Abstract
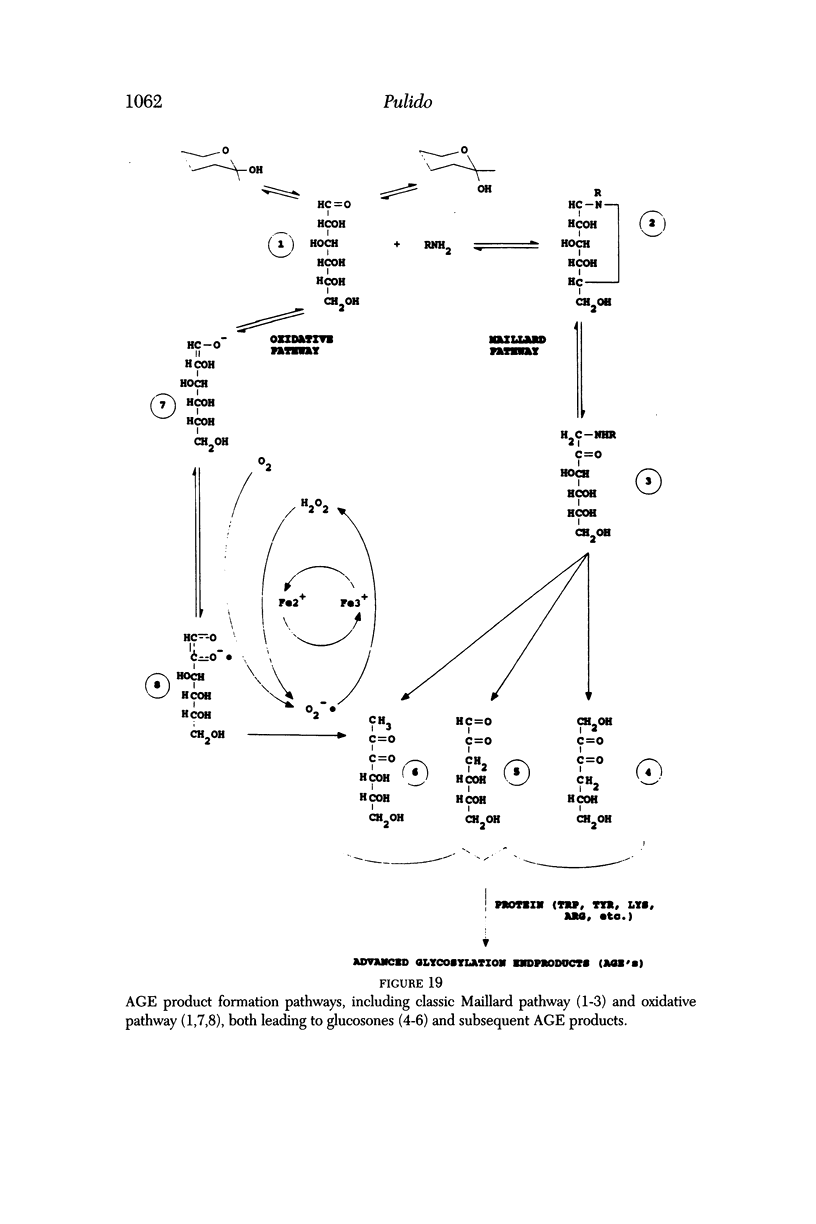
PURPOSE: To study the process of nonenzymatic glycosolation of vitreous collagen in vitro to determine the contributions of the classic Maillard pathway and the oxidative pathway, as well as to evaluate possible inhibitors of both pathways. METHODS: Bovine vitreous collagen was extracted and then incubated with hexoses in vitro. The amount of advanced glycosylation end (AGE) products was measured by fluorometry under varying conditions in the presence and absence of glycosolation inhibitors. Oxygen consumption studies and electron spin resonance spectroscopy with and without free-radical inhibitors were performed to differentiate oxidative from nonoxidative glycosolation. RESULTS: Vitreous collagen undergoes nonenzymatic glycosolation in the presence of glucose or galactose in vitro. Oxygen consumption data show that oxygen is consumed in glucose and galactose solutions. Oxygen consumption is decreased by known free-radical inhibitors and rutin but not aminoguanidine. Electron spin resonance spectroscopy demonstrated the presence of a carbon-centered radical, and known free-radical inhibitors decreased the carbon-centered signal. CONCLUSIONS: Nonenzymatic glycosolation of vitreous collagen can occur not only by the classic nonoxidative pathway, but also by a second oxidative pathway that is susceptible to a number of inhibitors.
Full text
PDF






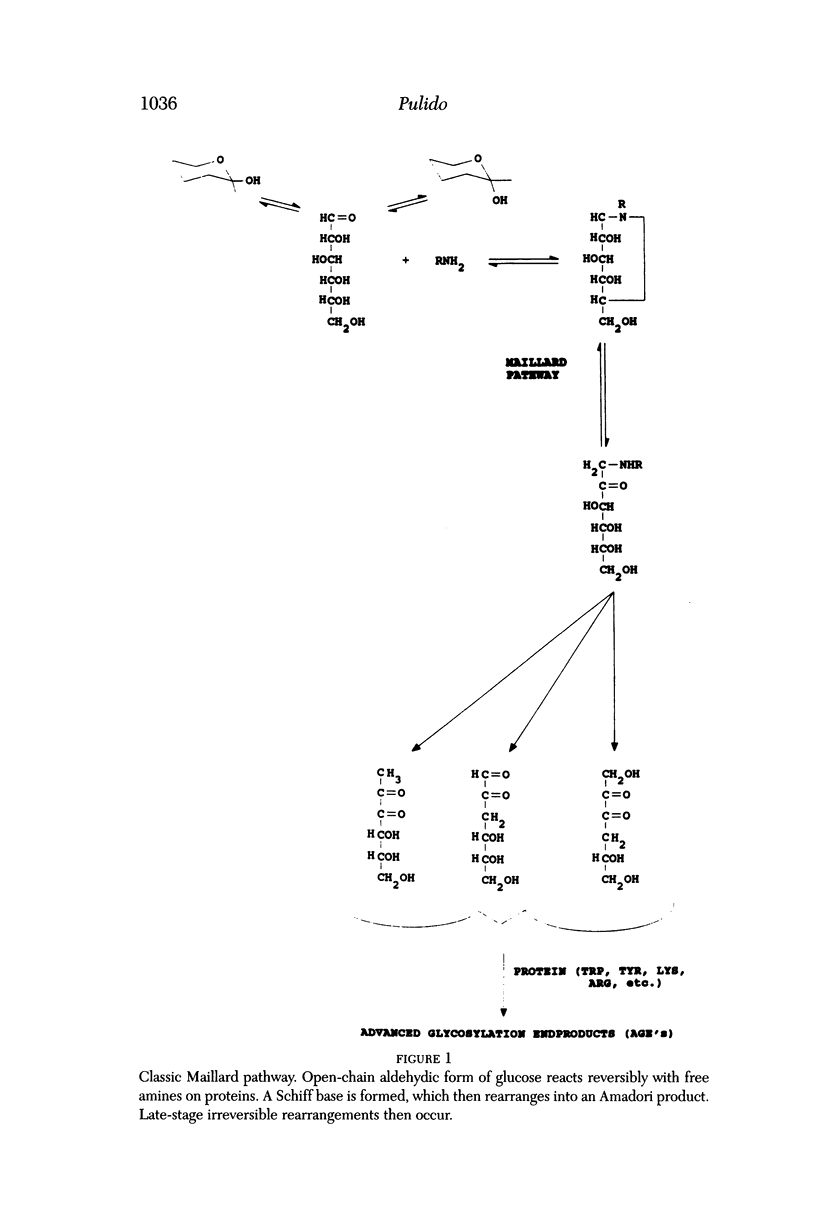



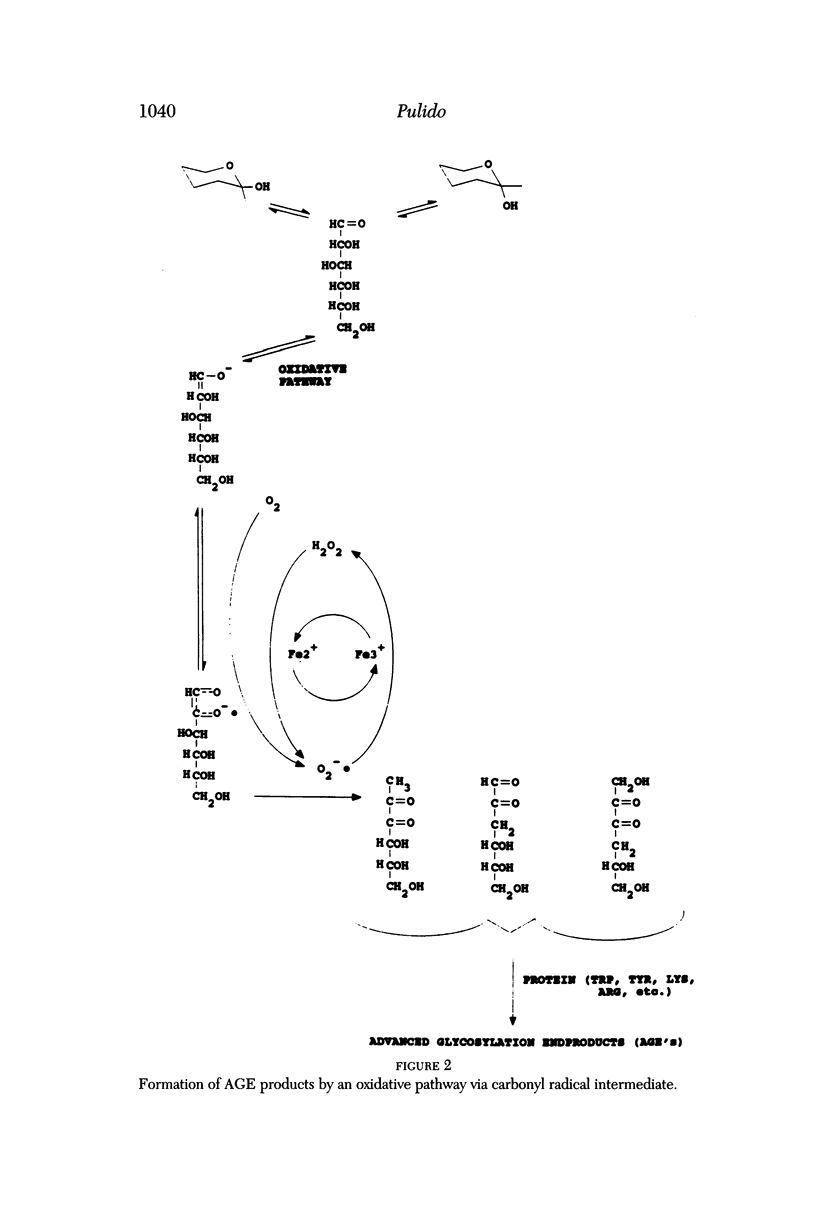






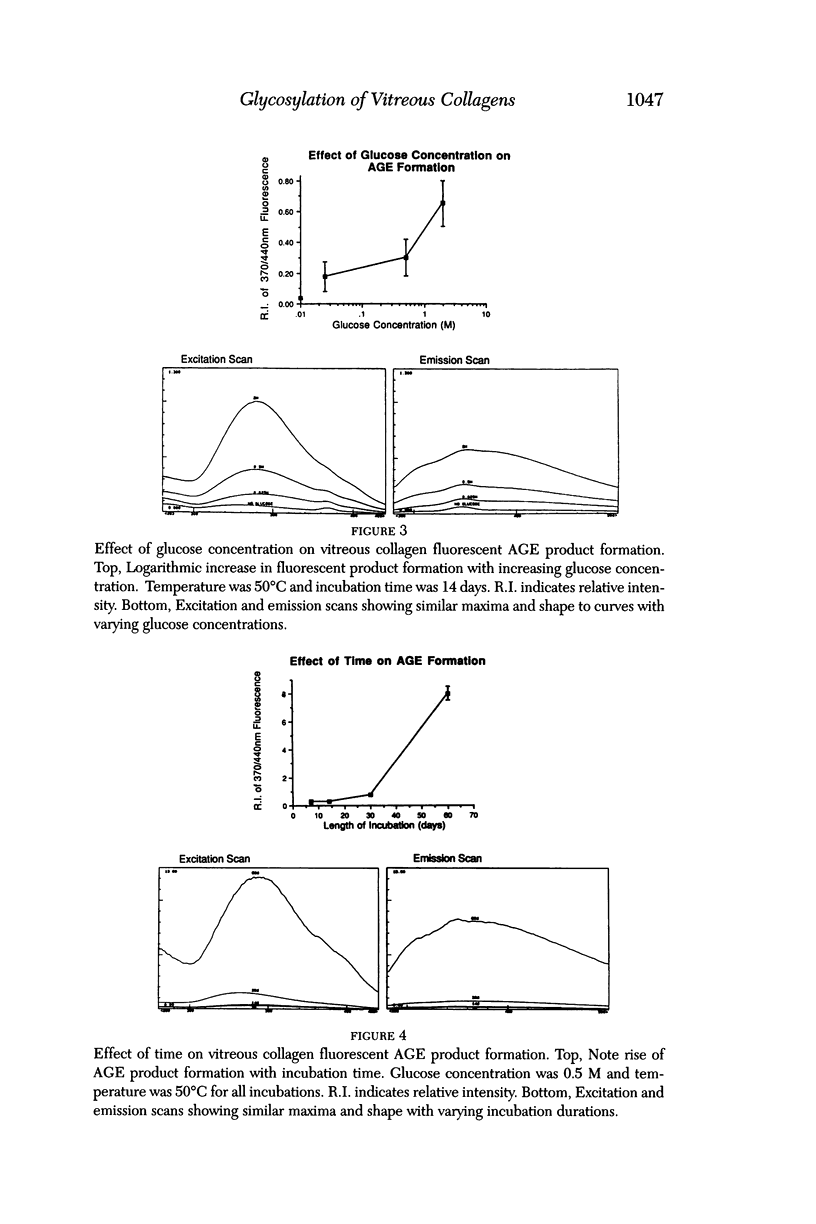
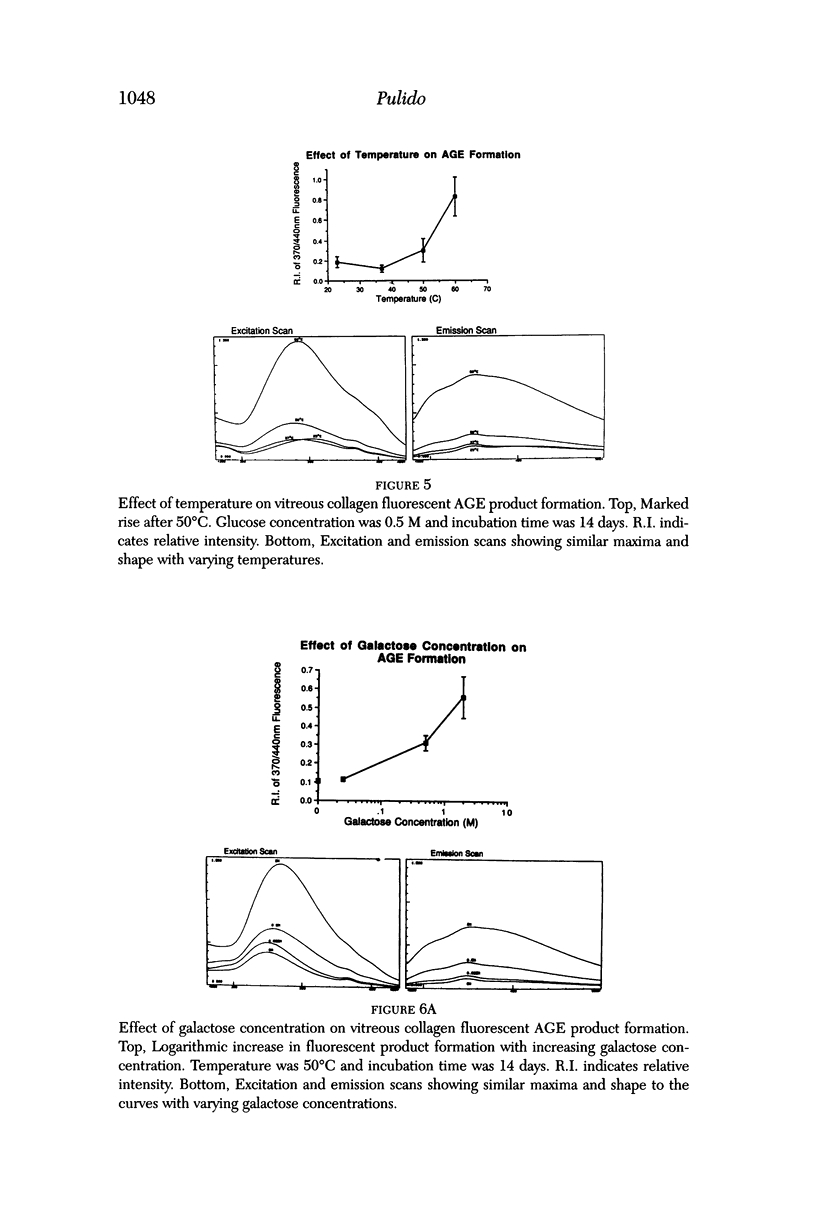

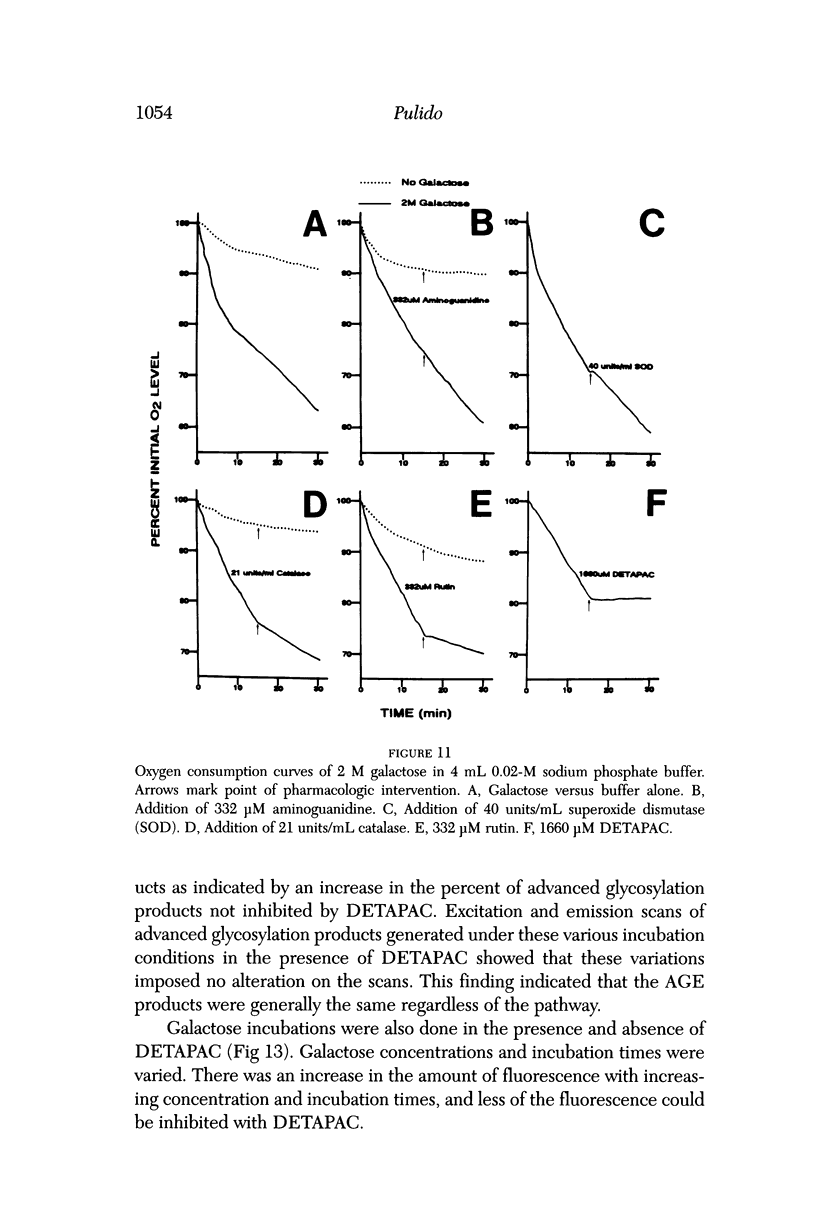
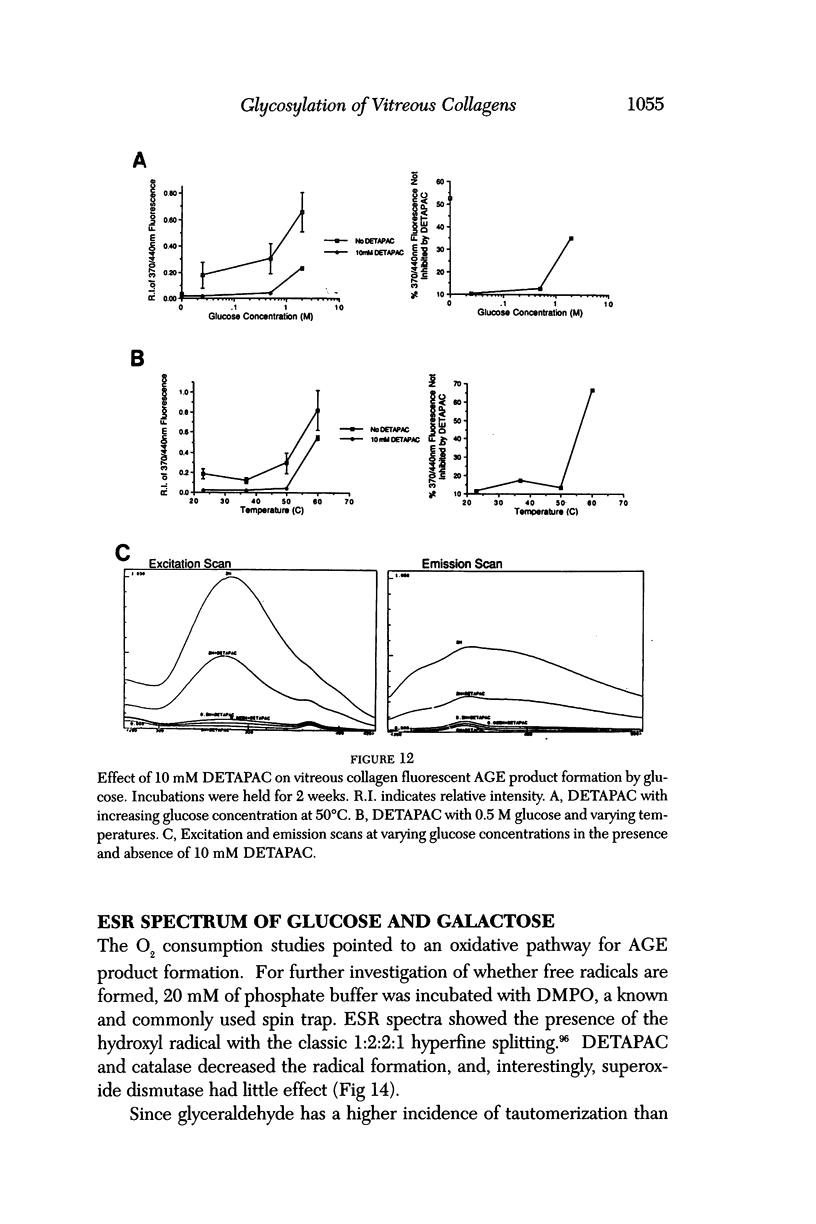
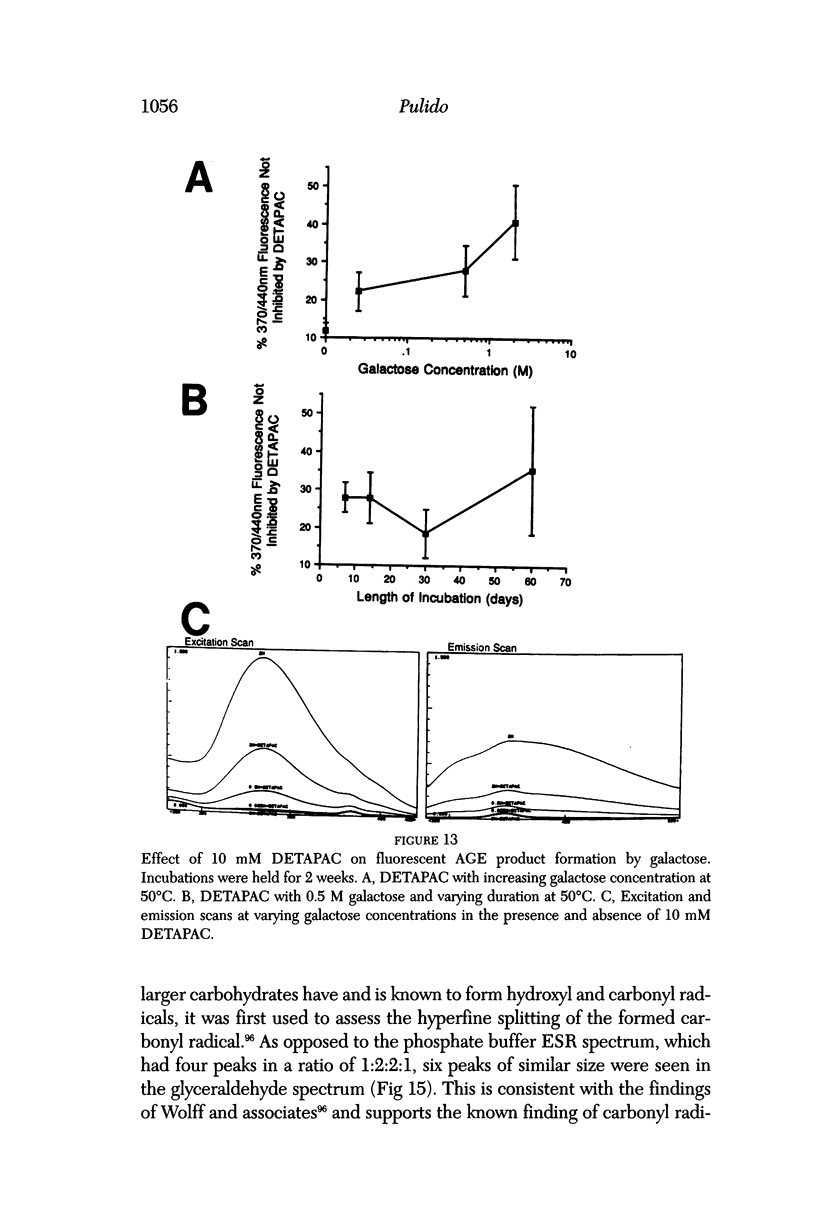
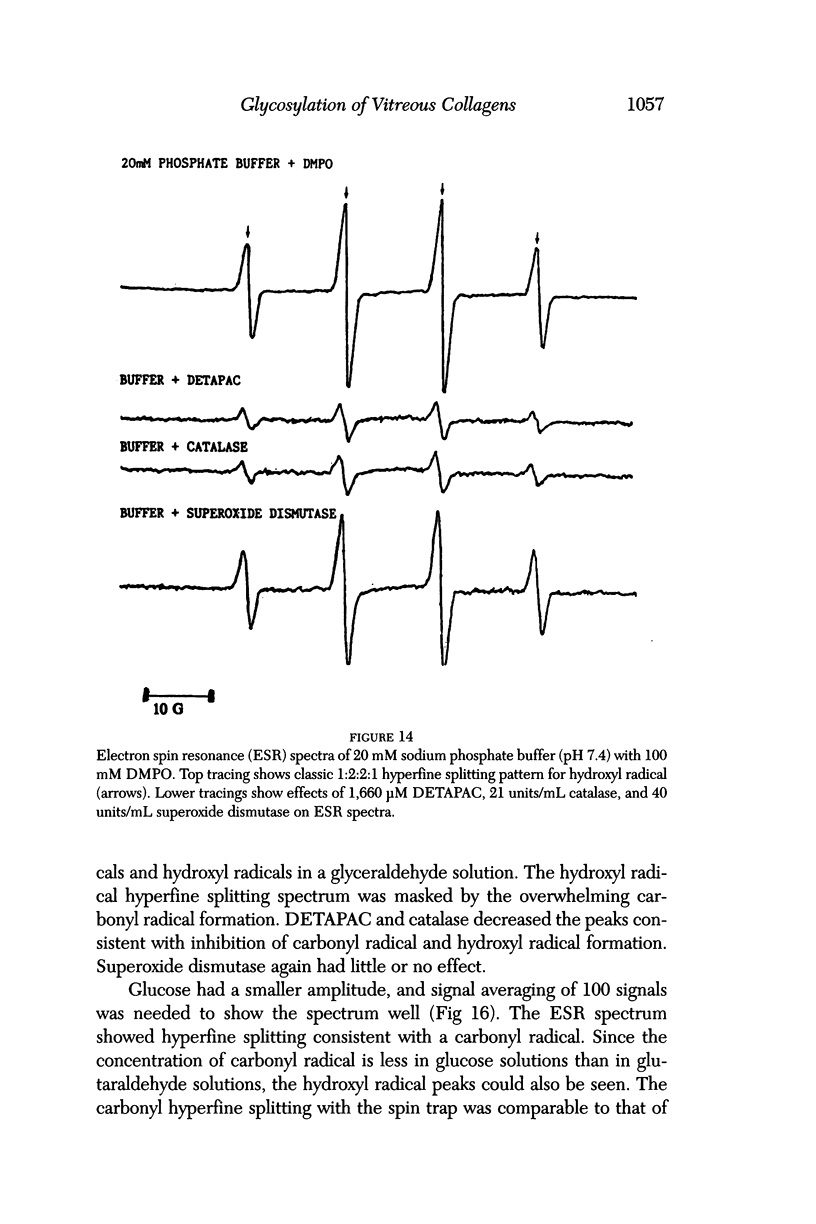










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi Y., Yajima Y., Kador P. F., Kuwabara T., Kinoshita J. H. Localization of aldose reductase in the human eye. Diabetes. 1984 Jun;33(6):562–566. doi: 10.2337/diab.33.6.562. [DOI] [PubMed] [Google Scholar]
- Akiba J., Arzabe C. W., Trempe C. L. Posterior vitreous detachment and neovascularization in diabetic retinopathy. Ophthalmology. 1990 Jul;97(7):889–891. doi: 10.1016/s0161-6420(90)32486-7. [DOI] [PubMed] [Google Scholar]
- Akiba J., Ueno N., Chakrabarti B. Age-related changes in the molecular properties of vitreous collagen. Curr Eye Res. 1993 Oct;12(10):951–954. doi: 10.3109/02713689309020402. [DOI] [PubMed] [Google Scholar]
- Akiba J., Ueno N., Chakrabarti B. Mechanisms of photo-induced vitreous liquefaction. Curr Eye Res. 1994 Jul;13(7):505–512. doi: 10.3109/02713689408999882. [DOI] [PubMed] [Google Scholar]
- Anderson S. S., Kim Y., Tsilibary E. C. Effects of matrix glycation on mesangial cell adhesion, spreading and proliferation. Kidney Int. 1994 Nov;46(5):1359–1367. doi: 10.1038/ki.1994.405. [DOI] [PubMed] [Google Scholar]
- Anderson S. S., Tsilibary E. C., Charonis A. S. Nonenzymatic glycosylation-induced modifications of intact bovine kidney tubular basement membrane. J Clin Invest. 1993 Dec;92(6):3045–3052. doi: 10.1172/JCI116929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S., Weiss J. B. A new look at vitreous-humour collagen. Biochem J. 1984 Mar 15;218(3):835–840. doi: 10.1042/bj2180835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Sims T. J., Avery N. C., Miles C. A. Chemistry of collagen cross-links: glucose-mediated covalent cross-linking of type-IV collagen in lens capsules. Biochem J. 1993 Dec 1;296(Pt 2):489–496. doi: 10.1042/bj2960489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes J. W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991 Apr;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Beyer-Mears A., Cruz E., Edelist T., Varagiannis E. Diminished proteinuria in diabetes mellitus by sorbinil, an aldose reductase inhibitor. Pharmacology. 1986;32(1):52–60. doi: 10.1159/000138152. [DOI] [PubMed] [Google Scholar]
- Beyer-Mears A., Ku L., Cohen M. P. Glomerular polyol accumulation in diabetes and its prevention by oral sorbinil. Diabetes. 1984 Jun;33(6):604–607. doi: 10.2337/diab.33.6.604. [DOI] [PubMed] [Google Scholar]
- Bishop P. N., Crossman M. V., McLeod D., Ayad S. Extraction and characterization of the tissue forms of collagen types II and IX from bovine vitreous. Biochem J. 1994 Apr 15;299(Pt 2):497–505. doi: 10.1042/bj2990497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair N. P., Tso M. O., Dodge J. T. Pathologic studies of the blood--retinal barrier in the spontaneously diabetic BB rat. Invest Ophthalmol Vis Sci. 1984 Mar;25(3):302–311. [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes. 1994 Jun;43(6):836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Higgins P. J. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981 Jul 10;213(4504):222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- Chace K. V., Carubelli R., Nordquist R. E. The role of nonenzymatic glycosylation, transition metals, and free radicals in the formation of collagen aggregates. Arch Biochem Biophys. 1991 Aug 1;288(2):473–480. doi: 10.1016/0003-9861(91)90223-6. [DOI] [PubMed] [Google Scholar]
- Chibber R., Molinatti P. A., Wong J. S., Mirlees D., Kohner E. M. The effect of aminoguanidine and tolrestat on glucose toxicity in bovine retinal capillary pericytes. Diabetes. 1994 Jun;43(6):758–763. doi: 10.2337/diab.43.6.758. [DOI] [PubMed] [Google Scholar]
- Cook C. S., McGahan M. C. Copper concentration in cornea, iris, normal, and cataractous lenses and intraocular fluids of vertebrates. Curr Eye Res. 1986 Jan;5(1):69–76. doi: 10.3109/02713688608995168. [DOI] [PubMed] [Google Scholar]
- Crowley S. T., Brownlee M., Edelstein D., Satriano J. A., Mori T., Singhal P. C., Schlondorff D. O. Effects of nonenzymatic glycosylation of mesangial matrix on proliferation of mesangial cells. Diabetes. 1991 May;40(5):540–547. doi: 10.2337/diab.40.5.540. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J., Faria de Abreu J. R., Campos A. J. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975 Nov;59(11):649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B. S., Hostetter T. H. Aldose reductase inhibition and glomerular abnormalities in diabetic rats. Diabetes. 1989 Aug;38(8):981–986. doi: 10.2337/diab.38.8.981. [DOI] [PubMed] [Google Scholar]
- Davis M. D. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol. 1965 Dec;74(6):741–751. doi: 10.1001/archopht.1965.00970040743003. [DOI] [PubMed] [Google Scholar]
- Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–11660. [PubMed] [Google Scholar]
- Dyer D. G., Dunn J. A., Thorpe S. R., Bailie K. E., Lyons T. J., McCance D. R., Baynes J. W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993 Jun;91(6):2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Aldose reductase inhibition fails to prevent retinopathy in diabetic and galactosemic dogs. Diabetes. 1993 Jun;42(6):820–825. doi: 10.2337/diab.42.6.820. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Experimental galactosemia produces diabetic-like retinopathy. Diabetes. 1984 Jan;33(1):97–100. doi: 10.2337/diab.33.1.97. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Retinopathy in animal models of diabetes. Diabetes Metab Rev. 1995 Jul;11(2):109–120. doi: 10.1002/dmr.5610110203. [DOI] [PubMed] [Google Scholar]
- Engerman R. Diabetes-like preproliferative retinal changes in galactose-fed dogs. Arch Ophthalmol. 1993 May;111(5):584–585. doi: 10.1001/archopht.1993.01090050018012. [DOI] [PubMed] [Google Scholar]
- Finegold D., Lattimer S. A., Nolle S., Bernstein M., Greene D. A. Polyol pathway activity and myo-inositol metabolism. A suggested relationship in the pathogenesis of diabetic neuropathy. Diabetes. 1983 Nov;32(11):988–992. doi: 10.2337/diab.32.11.988. [DOI] [PubMed] [Google Scholar]
- Foos R. Y., Kreiger A. E., Forsythe A. B., Zakka K. A. Posterior vitreous detachment in diabetic subjects. Ophthalmology. 1980 Feb;87(2):122–128. doi: 10.1016/s0161-6420(80)35269-x. [DOI] [PubMed] [Google Scholar]
- Frank R. N. On the pathogenesis of diabetic retinopathy. A 1990 update. Ophthalmology. 1991 May;98(5):586–593. doi: 10.1016/s0161-6420(91)32253-x. [DOI] [PubMed] [Google Scholar]
- Frank R. N. The aldose reductase controversy. Diabetes. 1994 Feb;43(2):169–172. doi: 10.2337/diab.43.2.169. [DOI] [PubMed] [Google Scholar]
- Fu M. X., Wells-Knecht K. J., Blackledge J. A., Lyons T. J., Thorpe S. R., Baynes J. W. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 1994 May;43(5):676–683. doi: 10.2337/diab.43.5.676. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Hasty K., Breslow J. L., Ellison R. C., Bunn H. F., Gallop P. M. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977 May;44(5):859–864. doi: 10.1210/jcem-44-5-859. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Sosenko J. M., Banuchi G. A., Mininsohn M. J., Flückiger R. Glycosylated hemoglobins: increased glycosylation of hemoglobin A in diabetic patients. Diabetes. 1979 Apr;28(4):337–340. doi: 10.2337/diab.28.4.337. [DOI] [PubMed] [Google Scholar]
- Giugliano D., Ceriello A., Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism. 1995 Mar;44(3):363–368. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Grandhee S. K., Monnier V. M. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem. 1991 Jun 25;266(18):11649–11653. [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Beswick H. T. The possible contribution of glucose autoxidation to protein modification of diabetes. Biochem J. 1988 Jan 15;249(2):617–618. doi: 10.1042/bj2490617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. A., Ege E. A. Active and passive mechanical properties of isolated arterioles from STZ-induced diabetic rats. Effect of aminoguanidine treatment. Diabetes. 1994 Dec;43(12):1450–1456. doi: 10.2337/diab.43.12.1450. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Dean R. T., Wolff S. P. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988 Nov 15;256(1):205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkh A., Takahashi M., Topilow H. W., Trempe C. L., McMeel J. W. Prognostic value of vitreous findings in diabetic retinopathy. Arch Ophthalmol. 1982 Mar;100(3):432–434. doi: 10.1001/archopht.1982.01030030434009. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y., Zhou Q. L., Eaton J. W., Koppenol W. H., Hunt J. V., Wolff S. P. Spirohydantoin inhibitors of aldose reductase inhibit iron- and copper-catalysed ascorbate oxidation in vitro. Biochem Pharmacol. 1991 Aug 22;42(6):1273–1278. doi: 10.1016/0006-2952(91)90265-7. [DOI] [PubMed] [Google Scholar]
- Judzewitsch R. G., Jaspan J. B., Polonsky K. S., Weinberg C. R., Halter J. B., Halar E., Pfeifer M. A., Vukadinovic C., Bernstein L., Schneider M. Aldose reductase inhibition improves nerve conduction velocity in diabetic patients. N Engl J Med. 1983 Jan 20;308(3):119–125. doi: 10.1056/NEJM198301203080302. [DOI] [PubMed] [Google Scholar]
- Kador P. F., Akagi Y., Takahashi Y., Ikebe H., Wyman M., Kinoshita J. H. Prevention of retinal vessel changes associated with diabetic retinopathy in galactose-fed dogs by aldose reductase inhibitors. Arch Ophthalmol. 1990 Sep;108(9):1301–1309. doi: 10.1001/archopht.1990.01070110117035. [DOI] [PubMed] [Google Scholar]
- Kador P. F., Robison W. G., Jr, Kinoshita J. H. The pharmacology of aldose reductase inhibitors. Annu Rev Pharmacol Toxicol. 1985;25:691–714. doi: 10.1146/annurev.pa.25.040185.003355. [DOI] [PubMed] [Google Scholar]
- Kador P. F. The role of aldose reductase in the development of diabetic complications. Med Res Rev. 1988 Jul-Sep;8(3):325–352. doi: 10.1002/med.2610080302. [DOI] [PubMed] [Google Scholar]
- Kalfa T. A., Gerritsen M. E., Carlson E. C., Binstock A. J., Tsilibary E. C. Altered proliferation of retinal microvascular cells on glycated matrix. Invest Ophthalmol Vis Sci. 1995 Nov;36(12):2358–2367. [PubMed] [Google Scholar]
- Khoo U. Y., Newman D. J., Miller W. K., Price C. P. The influence of glycation on the peroxidase activity of haemoglobin. Eur J Clin Chem Clin Biochem. 1994 Jun;32(6):435–440. doi: 10.1515/cclm.1994.32.6.435. [DOI] [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Kishi S., Shimizu K. Clinical manifestations of posterior precortical vitreous pocket in proliferative diabetic retinopathy. Ophthalmology. 1993 Feb;100(2):225–229. doi: 10.1016/s0161-6420(93)31666-0. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Cerami A. Hemoglobin A Ic and diabetes mellitus. Annu Rev Med. 1980;31:29–34. doi: 10.1146/annurev.me.31.020180.000333. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Peterson C. M., Jones R. L., Saudek C., Lehrman M., Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976 Aug 19;295(8):417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Peterson C. M., Kilo C., Cerami A., Williamson J. R. Hemoglobin AIc as an indicator of the degree of glucose intolerance in diabetes. Diabetes. 1976 Mar;25(3):230–232. doi: 10.2337/diab.25.3.230. [DOI] [PubMed] [Google Scholar]
- Lee A. Y., Chung S. K., Chung S. S. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis H., Abrams G. W., Blumenkranz M. S., Campo R. V. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992 May;99(5):753–759. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- Liang J. N., Chakrabarti B. Spectroscopic studies on pepsin-solubilized vitreous and cartilage collagens. Curr Eye Res. 1981;1(3):175–181. doi: 10.3109/02713688109001823. [DOI] [PubMed] [Google Scholar]
- Lightman S., Rechthand E., Terubayashi H., Palestine A., Rapoport S., Kador P. Permeability changes in blood-retinal barrier of galactosemic rats are prevented by aldose reductase inhibitors. Diabetes. 1987 Nov;36(11):1271–1275. doi: 10.2337/diab.36.11.1271. [DOI] [PubMed] [Google Scholar]
- Loy A., Lurie K. G., Ghosh A., Wilson J. M., MacGregor L. C., Matschinsky F. M. Diabetes and the myo-inositol paradox. Diabetes. 1990 Oct;39(10):1305–1312. doi: 10.2337/diab.39.10.1305. [DOI] [PubMed] [Google Scholar]
- Lundquist O., Osterlin S. Glucose concentration in the vitreous of nondiabetic and diabetic human eyes. Graefes Arch Clin Exp Ophthalmol. 1994 Feb;232(2):71–74. doi: 10.1007/BF00171666. [DOI] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- McCance D. R., Dyer D. G., Dunn J. A., Bailie K. E., Thorpe S. R., Baynes J. W., Lyons T. J. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993 Jun;91(6):2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahan M. C. Ascorbic acid levels in aqueous and vitreous humors of the rabbit: effects of inflammation and ceruloplasmin. Exp Eye Res. 1985 Sep;41(3):291–298. doi: 10.1016/s0014-4835(85)80019-1. [DOI] [PubMed] [Google Scholar]
- McGahan M. C., Fleisher L. N. Inflammation-induced changes in the iron concentration and total iron-binding capacity of the intraocular fluids of rabbits. Graefes Arch Clin Exp Ophthalmol. 1988;226(1):27–30. doi: 10.1007/BF02172712. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Vishwanath V., Frank K. E., Elmets C. A., Dauchot P., Kohn R. R. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986 Feb 13;314(7):403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- Nasrallah F. P., Jalkh A. E., Van Coppenolle F., Kado M., Trempe C. L., McMeel J. W., Schepens C. L. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988 Oct;95(10):1335–1339. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- Odetti P. R., Borgoglio A., De Pascale A., Rolandi R., Adezati L. Prevention of diabetes-increased aging effect on rat collagen-linked fluorescence by aminoguanidine and rutin. Diabetes. 1990 Jul;39(7):796–801. doi: 10.2337/diab.39.7.796. [DOI] [PubMed] [Google Scholar]
- Odetti P. R., Borgoglio A., Rolandi R. Age-related increase of collagen fluorescence in human subcutaneous tissue. Metabolism. 1992 Jun;41(6):655–658. doi: 10.1016/0026-0495(92)90059-j. [DOI] [PubMed] [Google Scholar]
- Oimomi M., Maeda Y., Baba S., Iga T., Yamamoto M. Relationship between levels of advanced-stage products of the Maillard reaction and the development of diabetic retinopathy. Exp Eye Res. 1989 Aug;49(2):317–320. doi: 10.1016/0014-4835(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Oimomi M., Maeda Y., Hata F., Kitamura Y., Matsumoto S., Baba S., Iga T., Yamamoto M. Glycation of cataractous lens in non-diabetic senile subjects and in diabetic patients. Exp Eye Res. 1988 Mar;46(3):415–420. doi: 10.1016/s0014-4835(88)80029-0. [DOI] [PubMed] [Google Scholar]
- Perejda A. J., Zaragoza E. J., Eriksen E., Uitto J. Nonenzymatic glucosylation of lysyl and hydroxylysyl residues in type I and type II collagens. Coll Relat Res. 1984 Dec;4(6):427–439. doi: 10.1016/s0174-173x(84)80010-2. [DOI] [PubMed] [Google Scholar]
- Pugliese G., Tilton R. G., Speedy A., Santarelli E., Eades D. M., Province M. A., Kilo C., Sherman W. R., Williamson J. R. Modulation of hemodynamic and vascular filtration changes in diabetic rats by dietary myo-inositol. Diabetes. 1990 Mar;39(3):312–322. doi: 10.2337/diab.39.3.312. [DOI] [PubMed] [Google Scholar]
- Rahbar S. An abnormal hemoglobin in red cells of diabetics. Clin Chim Acta. 1968 Oct;22(2):296–298. doi: 10.1016/0009-8981(68)90372-0. [DOI] [PubMed] [Google Scholar]
- Rahbar S., Blumenfeld O., Ranney H. M. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969 Aug 22;36(5):838–843. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- Reichard P., Nilsson B. Y., Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993 Jul 29;329(5):304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- Robison W. G., Jr, Laver N. M., Jacot J. L., Glover J. P. Sorbinil prevention of diabetic-like retinopathy in the galactose-fed rat model. Invest Ophthalmol Vis Sci. 1995 Nov;36(12):2368–2380. [PubMed] [Google Scholar]
- Robison W. G., Jr, Nagata M., Laver N., Hohman T. C., Kinoshita J. H. Diabetic-like retinopathy in rats prevented with an aldose reductase inhibitor. Invest Ophthalmol Vis Sci. 1989 Nov;30(11):2285–2292. [PubMed] [Google Scholar]
- Sebag J. Abnormalities of human vitreous structure in diabetes. Graefes Arch Clin Exp Ophthalmol. 1993 May;231(5):257–260. doi: 10.1007/BF00919101. [DOI] [PubMed] [Google Scholar]
- Sebag J., Buckingham B., Charles M. A., Reiser K. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch Ophthalmol. 1992 Oct;110(10):1472–1476. doi: 10.1001/archopht.1992.01080220134035. [DOI] [PubMed] [Google Scholar]
- Sebag J., Nie S., Reiser K., Charles M. A., Yu N. T. Raman spectroscopy of human vitreous in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1994 Jun;35(7):2976–2980. [PubMed] [Google Scholar]
- Seery C. M., Davison P. F. Collagens of the bovine vitreous. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1540–1550. [PubMed] [Google Scholar]
- Shimizu K., Furuya T., Takeo Y., Shirama K., Maekawa K. Decrease of collagen content in the intraocular fluid of senile rats. Acta Anat (Basel) 1981;109(1):44–46. doi: 10.1159/000145363. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Braddock K. J., Pulido J. S. Insulin in the vitreous of the normal and streptozotocin-induced diabetic rat. Peptides. 1992 Jul-Aug;13(4):671–675. doi: 10.1016/0196-9781(92)90171-x. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Faeth J. A., Pulido J. S. Nonenzymatic glycosylation of vitreous proteins in vitro and in the streptozotocin-treated diabetic rat. Retina. 1990;10(2):153–158. doi: 10.1097/00006982-199004000-00013. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Faeth J. A., Pulido J. S. Protein levels in the vitreous of rats with streptozotocin-induced diabetes mellitus. Brain Res Bull. 1993;30(1-2):85–90. doi: 10.1016/0361-9230(93)90042-a. [DOI] [PubMed] [Google Scholar]
- Snowden J. M., Swann D. A. Vitreous structure. V. The morphology and thermal stability of vitreous collagen fibers and comparison to articular cartilage (type II) collagen. Invest Ophthalmol Vis Sci. 1980 Jun;19(6):610–618. [PubMed] [Google Scholar]
- Swann D. A. Chemistry and biology of the vitreous body. Int Rev Exp Pathol. 1980;22:1–64. [PubMed] [Google Scholar]
- Swann D. A., Sotman S. S. The chemical composition of bovine vitreous-humour collagen fibres. Biochem J. 1980 Mar 1;185(3):545–554. doi: 10.1042/bj1850545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa H., McMeel J. W., Furukawa H., Quiroz H., Murakami K., Takahashi M., Trempe C. L. Role of the vitreous in diabetic retinopathy. I. Vitreous changes in diabetic retinopathy and in physiologic aging. Ophthalmology. 1986 May;93(5):596–601. doi: 10.1016/s0161-6420(86)33690-x. [DOI] [PubMed] [Google Scholar]
- Tagawa H., McMeel J. W., Trempe C. L. Role of the vitreous in diabetic retinopathy. II. Active and inactive vitreous changes. Ophthalmology. 1986 Sep;93(9):1188–1192. doi: 10.1016/s0161-6420(86)33608-x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Wyman M., Ferris F., 3rd, Kador P. F. Diabeteslike preproliferative retinal changes in galactose-fed dogs. Arch Ophthalmol. 1992 Sep;110(9):1295–1302. doi: 10.1001/archopht.1992.01080210113037. [DOI] [PubMed] [Google Scholar]
- Tasman W. Diabetic vitreous hemorrhage and its relationship to hypoglycemia. Mod Probl Ophthalmol. 1979;20:413–414. [PubMed] [Google Scholar]
- Tilton R. G., Chang K., Hasan K. S., Smith S. R., Petrash J. M., Misko T. P., Moore W. M., Currie M. G., Corbett J. A., McDaniel M. L. Prevention of diabetic vascular dysfunction by guanidines. Inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993 Feb;42(2):221–232. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Vlassara H. Recent progress on the biologic and clinical significance of advanced glycosylation end products. J Lab Clin Med. 1994 Jul;124(1):19–30. [PubMed] [Google Scholar]
- Watala C., Gwozdzinski K., Malek M. Direct evidence for the alterations in protein structure and conformation upon in vitro nonenzymatic glycosylation. Int J Biochem. 1992 Aug;24(8):1295–1302. doi: 10.1016/0020-711x(92)90204-e. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Williamson J. R., Easom R. A., Chang K., Sherman W. R., Turk J. Diacylglycerol accumulation and microvascular abnormalities induced by elevated glucose levels. J Clin Invest. 1991 Jan;87(1):31–38. doi: 10.1172/JCI114988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. P., Dean R. T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem J. 1987 Jul 1;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. P., Jiang Z. Y., Hunt J. V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10(5):339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]


