdc25A is destabilized after release from metaphase arrest. Cells were released from a nocodazole block by replating into a drug-free medium. At the indicated times, cell lysates were processed for western blotting or cyclin B–Cdk1 activity measurement.
First, we excluded that the mitotic accumulation of Cdc25A reflected increased transcription. In fact, a semi-quantitative RT–PCR analysis showed that the Cdc25A (but not Cdc25C) mRNA was downregulated in nocodazole-arrested cells (Figure 1D), consistent with Cdc25A gene expression being positively regulated by E2F (Vigo et al., 1999), and with E2F silencing upon S-phase exit (Krek et al., 1994). Secondly, while treatment of asynchronous U-2-OS cells with a proteasome inhibitor stabilized the endogenous Cdc25A protein, it did not have any effect on Cdc25A in nocodazole-arrested cells (Figure 1E), suggesting that the mitotic form of Cdc25A is already fully stable. Finally, we found that the Cdc25A protein was also highly elevated in cells naturally progressing through mitosis (Figure 1F), eliminating the possibility that the observed stabilization could be a consequence of any side effects elicited by nocodazole. We conclude that the accumulation of human Cdc25A in mitosis reflects its protein stabilization.
Next, we determined whether the stabilized form of Cdc25A retains its activity. Indeed, in vitro phosphatase assays showed that Cdc25A immunoprecipitated from mitotic cells was highly active, as was the known regulator of mitosis, Cdc25C (Figure 1G). Like Cdc25C, the mitotic Cdc25A was modified, as indicated by its retarded electrophoretic migration (Figure 1B and E–H). Treatment of immunopurified Cdc25A and C with λ phosphatase reverted their migration back to the pattern seen in interphase cells (Figure 1G), indicating that their M-phase shifts were due to phosphorylation. Despite these similarities, at least three pieces of evidence suggest that the regulation of Cdc25A and C in mitosis differs profoundly. First, while the activity of Cdc25C was strictly associated with its shifted, phosphorylated form, Cdc25A was already active during the interphase (Figure 1G; data not shown). Secondly, dephosphorylation by the λ phosphatase completely inhibited the mitotic increase in Cdc25C activity, while it had little effect on the activity of the mitotic Cdc25A (Figure 1G). Thirdly, physiological dephosphoryl ation upon exit from mitosis correlated with gradual and complete destruction of the Cdc25A protein, but did not alter the abundance of Cdc25C (Figure 1H). Together, these data suggest that in addition to activating Cdc25C, the mitosis-specific phosphorylation helps to build up the total activity of Cdc25A through its stabilization.
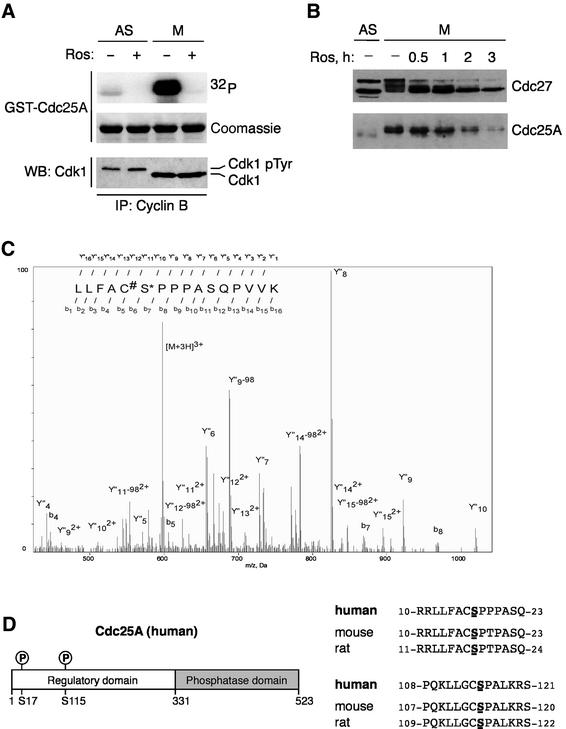
Mitotic Cdc25A is stabilized via cyclin B–Cdk1-mediated phosphorylation of Ser17 and Ser115
The mobility shift and the appearance of stable Cdc25A coincided with the peak of activity of cyclin B–Cdk1, the main mitosis-promoting kinase (Figure 1G). Two additional results supported the link between cyclin B–Cdk1 and phosphorylation-dependent stabilization of Cdc25A. First, immunopurified cyclin B–Cdk1 phosphorylated glutathione S-transferase (GST)–Cdc25A, resulting in a mobility shift similar to that observed on endogenous Cdc25A in mitosis (Figure 2A). This was completely reversed by roscovitine (Meijer et al., 1997), an inhibitor of Cdk2 and Cdk1 (Figure 2A). Secondly, if the stability of the mitotic Cdc25A was dependent on CDK-mediated phosphorylation, inhibition of Cdk1 should destabilize Cdc25A. Indeed, treatment of nocodazole-arrested cells with roscovitine reduced the mitosis-specific mobility shift of Cdc25A and led to a rapid disappearance of the endogenous Cdc25A protein with kinetics closely following those of dephosphorylation of known targets of cyclin B–Cdk1 such as Cdc27 (Figure 2B; data not shown). The major target of roscovitine under these conditions must have been cyclin B–Cdk1 because the other plausible candidates, cyclin A–Cdk2 or cyclin A–Cdk1, are absent in nocodazole-arrested cells due to cyclin A degradation (den Elzen and Pines, 2001; Geley et al., 2001).
Fig. 2. Cyclin B–Cdk1-targeted phosphorylation sites of Cdc25A. (A) Cyclin B–Cdk1 phosphorylates Cdc25A in vitro. Cyclin B–Cdk1 immunopre cipitated (IP) from asynchronous or nocodazole-arrested cells was assayed using full-length GST–Cdc25A as substrate. Ros, roscovitine; WB, western blot; Cdk1pTyr, inactive, Tyr15-phosphorylated Cdk1. (B) Inhibition of cyclin B–Cdk1 destabilizes Cdc25A in mitosis. Mitotic cells prepared by nocodazole treatment and shake-off were treated with roscovitine for the indicated times. Cdc25A and Cdc27 protein levels and SDS gel migration were analyzed by western blotting. (C) Mass spectrometric identification of Ser17 as an in vivo phosphorylation site on Cdc25A. Nanoelectrospray tandem mass spectrum of a tryptic peptide derived from the in-gel digestion of CDC25A from mitotic cells. Collision fragmentation of triply charged precursor ion at m/z 597.588 led to overlapping series of singly Y′′ and doubly Y′′2+ charged ions. The partial sequences were determined and sequence tags assigned to both Y′′ and Y′′2+ series allowing identification of CDC25A peptide and localization of a phospho-group on Ser17. The peptide sequence and localization of phospho-serine residue were confirmed by the existence of partial b and Y′′-98 ion series, corresponding to the loss of phosphoric acid from fragment ions containing Ser17. (D) Ser17 and Ser115 of Cdc25A are conserved across species.
To identify the residues of Cdc25A targeted by cyclin B–Cdk1 in vivo, we subjected the Cdc25A protein, isolated from either asynchronous or nocodazole-arrested cells, to mass spectrometry. To obtain enough material for this analysis, we employed our U-2-OS cell line conditionally expressing ectopic Cdc25A (Mailand et al., 2000). Mass spectrometry analysis identified the peptides 11RLLFACS*PPPASQPVVK27, 12LLFACS*PPPASQPV VK27, 111LLGCS*PALK119 and 278SQEES*PPGSTK288 as phosphorylated in mitotic cells (Figure 2C). Comparisons of CDC25A from synchronized and non-synchronized cells showed that phosphorylation on Ser17 and Ser115 was specific to mitotic cells. The regions surrounding these serines are highly homologous in human, mouse and rat proteins (Figure 2D), suggesting that modification of Ser17/Ser115 by cyclin B–Cdk1 could represent a conserved regulatory mechanism.
Mutation of Ser17/Ser115 abolishes Cdc25A phosphorylation in vitro, and restores ubiquitylation and instability of the mitotic Cdc25A in vivo
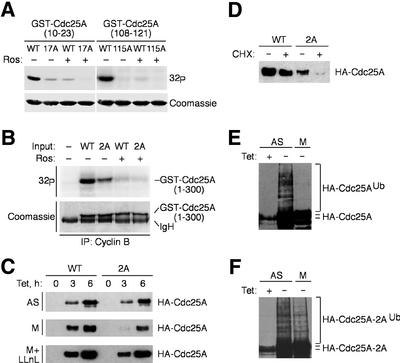
To test whether the identified serines were phosphorylated in mitosis, we purified, from bacteria, short GST-tagged fragments of Cdc25A spanning Ser17 and Ser115, respectively, and their derivatives where the serines were substituted by alanines. While both fusion proteins with the wild-type serines were phosphorylated efficiently by cyclin B–Cdk1, the alanine mutants remained virtually unmodified (Figure 3A). Furthermore, concomitant mutations of Ser17 and Ser115 in the context of the entire regulatory domain of Cdc25A containing most of the CDK consensus sites also impaired (although did not completely abolish) its phosphorylation by cyclin B–Cdk1 in this in vitro kinase assay (Figure 3B).
Fig. 3. Phosphorylation by cyclin B–Cdk1 uncouples Cdc25A from ubiquitylation and degradation. (A) Cyclin B–Cdk1 phosphorylates Cdc25A on Ser17 and Ser115 in vitro. Cyclin B–Cdk1 was immunoprecipitated from mitotic U-2-OS cells and assayed using the indicated GST-tagged fragments of Cdc25A containing Ser17 or Ser115, or alanine substitutions as substrates, with or without roscovitine (Ros). (B) Reduced phosphorylation of the Ser17/Ser115(2A) mutant in the context of the entire regulatory region of Cdc25A. Experimental conditions were as in (A); incorporation of 32P was reduced to ∼55% in the 2A mutant. (C) U-2-OS T-Rex cells were transfected with plasmids encoding wild-type Cdc25A or the Ser17/Ser115(2A) mutant, treated with nocodazole (M) or left asynchronous (AS), and induced by addition of tetracycline for 3 or 6 h. Where indicated, LLnL was added to the medium at the time of the transgene induction. Cdc25A proteins were analyzed by western blotting. (D) Destabilization of Cdc25A(2A) in mitosis. U-2-OS T-Rex cells were treated as in (C), followed by addition of cycloheximide for 2 h, and Cdc25A was assessed by western blotting. (E) Cdc25A is not ubiquitylated in mitosis. Asynchronous (AS) or mitotic (M) U-2-OS/B3C4 cells transiently transfected with His-ubiquitin were kept uninduced or induced to express ectopic Cdc25A for 12 h as indicated, and processed for detection of Cdc25A-associated ubiquitin (Ub) conjugates. (F) Mutation of the cyclin B–Cdk1-targeted sites in Cdc25A restores its ubiquitylation in mitosis. U-2-OS/Cdc25A(2A) cells were treated and analyzed as in (E).
Next, we substituted both Ser17 and Ser115 with alanines in the full-length Cdc25A and generated U-2-OS clones conditionally expressing the wild-type or the double alanine (2A) mutant of Cdc25A in a tetracycline-dependent manner. Upon induction in asynchronous cells, both forms of Cdc25A accumulated with very similar kinetics (Figure 3C). Strikingly, when induced in nocodazole-arrested cells, the phosphorylation-deficient Cdc25A(2A) mutant accumulated more slowly than the wild-type protein (Figure 3C), a difference abolished in the presence of the proteasome inhibitor N-acetyl-Leu-Leu-norleucine (LLnL) (Figure 3C). These results are consistent with the interpretation that the cyclin B–Cdk1-mediated phosphorylation of Ser17/Ser115 is instrumental for stabilization and accumulation of Cdc25A specifically in mitotic cells. This conclusion was also supported by direct protein stability estimation, as wild-type Cdc25A was stable in mitosis, while the Cdc25A(2A) mutant (but not the one where both serines were substituted by acidic, and presumably phospho-mimicking, amino acids) was turned over rapidly (Figure 3D; data not shown).
Finally, if the lack of Ser17/Ser115 phosphorylation indeed caused destruction of Cdc25A in mitosis, it should be reflected by the increased polyubiquitylation of the mutated protein, a prerequisite for targeting of many proteins by the proteasome (Hershko and Ciechanover, 1998). An established in vivo assay (Treier et al., 1994) showed that wild-type Cdc25A was ubiquitylated in asynchronous cells but not in mitosis (Figure 3E). In contrast, Cdc25A(2A) also became polyubiquitylated in mitotic cells (Figure 3F). These data demonstrate that the mitotic phosphorylation of Ser17/Ser115 uncouples Cdc25A from the ubiquitin–proteasome-mediated degradation and leads to accumulation of an active Cdc25A phosphatase.
Cdc25A activates cyclin B–Cdk1 and is rate limiting for the G2/M transition
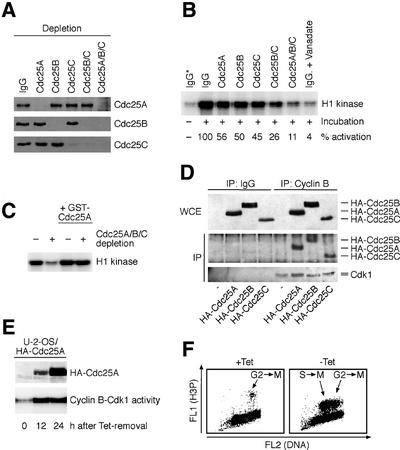
Next, we asked what is the purpose of preserving the activity of Cdc25A at and beyond the G2/M transition. It is well known that the sharp increase of cyclin B–Cdk1 activity early in mitosis is essential for proper execution of mitotic events (Nigg, 2001). We reasoned that Cdc25A may help to generate and/or maintain the threshold required for mitosis-promoting CDK activity once cells become committed to cell division. To address this issue, we first immunodepleted each member of the Cdc25 family (Figure 4A) and assayed the ability of the resulting cell extracts to activate cyclin B–Cdk1 in vitro. Depletion of each individual Cdc25 phosphatase reduced the total cellular cyclin B–Cdk1-activating potential to ∼50% (Figure 4B). Combined depletion of Cdc25B and C was more effective, yet a complete inhibition, mimicking the effect of the phosphatase inhibitor, sodium vanadate, required depletion of all three Cdc25s (Figure 4B). Re-addition of purified GST–Cdc25A into the cell extracts depleted for all the Cdc25s substantially restored the lysate’s capacity to activate the cyclin B–Cdk1 kinase complex (Figure 4C). These data suggest that Cdc25A, together with Cdc25B and C, jointly generate a cellular phosphatase pool required for full activation of cyclin B–Cdk1.
Fig. 4. Cdc25A activates cyclin B–Cdk1 and promotes mitotic entry. (A) Western blot-verified depletion of Cdc25A, B and C from U-2-OS cells lysed in a kinase buffer. Individual and/or the indicated combinations of Cdc25 proteins were depleted by specific antibodies or control non-specific immunoglobulin (IgG). (B) Cdc25 isoforms contribute to activation of cyclin B–Cdk1. Cell lysates were prepared and depleted as in (A), supplemented with 10 mM EDTA to inhibit endogenous kinases and incubated for 20 min at 30°C. Cyclin B–Cdk1 was then immunoprecipitated (IP) and its activity measured using histone H1 as a substrate. Sodium vanadate was added into the control reaction to inhibit all Cdc25 phosphatase activity. Numbers indicate the extent (%) of cyclin B–Cdk1 activation relative to the depletion with non-specific immunoglobulin (asterisk: control reaction where the lysate was not incubated at 30°C). (C) U-2-OS cell lysates were depleted with non-specific (–) or Cdc25A/B/C antibodies (+) as in (A) and incubated for 20 min at 30°C in the presence of 10 mM EDTA. Where indicated, 100 ng of purified GST–Cdc25A was added to the reaction. (D) Cyclin B–Cdk1 interacts with all Cdc25 family members. U-2-OS cells were transfected with the indicated plasmids (bottom) and the presence of HA-tagged Cdc25s and Cdk1 was analyzed in anti-cyclin B immunoprecipitates (IgG, non-specific control antibody). (E) U-2-OS/HA-Cdc25A cells were induced to express the transgene, and histone H1 kinase activities were measured in lysates harvested at the indicated times. (F) Elevated Cdc25A induces premature mitotic entry. U-2-OS/B3C4 cells were arrested in S phase by a double thymidine block, released and induced to express ectopic Cdc25A (–Tet) or kept uninduced (+Tet). After 5 h, cells were analyzed by flow cytometry for phospho-histone H3, a marker of productive entry into mitosis. About 60% of the phospho-H3-positive cells also displayed other morphological signs of mitosis such as condensed chromatin.
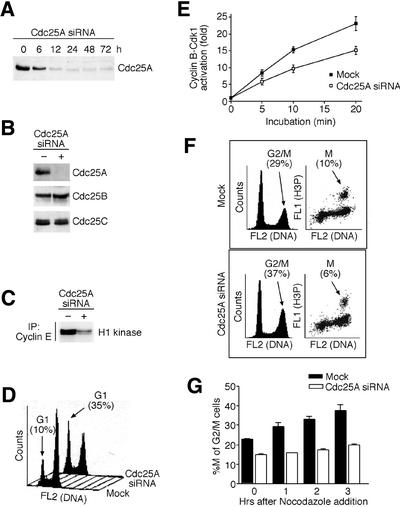
Such a conclusion predicts that Cdc25A can activate cyclin B–Cdk1 in vivo and thereby modulate G2/M progression. Indeed, conditionally elevated Cdc25A bound physically to cyclin B and Cdk1 in vivo in a manner comparable with Cdc25B and C (Figure 4D), and increased the endogenous cyclin B–Cdk1 kinase activity (Figure 4E). Consistent with activation of cyclin B–Cdk1, transient induction of wild-type Cdc25A accelerated entry into mitosis from G2, and induced premature mitosis in cells with incompletely replicated DNA (Figure 4F), which indicated (but did not prove) that Cdc25A might collaborate with Cdc25B and C to control G2/M transition. To address this issue directly, we employed RNA interference (RNAi) technology and found that quantitative and specific downregulation of endogenous Cdc25A by short inhibitory RNA (siRNA) duplexes (Figure 5A and B) not only inhibited the cyclin E–Cdk2 kinase activity (Figure 5C) and S-phase entry (Figure 5D), as expected, but also impaired a full-scale activation of the cyclin B–Cdk1 kinase complex (Figure 5E) and delayed the G2/M transition (Figure 5F and G). Remarkably, the G2/M delay in cells lacking endogenous Cdc25A occurred in spite of the fact that the levels and activities of Cdc25B and Cdc25C remained unchanged (Figure 5B; data not shown). Thus, apart from its S-phase-promoting effect, Cdc25A physically and functionally interacts with the main mitosis-promoting cyclin–CDK complex and generates a rate-limiting stimulus for the G2/M transition, and the lack of its activity can delay completion of the cell division cycle.
Fig. 5. Downregulation of Cdc25A by siRNA impairs both G1/S and G2/M transitions. (A) HeLa cells were transfected with Cdc25A siRNA duplexes, and the level of Cdc25A determined by western blotting at the indicated times after transfection. (B) Cdc25A-specific siRNA does not affect the levels of Cdc25B and C. HeLa cells were treated as in (A) and assayed by western blotting 24 h after transfection for the indicated Cdc25 proteins. (C) Downregulation of cyclin E–Cdk2 activity in cells treated with Cdc25A siRNA. HeLa cells were treated as in (A) and the cyclin E-associated histone H1 kinase activity was measured 24 h after transfection. (D) siRNA-mediated downregulation of endogenous Cdc25A delays G1/S transition. HeLa cells were transfected with Cdc25A siRNA for 24 h, treated with nocodazole for an additional 12 h to prevent re-entry of the transfected cells into G1, and analyzed by flow cytometry. The numbers indicate the G1 fractions in mock- and Cdc25A siRNA-treated cells, respectively, and demonstrate a substantial retention of the latter in G1. (E) Lysates from Cdc25A siRNA-treated cells show attenuated cyclin B–Cdk1 activation. HeLa cells were treated as in (A). After 24 h, the cell lysates were prepared and induced to activate cyclin B–Cdk1 as in Figure 4B for the indicated times. Results of two independent experiments are shown. (F) siRNA-mediated downregulation of endogenous Cdc25A impairs G2/M transition. HeLa cells were transfected as in (E) but exposed only to a short (2 h) pulse of nocodazole to prevent mitotic exit of cells acutely progressing through the G2/M transition. Subsequently, the cells were analyzed by flow cytometry, for either DNA content alone (left panels) or DNA content together with the phospho-histone H3 fluorescence associated specifically with mitotic cells (right panels). Numbers indicate the total percentages of cells in G2/M and pure mitotic (M) compartments, respectively, and show that despite a higher overall G2/M population, the Cdc25A siRNA-treated cells were impaired to proceed beyond this transition into mitosis. (G) Quantification of two independent experiments described in (F) and performed after various times of nocodazole treatment as indicated. The results are presented as a percentage of purely mitotic cells (M) from the total amount of G2/M cells at each time point. The lack of increase of this ratio in the Cdc25A siRNA-treated cells reflects their arrest at the G2/M boundary.
Destruction of Cdc25A is required to prevent entry into mitosis in response to DNA damage
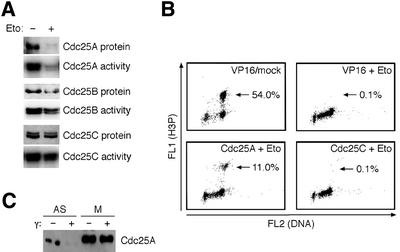
The above data implicate Cdc25A as a potential target of the checkpoint pathways that delay entry into mitosis under various stress conditions. To test this possibility, we transiently treated U-2-OS cells with the topoisomerase II inhibitor etoposide, a drug known to elicit a robust G2 arrest (Kaufmann and Kies, 1998). Exposure to etoposide caused rapid and quantitative destruction of the Cdc25A protein and loss of its phosphatase activity (Figure 6A). While Cdc25B was also downregulated (although to a much lower extent than Cdc25A), Cdc25C remained unchanged (Figure 6A), indicating that neither the protein level nor the activity of Cdc25C represents a major target in cellular response to topoisomerase poisons. Addition of either the proteasome inhibitor LLnL or caffeine, which inhibits the ATM/ATR kinases (Blasina et al., 1999; Sarkaria et al., 1999), rescued the downregulation of Cdc25A in the etoposide-treated cells (data not shown). Thus, similarly to cells exposed to genotoxic stress in late G1 or S phase (Mailand et al., 2000; Falck et al., 2001), the G2 checkpoint was also accompanied by the accelerated proteolysis of Cdc25A.
Fig. 6. Cdc25A degradation in response to DNA damage in G2. (A) Etoposide-induced G2/M arrest causes loss of Cdc25A protein and associated activity. Asynchronous U-2-OS cells were treated with etoposide for 12 h to arrest cells in G2, and levels and activities of Cdc25A, B and C were determined. (B) Overexpression of Cdc25A but not Cdc25C compromises the G2/M checkpoint. Etoposide was added to asynchronous Cdc25A- and Cdc25C-inducible, or parental U-2-OS/TA cells. After 4 h, nocodazole was added to the medium and cells concomitantly were induced to express the transgenes. After an additional 16 h, cells were analyzed by flow cytometry for phospho-histone H3. Following Cdc25A induction, 72% of the phospho-H3-positive cells also showed signs of premature chromosome condensation. (C) The mitotic form of Cdc25A is not destabilized by DNA damage. Asynchronous or mitotic U-2-OS cells were γ-irradiated (10 Gy), and Cdc25A analyzed by western blotting 1 h later.
To test whether the Cdc25A degradation was essential for the G2 checkpoint, we exposed the U-2-OS clones conditionally expressing Cdc25A to etoposide. In control experiments, the parental U-2-OS/TA or its derivative overexpressing Cdc25C responded to etoposide by a robust cessation of mitotic entry (Figure 6B). In contrast, short pre-induction of Cdc25A to the levels sufficient to compensate its degradation (Falck et al., 2001) caused partial but reproducible abrogation of the G2 checkpoint and resulted in reduced but steady progression of the damaged cells into mitosis (Figure 6B). Strikingly, the mitotic, cyclin B–Cdk1-phosphorylated form of Cdc25A was stable even after exposure to relatively high doses of ionizing radiation (Figure 6C). This indicates that the stabilizing phosphorylations (including those on Ser17/Ser115 identified here) are dominant and sufficient to uncouple Cdc25A from the regulatory mechanisms, which normally determine the basic interphase turnover as well as the accelerated proteolysis in response to DNA damage.
Discussion
Phosphorylation-dependent regulation of Cdc25A at the G2/M transition
Arguably the most unexpected finding of this study was that entry into mitosis is accompanied by an abrupt switch, which converts Cdc25A from a labile to a stable protein. Hence, while acceleration of the basal Cdc25A protein turnover participates in response to genotoxic stress or stalled replication in interphase (Mailand et al., 2000; Molinari et al., 2000; Falck et al., 2001), uncoupling of Cdc25A from the ubiquitin–proteasome-dependent degradation plays an important role once the cells become committed to undergo cell division. Whereas destruction of the phosphatase guards against premature mitosis of cells with an incompletely replicated and/or damaged genome, stabilization of Cdc25A ensures that even in the absence of de novo mRNA synthesis, Cdc25A is abundant and active during initial stages of mitotis. Both reduction and extension of the Cdc25A protein half-life rely on phosphorylations within its N-terminal regulatory region. We have reported previously that in S-phase cells, phosphorylation of Ser123 by the ATM–Chk2 kinase cascade is required to induce degradation of Cdc25A in response to ionizing radiation (Falck et al., 2001). Here we show that such a mechanism may also operate in the DNA damage-induced G2 checkpoint. We further show that the mitotic stabilization of Cdc25A also reflects site-specific phosphorylation. Both Ser17 and Ser115 fulfill the minimum requirements for a CDK consensus site (Nigg, 1995), and we demonstrate that phosphorylation of Ser17 and Ser115 is mediated by cyclin B–Cdk1 in vitro, and is detectable in vivo only after entry into mitosis when the cyclin B–Cdk1 complex becomes physiologically active. Although the integrity of Ser17 and Ser115 appears crucial for Cdc25A stabilization, our data do not exclude the potential involvement of other residues. Thus, while concomitant mutation of Ser17 and Ser115 does confer marked instability in mitosis, the protein turnover of such a Cdc25A(2A) mutant could still be accelerated moderately by roscovitine, and the Ser17/Ser115-deficient protein still undergoes a partial mitosis-associated electrophoretic mobility shift (our unpublished observations). This indicates that cyclin B–Cdk1 might phosphorylate more residues than those identified by our mass spectrometry analysis. Moreover, the fact that the endogenous Cdc25A protein remains partly shifted in roscovitine-treated mitotic cells (Figure 2B) suggests that other kinases (such as Plk1), specifically activated at the G2/M transition (Nigg, 2001), assist in inducing and/or maintaining the stability of Cdc25A.
The role(s) for Cdc25A in G2/M control
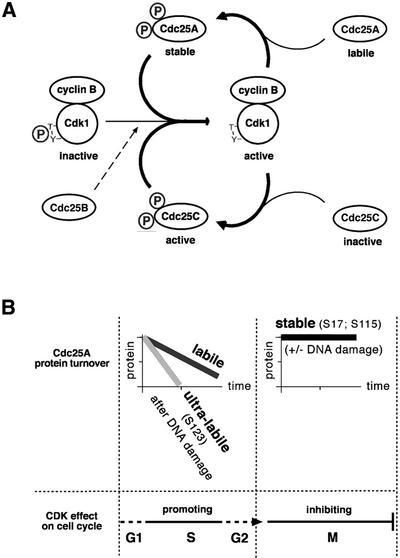
An intriguing question raised by our present findings is why should Cdc25A be stabilized in mitosis at all? At first glance, this seems surprising, given the overwhelming evidence for the role of Cdc25A in promoting G1/S transition through stimulation of cyclin E(A)–Cdk2 complexes (Nilsson and Hoffmann, 2000). However, a putative involvement of Cdc25A in later stages of the cell cycle is not entirely unprecedented. Several laboratories reported the presence of Cdc25A in G2/M cells (Hoffmann et al., 1994; Bernardi et al., 2000). In addition, overexpression of Cdc25A induces premature chromosome condensation and can lead to an abortive mitosis (Molinari et al., 2000). Finally, although the prevailing outcome of microinjection of Cdc25A antibodies into live cells was a G1/S arrest (Hoffmann et al., 1994; Jinno et al., 1994; Vigo et al., 1999), the initial report on Cdc25A also noted an inhibitory effect at G2/M (Galaktionov and Beach, 1991). Our study provides the first direct mechanistic insight into the role of Cdc25A in mitosis. It can be integrated into a model of how distinct members of the Cdc25 phosphatase family contribute to achieve and maintain the high activity of the major mitosis-promoting CDK complex (Figure 7A). This model accommodates the possible ‘starting’ effect of Cdc25B, proposed to generate the initial stimulation of cyclin B–Cdk1 at the G2/M transition (Lammer et al., 1998). Owing to the cyclin A–Cdk1-promoted destruction of Cdc25B (Baldin et al., 1997), such an initializing effect is probably only transient. Perhaps the interphasic, labile form of Cdc25A may also contribute to such a ‘starting’ effect. The resulting first wave of cyclin B–Cdk1 activation would then trigger amplification of the mitosis-promoting signal by a dual autocatalytic loop (Figure 7A). First, phosphorylation by cyclin B–Cdk1 is essential for the phosphatase activity of Cdc25C (Izumi et al., 1992; Hoffmann et al., 1993; Strausfeld et al., 1994). Secondly, we show that cyclin B–Cdk1-dependent phosphorylation stabilizes Cdc25A, thereby further increasing the cellular phosphatase potential that keeps Cdk1 in its active state. Only after reaching this stage may cells become irreversibly committed to undergo cell division. The robust Cdk1-activating stimulus jointly achieved by all Cdc25s may ensure that cyclin B–Cdk1 can no longer be targeted by cell cycle checkpoints and that its activity does not drop below the critical threshold until the destruction of cyclin B in metaphase (Clute and Pines, 1999). As mentioned before, cells lacking Cdc25C can still regulate Cdk1 normally and show no mitotic abnormalities (Chen et al., 2001). We propose that the Cdc25A ‘branch’ of the autocatalytic loop might help to compensate for the loss of at least one Cdc25 family member.
Fig. 7. Models of Cdc25A regulation and function in the mammalian cell cycle. (A) A dual positive feedback loop leading to maximal and irreversible activation of the M-phase-promoting cyclin B–Cdk1 kinase. See Discussion for details. (B) Phosphorylation-dependent switches among three differentially stable forms of Cdc25A, designated labile, ultra-labile and stable, determine the thresholds and roles of Cdc25A in unperturbed or DNA-damaged interphase and mitotic cells, respectively. See Discussion for details.
Multiple phosphorylations, protein stability states and biological thresholds
From a broader perspective, the regulation and function of Cdc25A through its multiple protein stability states exemplify a concept of flexible switches to reach distinct threshold activities during the cell cycle (Figure 7B). The Cdc25A protein is equally labile in G1, S and G2 phases, a feature allowing acute and precise adjustment of Cdc25A abundance as a function of its mRNA levels. From G1 to G2, CDK activity is a key driving force of the cell cycle dependent on a threshold activity of Cdc25A provided by its common, ‘labile’ form. Consistent with its rate-limiting role in multiple cell cycle transitions, Cdc25A is a convenient target of the G1 (Mailand et al., 2000), intra-S (Falck et al., 2001), S/M (Molinari et al., 2000) and G2 (this study) checkpoints. All these checkpoint pathways achieve the desired subtreshold levels of Cdc25A by a switch of its ‘labile’ form into the ‘ultra-labile’ state, reflecting phosphorylation-induced, accelerated turnover of Cdc25A (Figure 7B). Remarkably, around the G2/M transition, Cdc25A may exist in three distinct protein stability states, including the mitotic, ‘stable’ form coinciding with the time when cellular CDK activity peaks and then ceases to drive the cell cycle (Figure 7). The requirement for multiple phosphorylations to switch irreversibly to the proteolysis-resistant Cdc25A state may safeguard against precocious commitment to mitosis due to small fluctuations in cyclin B–Cdk1 activity, thereby providing a more reliable timing device for onset of cell division.
This concept of Cdc25A protein stability switches and thresholds may also have important implications for pathogenesis of some diseases, particularly cancer. The cell cycle machinery is commonly targeted in oncogenesis (Hunter and Pines, 1994), and Cdc25A is a proto-oncogene overexpressed in diverse human malignancies (Galaktionov et al., 1995; Gasparotto et al., 1997; Wu et al., 1998; Cangi et al., 2000). The stabilizing effect of cyclin B–Cdk1-mediated phosphorylation reported here might provide a previously unrecognized means for cancer cells to overaccumulate Cdc25A and thereby undermine their cell cycle control and/or response to DNA damage. Such a possibility is consistent with unscheduled expression of cyclin B in various tumor types (Gong et al., 1994; Collecchi et al., 2000). Our data imply that overexpression of Cdc25A deregulates not only G1/S control, but also G2/M events including the G2 checkpoint. Thus, in addition to uninhibited replication of damaged DNA (Abraham, 2001; Falck et al., 2001; Kastan, 2001), the inability to destroy Cdc25A in a timely fashion may trigger precocious cell division, and thereby further destabilize the genome.
Materials and methods
Cell culture
Derivatives of the U-2-OS/TA cell line (Lukas et al., 1999) expressing hemagglutinin (HA)-tagged alleles of Cdc25A in a tetracycline-dependent manner were generated and induced as described previously (Mailand et al., 2000). U-2-OS T-Rex cells (Invitrogen) were used for transient transfection experiments of Tet-regulatable constructs (Figure 3C and D), and treated according to the manufacturer’s instructions. Synchronization of cells in early S phase by a double thymidine block has been described previously (Clute and Pines, 1999). Pure fractions of mitotic cells were obtained by shaking off the rounded cells following 12 h treatment with nocodazole (40 ng/ml; Sigma). Roscovitine (25 µM; Calbiochem), LLnL (25 µM; Sigma) or etoposide (0.5 µg/ml; Sigma) were added to the culture medium for the times indicated in the figure legends. For estimation of the protein half-life, the cultures were either treated with cycloheximide (25 µg/ml) for the time indicated in the figure legends, or the cells were metabolically labeled with [35S]methionine and subjected to pulse–chase analysis (Lukas et al., 1995).
Plasmids and mutagenesis
Human Cdc25A cDNA was tagged at the N-terminus with the HA epitope and subcloned into the pBI tetracycline-responsive plasmid (Clontech). Phosphatase-dead (C430S) and A/A (Ser17A/Ser115A) mutants were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). For tetracycline-inducible expression in U-2-OS T-Rex cells, HA-tagged Cdc25A alleles were subcloned into the pcDNA4/TO plasmid (Invitrogen).
RT–PCR
Total RNA was isolated using a Triazol reagent kit (Gibco-BRL). Conditions for reverse transcription and PCR amplification of Cdc25A and the porphobilinogen aminase (PBGD) have been described previously (Santoni-Rugiu et al., 2000). For RT–PCR of Cdc25C mRNA, we used the primers: 5′-ATGTCTACGGAACTCTTCTCA-3′ (forward) and 5′-TGGAACAGTAGTAATGGGACT-3′ (reverse).
Immunochemical techniques
Mouse monoclonal antibodies to Cdc25A (DCS-122 and DCS-124), Cdc25B (DCS-162 and DCS-164) and Cdc25C (DCS-193) were described in Mailand et al. (2000). Other reagents included mouse monoclonal antibodies to Cdc25A (F-6; Santa Cruz), cyclin B (C23420; Transduction Laboratories), Cdc27 (SC-9972; Santa Cruz) and the HA tag (12CA5; Lukas et al., 1997), rabbit serum (PC25) to Cdk1 (Calbiochem), and a phospho-specific antibody (06-570) to histone H3 (Upstate Biotechnology). Immunoprecipitation, immunoblotting (Lukas et al., 1997) and Cdc25 phosphatase assays and measurement of cyclin B–Cdk1 kinase activity were described in Mailand et al. (2000). For immunodepletion of Cdc25s, whole-cell lysates of asynchronous U-2-OS/TA cells in kinase assay buffer (50 mM HEPES pH 7.5, 10 mM MgCl2, 5 mM EGTA) were subjected to three successive immunoprecipitations with Cdc25 antibodies or pre-immune mouse serum. For in vitro dephosphorylation assays, λ phosphatase (New England Biolabs) was used as recommended by the manufacturer. Cdc25A ubiquitylation was determined as described previously (Mailand et al., 2000).
Flow cytometry
The DNA distribution of propidium iodide (PI)-stained cell nuclei was determined using a FACSCalibur flow cytometer (Becton Dickinson). Identification of mitotic cells was performed by staining of chromosomal DNA by PI combined with immunofluorescent detection of phosphorylated histone H3 essentially as described in Xu et al. (2001).
Mass spectrometry
Protein bands excised from gels were reduced, alkylated and digested with trypsin (Shevchenko et al., 1996). MALDI peptide maps were recorded on a Reflex III mass spectrometer (Bruker-Daltonics) using 1–2% of the peptide mixtures. The remaining samples were desalted and eluted into nanoelectrospray (Wilm and Mann, 1996) needles using a combination of POROS R2 and POROS R3 microcolumns (PerSeptive Biosystems). Electrospray analysis was performed on a quadrupole/time-of-flight mass spectrometer (Shevchenko et al., 1997) QSTAR (PE-Sciex). Data interpretation and database searches were carried out using the Protein and Peptide Software Suite from MDS Proteomics (Odense, Denmark).
RNA interference
RNAi was carried out as described (Mailand et al., 2002). The sequence of the region targeted by the siRNA, 5′-AAGGAAAATGAAGCCTTTGAG-3′, corresponded to bases 418–438 downstream of the first nucleotide of the start codon of human Cdc25A cDNA. HeLa cells were transfected by Oligofectamine (Invitrogen). The final concentration of the siRNA duplex in the culture medium was 100 nM.
Acknowledgments
Acknowledgements
We thank the Danish Cancer Society, Læge Sofus Carl Emil Friis og hustru Olga Doris Friis’ Legat, Eva and Henry Frænkels Mindefond, the European Union and the Danish Research Council for grant support.
References
- Abraham R.T. (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev., 15, 2177–2196. [DOI] [PubMed] [Google Scholar]
- Atherton-Fessler S., Parker,L.L., Geahlen,R.L. and Piwnica-Worms,H. (1993) Mechanisms of p34cdc2 regulation. Mol. Cell. Biol., 13, 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V., Cans,C., Knibiehler,M. and Ducommun,B. (1997) Phosphorylation of human CDC25B phosphatase by CDK1–cyclin A triggers its proteasome-dependent degradation. J. Biol. Chem., 272, 32731–32734. [DOI] [PubMed] [Google Scholar]
- Bernardi R., Liebermann,D.A. and Hoffman,B. (2000) Cdc25A stability is controlled by the ubiquitin–proteasome pathway during cell cycle progression and terminal differentiation. Oncogene, 19, 2447–2454. [DOI] [PubMed] [Google Scholar]
- Blasina A., Price,B.D., Turenne,G.A. and McGowan,C.H. (1999) Caffeine inhibits the checkpoint kinase ATM. Curr. Biol., 9, 1135–1138. [DOI] [PubMed] [Google Scholar]
- Blomberg I. and Hoffmann,I. (1999) Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell. Biol., 19, 6183–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R.N., Holman,P.S. and Fattaey,A. (1997) Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J. Biol. Chem., 272, 22300–22306. [DOI] [PubMed] [Google Scholar]
- Bulavin D.V., Higashimoto,Y., Popoff,I.J., Gaarde,W.A., Basrur,V., Potapova,O., Appella,E. and Fornace,A.J.,Jr (2001) Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature, 411, 102–107. [DOI] [PubMed] [Google Scholar]
- Cangi M.G., Cukor,B., Soung,P., Signoretti,S., Moreira,G.,Jr, Ranashinge,M., Cady,B., Pagano,M. and Loda,M. (2000) Role of the Cdc25A phosphatase in human breast cancer. J. Clin. Invest., 106, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.S., Hurov,J., White,L.S., Woodford-Thomas,T. and Piwnica-Worms,H. (2001) Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol. Cell. Biol., 21, 3853–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P. and Pines,J. (1999) Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol., 1, 82–87. [DOI] [PubMed] [Google Scholar]
- Collecchi P., Santoni,T., Gnesi,E., Giuseppe Naccarato,A., Passoni,A., Rocchetta,M., Danesi,R. and Bevilacqua,G. (2000) Cyclins of phases G1, S and G2/M are overexpressed in aneuploid mammary carcinomas. Cytometry, 42, 254–260. [DOI] [PubMed] [Google Scholar]
- Costanzo V., Robertson,K., Ying,C.Y., Kim,E., Avvedimento,E., Gottesman,M., Grieco,D. and Gautier,J. (2000) Reconstitution of an ATM-dependent checkpoint that inhibits chromosomal DNA replication following DNA damage. Mol. Cell, 6, 649–659. [DOI] [PubMed] [Google Scholar]
- den Elzen N. and Pines,J. (2001) Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol., 153, 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck J., Mailand,N., Syljuasen,R.G., Bartek,J. and Lukas,J. (2001) The ATM–Chk2–Cdc25A checkpoint pathway guards against radio resistant DNA synthesis. Nature, 410, 842–847. [DOI] [PubMed] [Google Scholar]
- Galaktionov K. and Beach,D. (1991) Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell, 67, 1181–1194. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Lee,A.K., Eckstein,J., Draetta,G., Meckler,J., Loda,M. and Beach,D. (1995) CDC25 phosphatases as potential human oncogenes. Science, 269, 1575–1577. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Chen,X. and Beach,D. (1996) Cdc25 cell-cycle phosphatase as a target of c-myc. Nature, 382, 511–517. [DOI] [PubMed] [Google Scholar]
- Gasparotto D., Maestro,R., Piccinin,S., Vukosavljevic,T., Barzan,L., Sulfaro,S. and Boiocchi,M. (1997) Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res., 57, 2366–2368. [PubMed] [Google Scholar]
- Geley S., Kramer,E., Gieffers,C., Gannon,J., Peters,J.M. and Hunt,T. (2001) Anaphase-promoting complex/cyclosome-dependent proteo lysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol., 153, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Ardelt,B., Traganos,F. and Darzynkiewicz,Z. (1994) Unscheduled expression of cyclin B1 and cyclin E in several leukemic and solid tumor cell lines. Cancer Res., 54, 4285–4288. [PubMed] [Google Scholar]
- Hershko A. and Ciechanover,A. (1998) The ubiquitin system. Annu. Rev. Biochem., 67, 425–479. [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Clarke,P.R., Marcote,M.J., Karsenti,E. and Draetta,G. (1993) Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J., 12, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann I., Draetta,G. and Karsenti,E. (1994) Activation of the phosphatase activity of human cdc25A by a cdk2–cyclin E dependent phosphorylation at the G1/S transition. EMBO J., 13, 4302–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. and Pines,J. (1994) Cyclins and cancer, II: cyclin D and CDK inhibitors come of age. Cell, 79, 573–582. [DOI] [PubMed] [Google Scholar]
- Izumi T., Walker,D.H. and Maller,J.L. (1992) Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol. Biol. Cell, 3, 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S., Suto,K., Nagata,A., Igarashi,M., Kanaoka,Y., Nojima,H. and Okayama,H. (1994) Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J., 13, 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M.B. (2001) Cell cycle. Checking two steps. Nature, 410, 766–767. [DOI] [PubMed] [Google Scholar]
- Kaufmann W.K. and Kies,P.E. (1998) DNA signals for G2 checkpoint response in diploid human fibroblasts. Mutat. Res., 400, 153–167. [DOI] [PubMed] [Google Scholar]
- Krek W., Ewen,M.E., Shirodkar,S., Arany,Z., Kaelin,W.G.,Jr and Livingston,D.M. (1994) Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell, 78, 161–172. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Yakowec,P.S. and Dunphy,W.G. (1998) 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol. Biol. Cell, 9, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammer C., Wagerer,S., Saffrich,R., Mertens,D., Ansorge,W. and Hoffmann,I. (1998) The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J. Cell Sci., 111, 2445–2453. [DOI] [PubMed] [Google Scholar]
- Lew D.J. and Kornbluth,S. (1996) Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol., 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Lukas J., Bartkova,J., Rohde,M., Strauss,M. and Bartek J. (1995) Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol. Cell. Biol., 15, 2600–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Herzinger,T., Hansen,K., Moroni,M.C., Resnitzky,D., Helin,K., Reed,S.I. and Bartek,J. (1997) Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev., 11, 1479–1492. [DOI] [PubMed] [Google Scholar]
- Lukas J., Sorensen,C.S., Lukas,C., Santoni-Rugiu,E. and Bartek,J. (1999) p16INK4a, but not constitutively active pRb, can impose a sustained G1 arrest: molecular mechanisms and implications for oncogenesis. Oncogene, 18, 3930–3935. [DOI] [PubMed] [Google Scholar]
- Mailand N., Falck,J., Lukas,C., Syljuasen,R.G., Welcker,M., Bartek,J. and Lukas,J. (2000) Rapid destruction of human Cdc25A in response to DNA damage. Science, 288, 1425–1429. [DOI] [PubMed] [Google Scholar]
- Mailand N., Lukas,C., Kaiser,B.K., Jackson,P.K., Bartek,J. and Lukas,J. (2002) Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat. Cell Biol., 4, 318–322. [DOI] [PubMed] [Google Scholar]
- Meijer L., Borgne,A., Mulner,O., Chong,J.P., Blow,J.J., Inagaki,N., Inagaki,M., Delcros,J.G. and Moulinoux,J.P. (1997) Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem., 243, 527–536. [DOI] [PubMed] [Google Scholar]
- Molinari M., Mercurio,C., Dominguez,J., Goubin,F. and Draetta,G.F. (2000) Human Cdc25 A inactivation in response to S phase inhibition and its role in preventing premature mitosis. EMBO rep., 1, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.O. (1995) Principles of CDK regulation. Nature, 374, 131–134. [DOI] [PubMed] [Google Scholar]
- Nagata A., Igarashi,M., Jinno,S., Suto,K. and Okayama,H. (1991) An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol., 3, 959–968. [PubMed] [Google Scholar]
- Nigg E.A. (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays, 17, 471–480. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell. Biol., 2, 21–32. [DOI] [PubMed] [Google Scholar]
- Nilsson I. and Hoffmann,I. (2000) Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res., 4, 107–114. [DOI] [PubMed] [Google Scholar]
- Nurse P., Masui,Y. and Hartwell,L. (1998) Understanding the cell cycle. Nat. Med., 4, 1103–1106. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves,P.R., Thoma,R.S., Wu,Z., Shaw,A.S. and Piwnica-Worms,H. (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science, 277, 1501–1505. [DOI] [PubMed] [Google Scholar]
- Sadhu K., Reed,S.I., Richardson,H. and Russell,P. (1990) Human homolog of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc. Natl Acad. Sci. USA, 87, 5139–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni-Rugiu E., Falck,J., Mailand,N., Bartek,J. and Lukas,J. (2000) Involvement of Myc activity in a G1/S-promoting mechanism parallel to the pRb/E2F pathway. Mol. Cell. Biol., 20, 3497–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkaria J.N., Busby,E.C., Tibbetts,R.S., Roos,P., Taya,Y., Karnitz,L.M. and Abraham,R.T. (1999) Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res., 59, 4375–4382. [PubMed] [Google Scholar]
- Sexl V., Diehl,J.A., Sherr,C.J., Ashmun,R., Beach,D. and Roussel,M.F. (1999) A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene, 18, 573–582. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Jensen,O.N., Podtelejnikov,A.V., Sagliocco,F., Wilm,M., Vorm,O., Mortensen,P., Boucherie,H. and Mann,M. (1996) Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc. Natl Acad. Sci. USA, 93, 14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A., Chernushevich,I., Ens,W., Standing,K.G., Thomson,B., Wilm,M. and Mann,M. (1997) Rapid ‘de novo’ peptide sequencing by a combination of nanoelectrospray, isotopic labeling and a quadrupole/time-of-flight mass spectrometer. Rapid Commun. Mass Spectrom., 11, 1015–1024. [DOI] [PubMed] [Google Scholar]
- Strausfeld U., Fernandez,A., Capony,J.P., Girard,F., Lautredou,N., Derancourt,J., Labbe,J.C. and Lamb,N.J. (1994) Activation of p34cdc2 protein kinase by microinjection of human cdc25C into mammalian cells. Requirement for prior phosphorylation of cdc25C by p34cdc2 on sites phosphorylated at mitosis. J. Biol. Chem., 269, 5989–6000. [PubMed] [Google Scholar]
- Treier M., Staszewski,L.M. and Bohmann,D. (1994) Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell, 78, 787–798. [DOI] [PubMed] [Google Scholar]
- Vigo E., Muller,H., Prosperini,E., Hateboer,G., Cartwright,P., Moroni,M.C. and Helin,K. (1999) CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol., 19, 6379–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm M. and Mann,M. (1996) Analytical properties of the nanoelectrospray ion source. Anal. Chem., 68, 1–8. [DOI] [PubMed] [Google Scholar]
- Wu W., Fan,Y.H., Kemp,B.L., Walsh,G. and Mao,L. (1998) Overexpression of cdc25A and cdc25B is frequent in primary non-small cell lung cancer but is not associated with overexpression of c-myc. Cancer Res., 58, 4082–4085. [PubMed] [Google Scholar]
- Xu B., Kim,S. and Kastan,M.B. (2001) Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol., 21, 3445–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.B. and Elledge,S.J. (2000) The DNA damage response: putting checkpoints in perspective. Nature, 408, 433–439. [DOI] [PubMed] [Google Scholar]