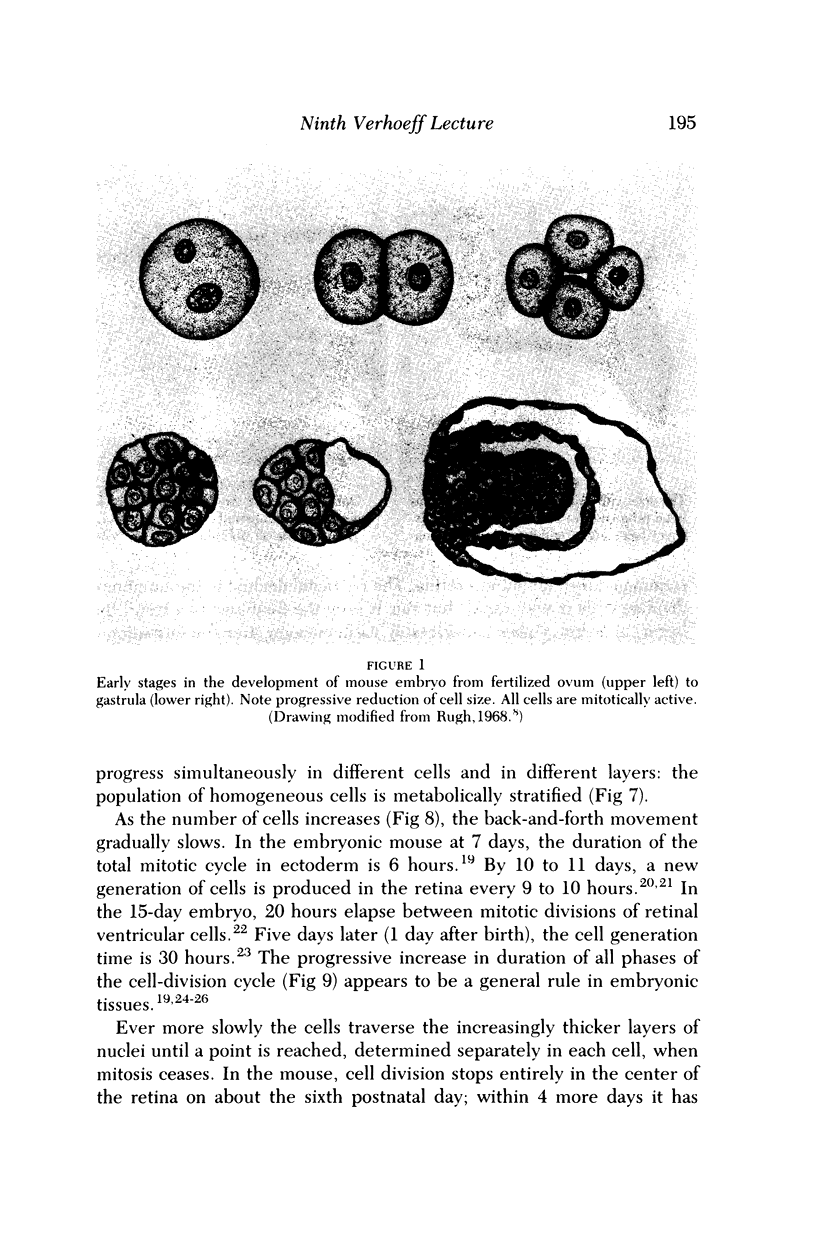
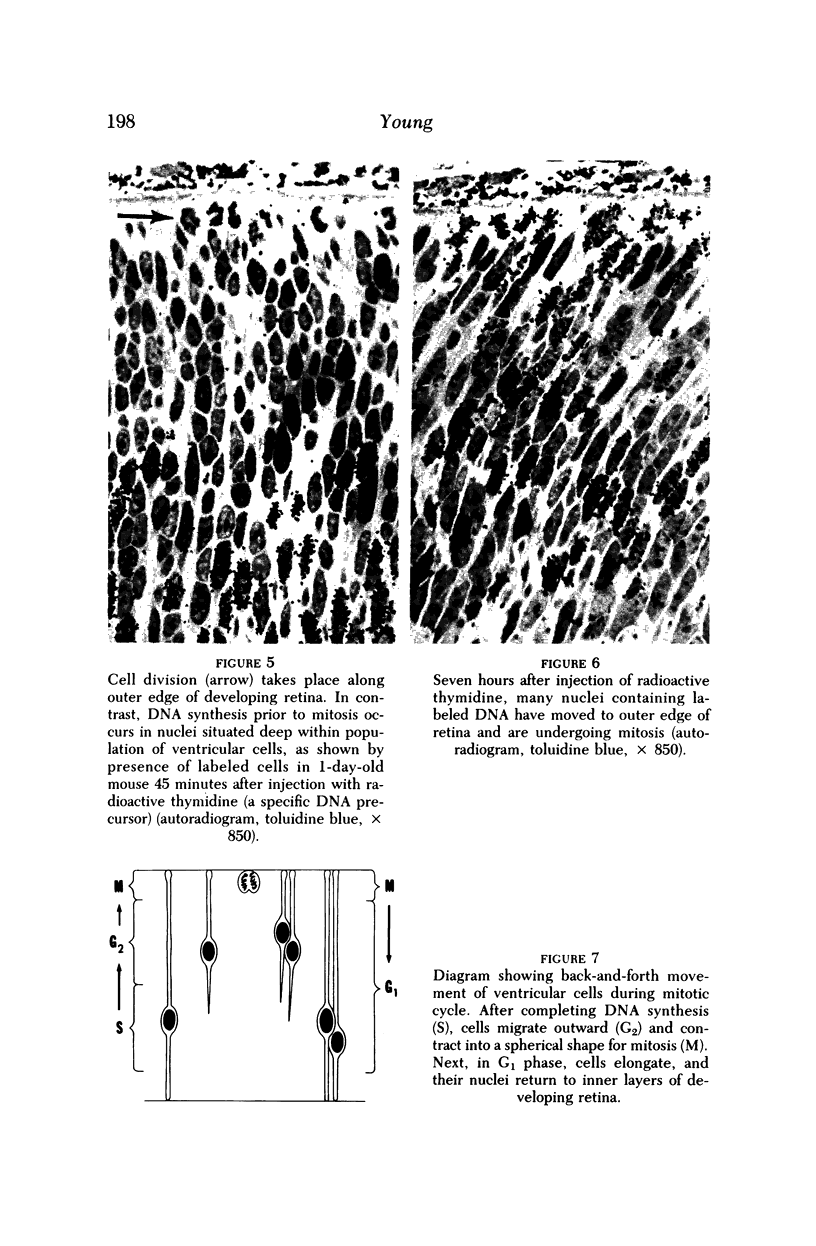
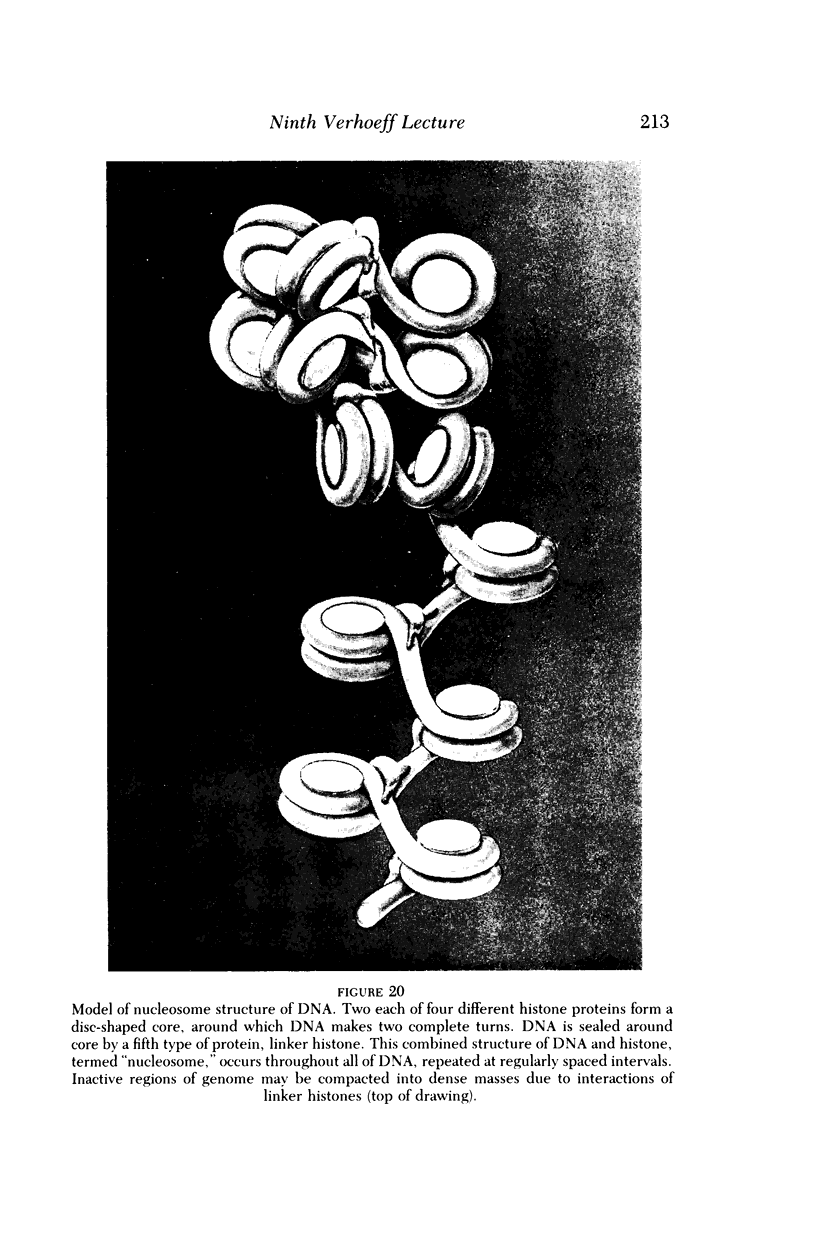
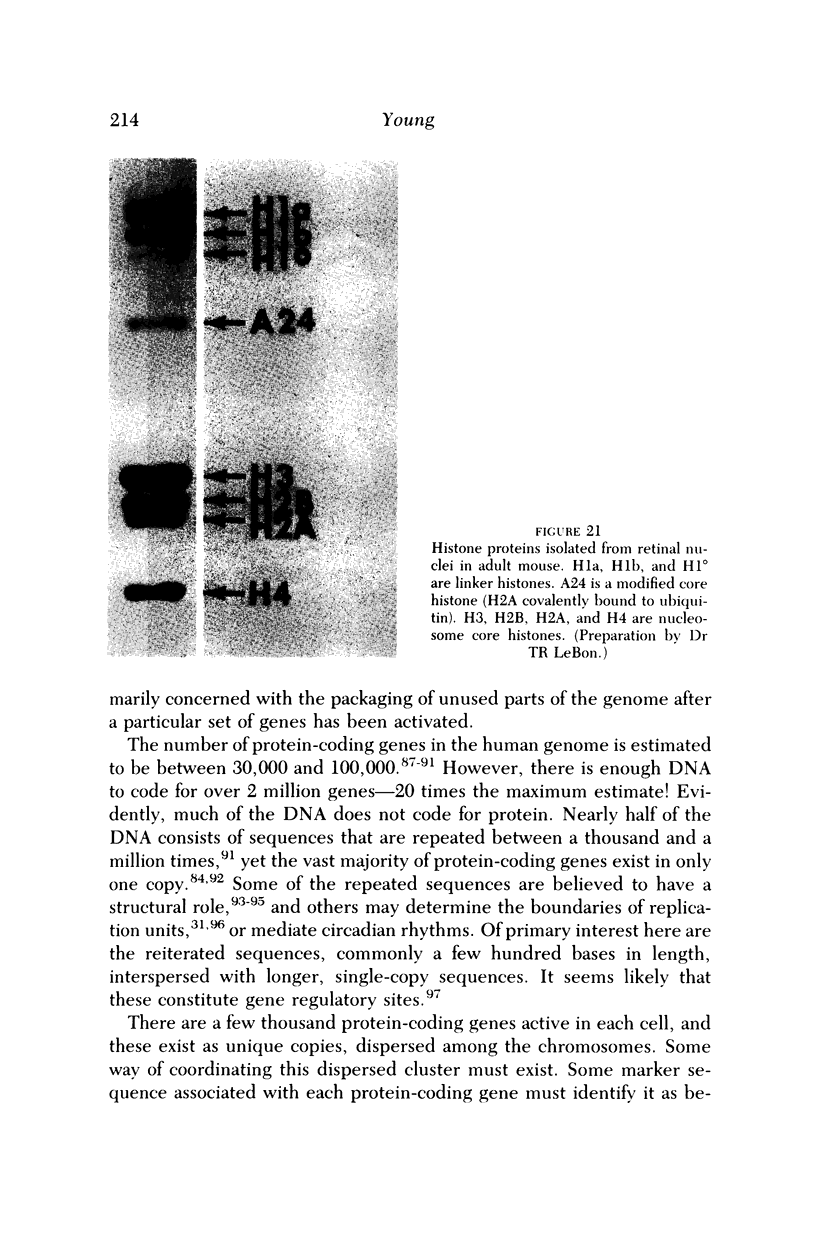
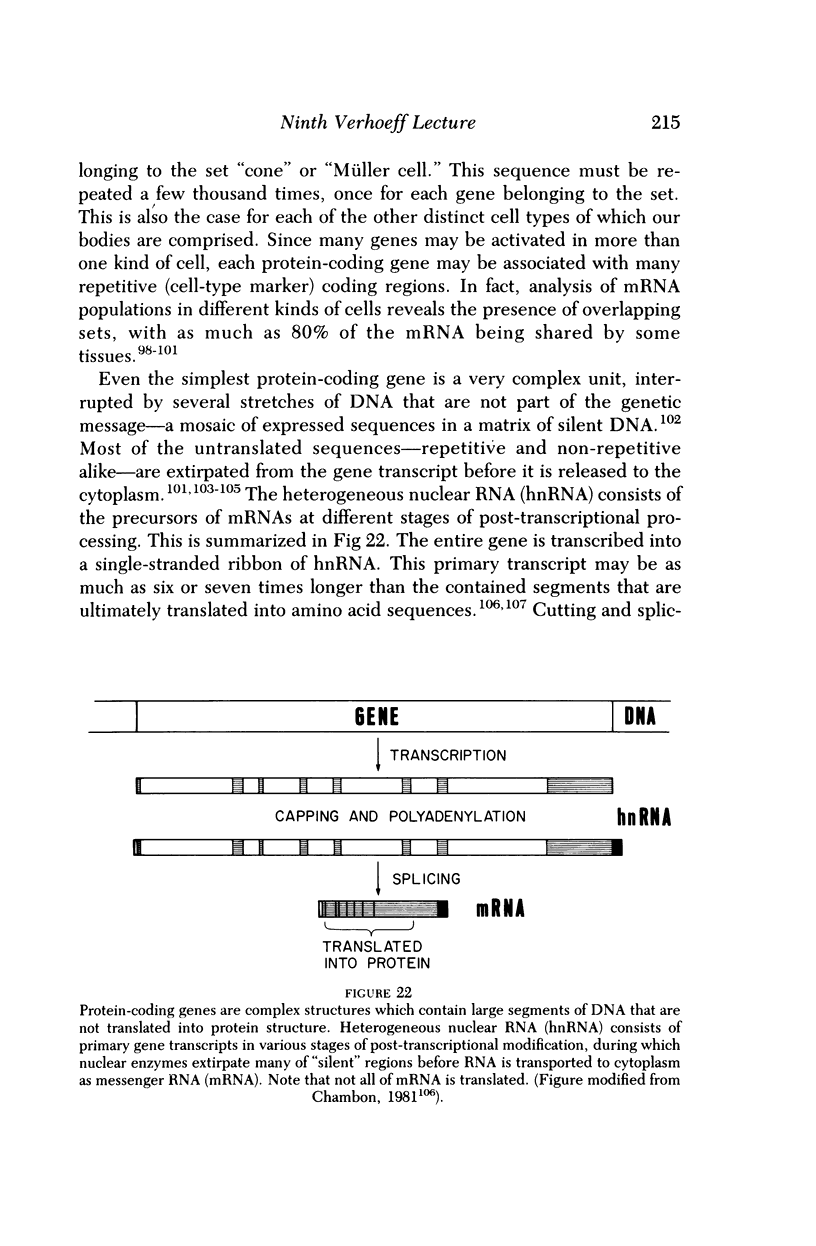
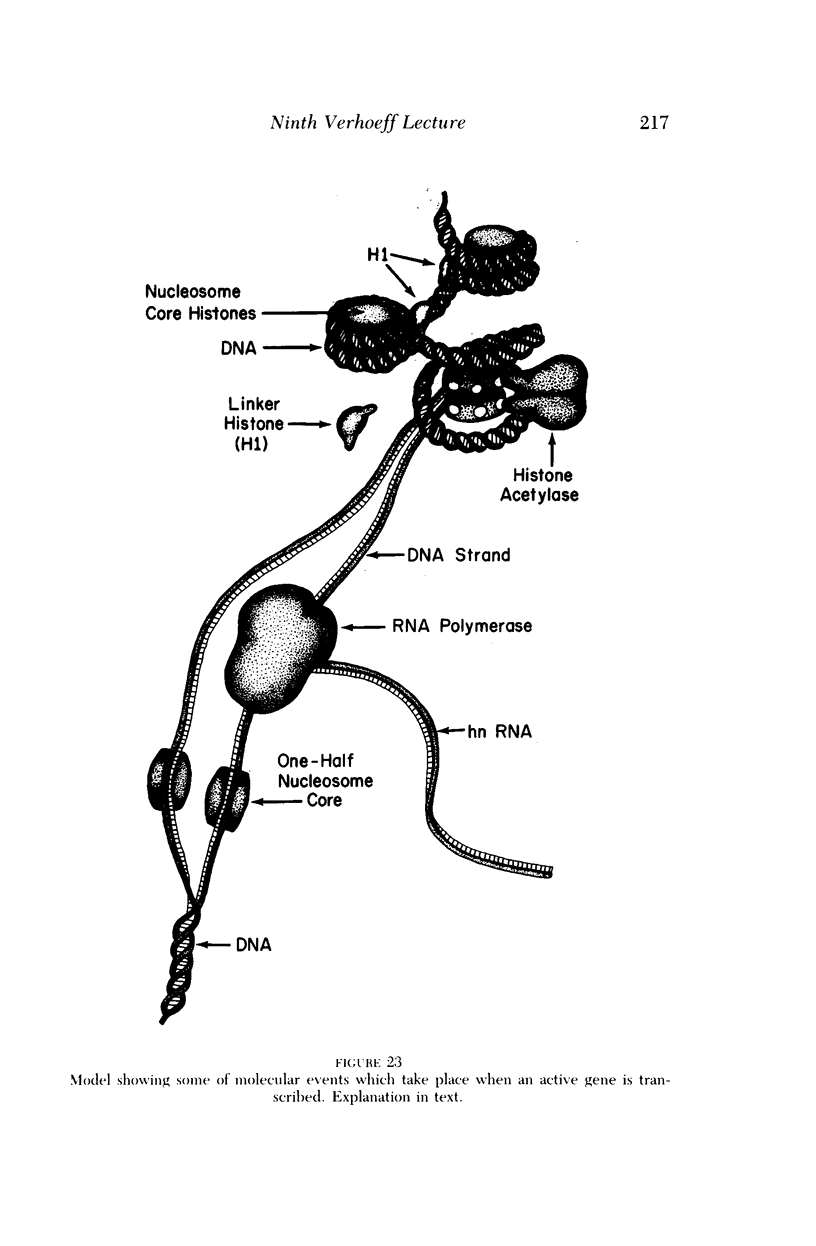
Full text
PDF



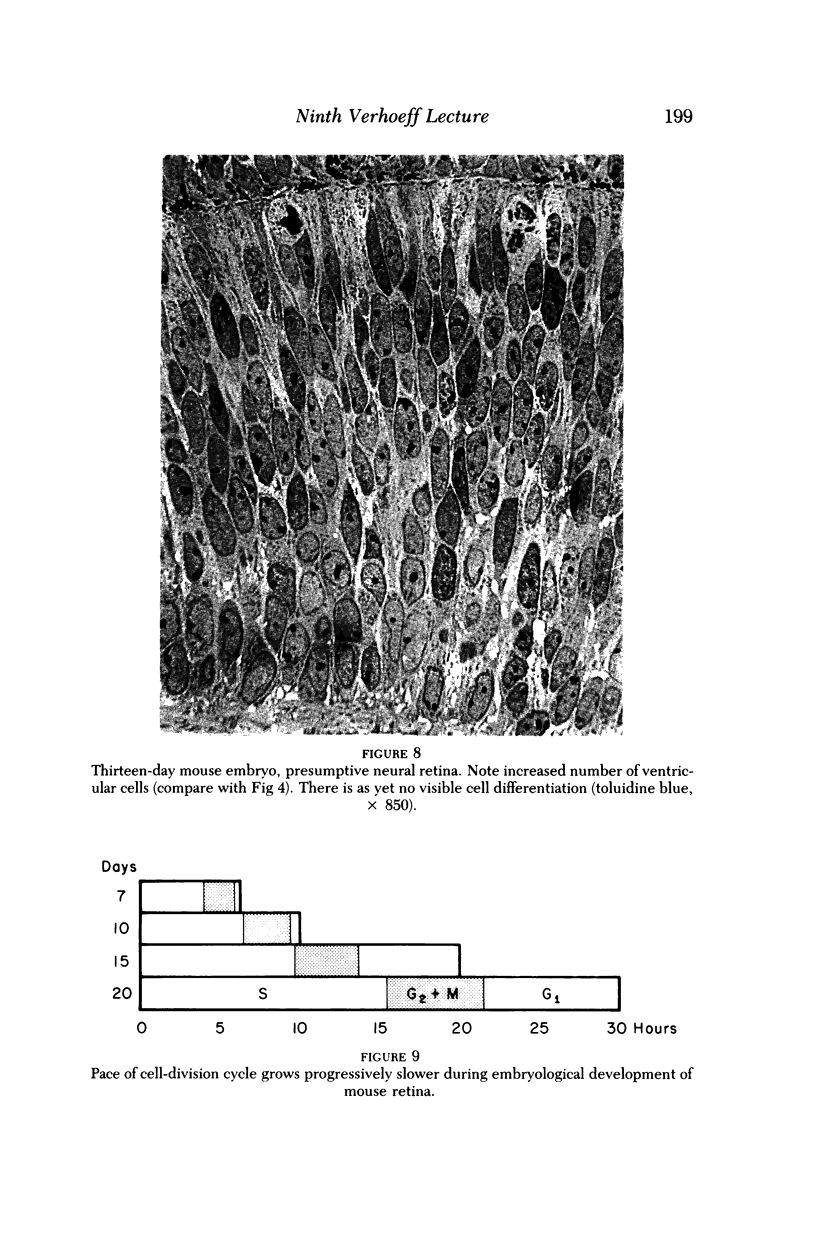
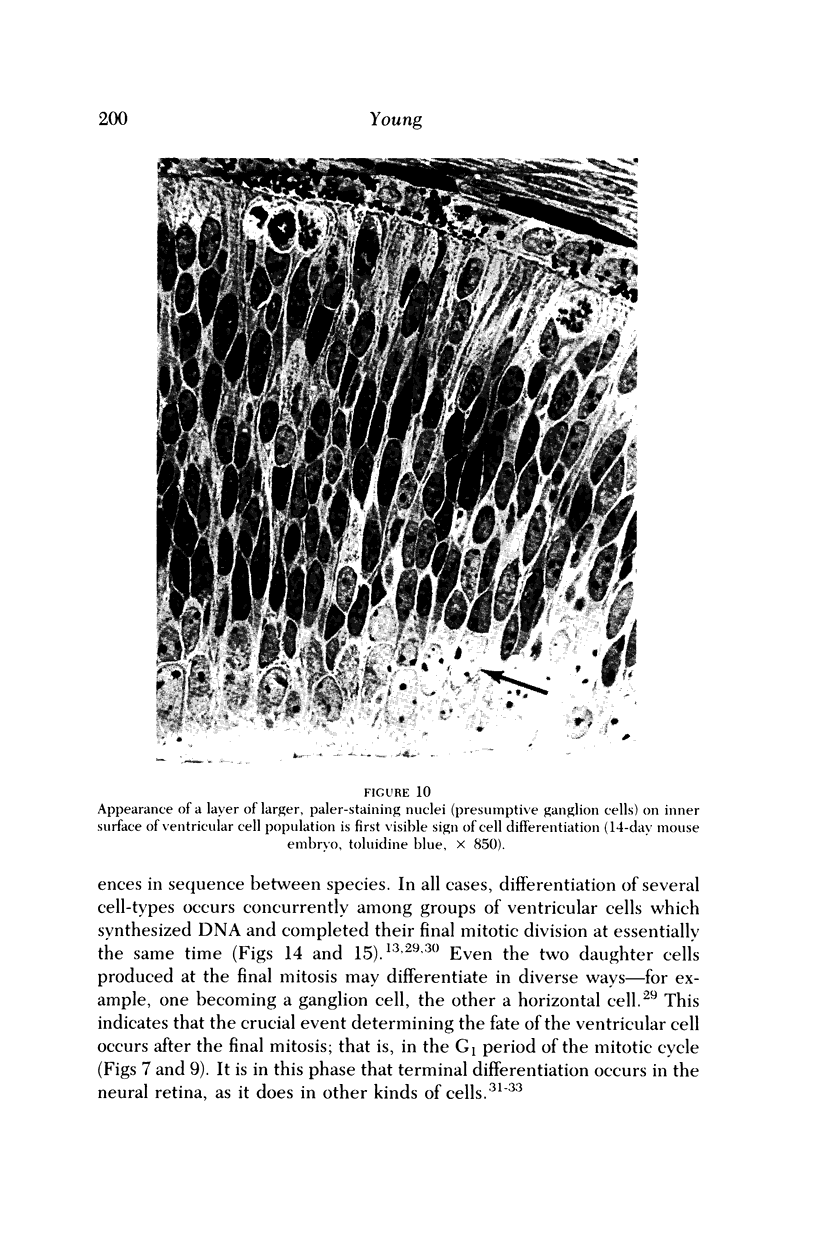
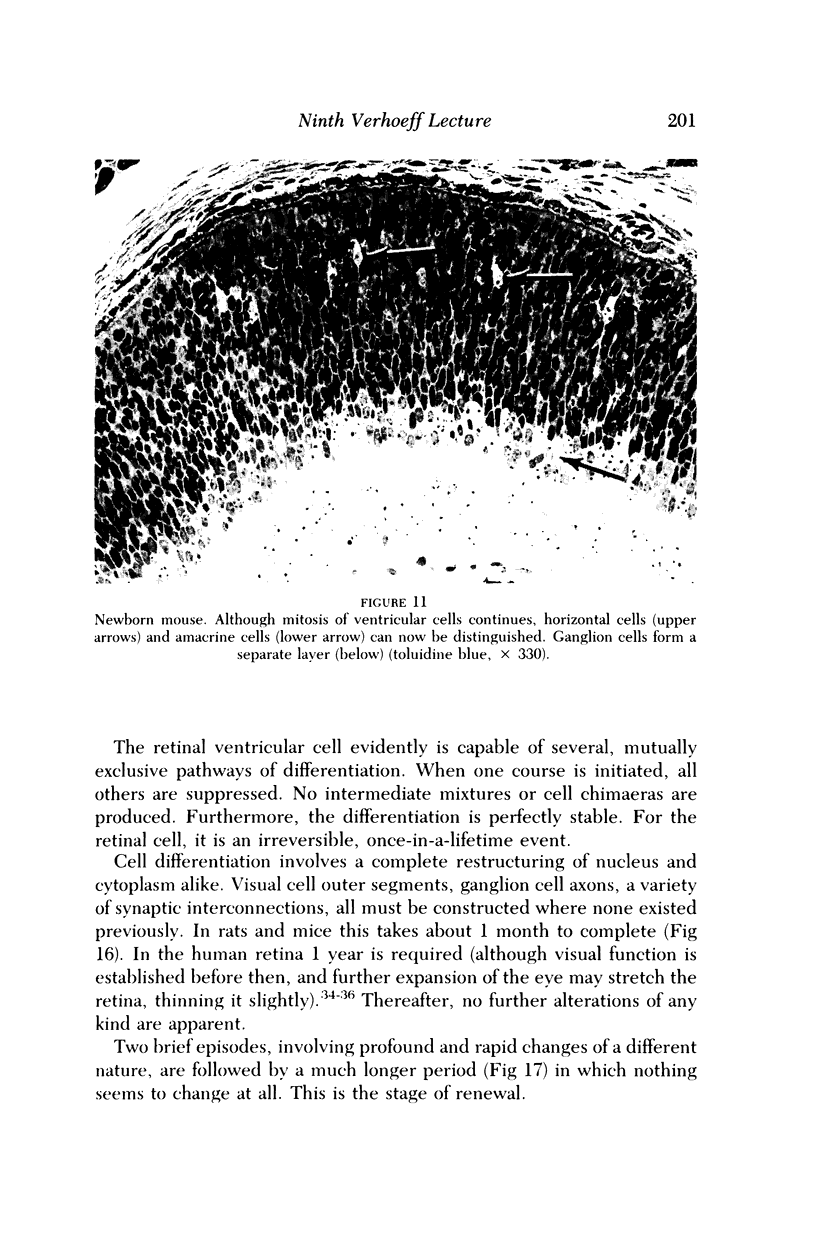
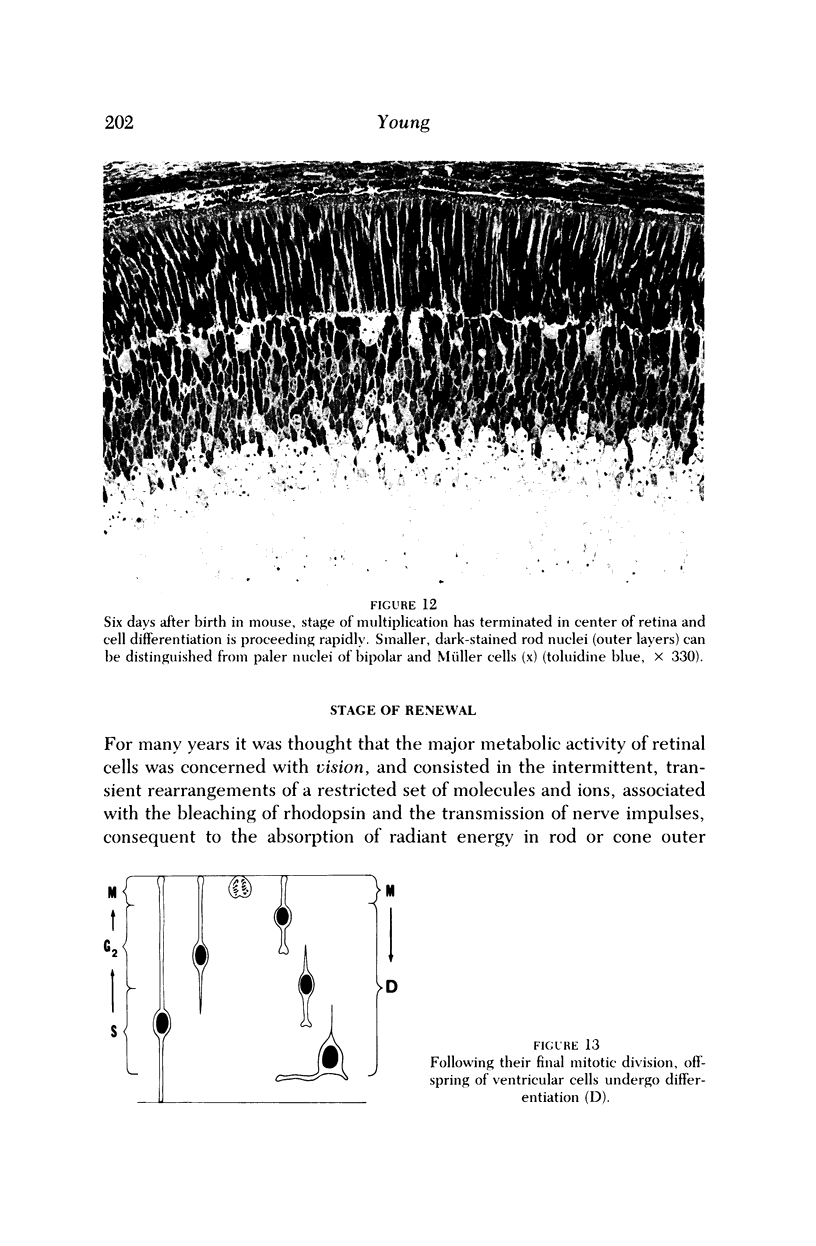
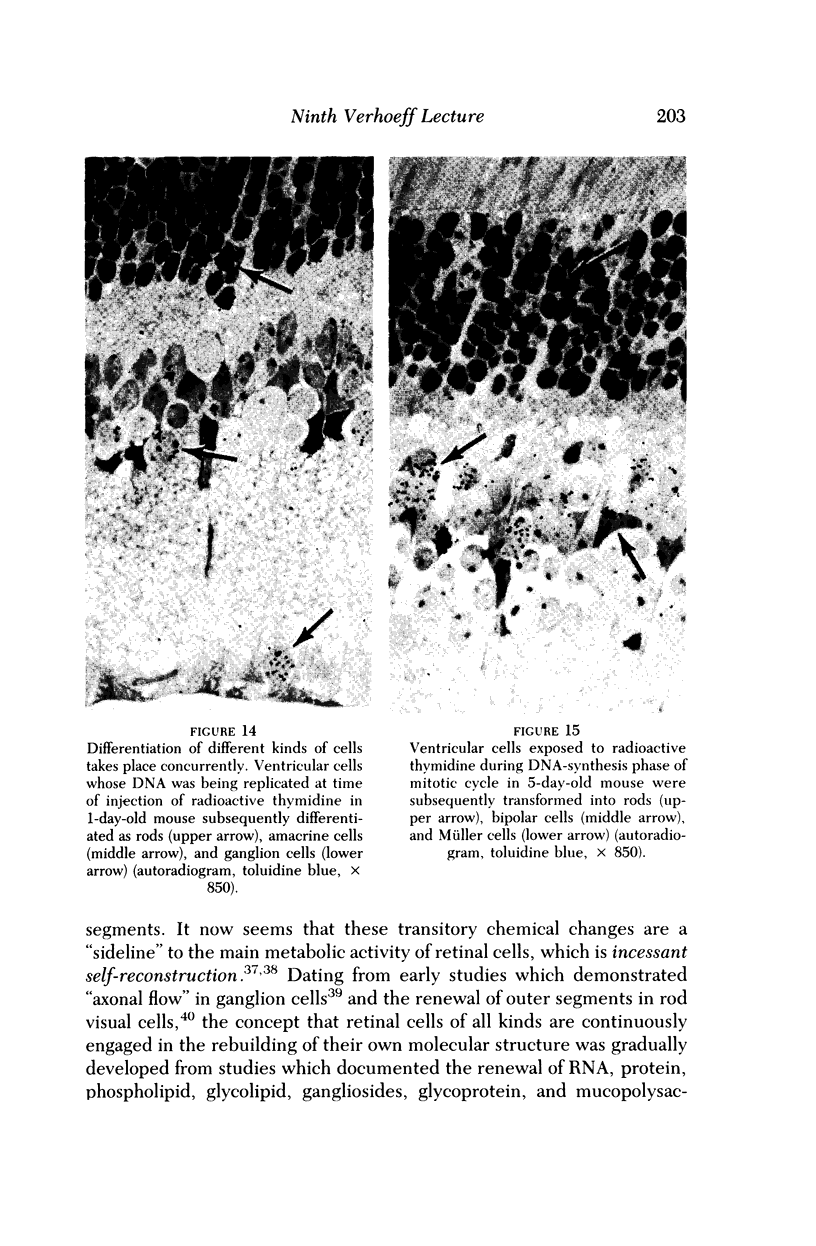

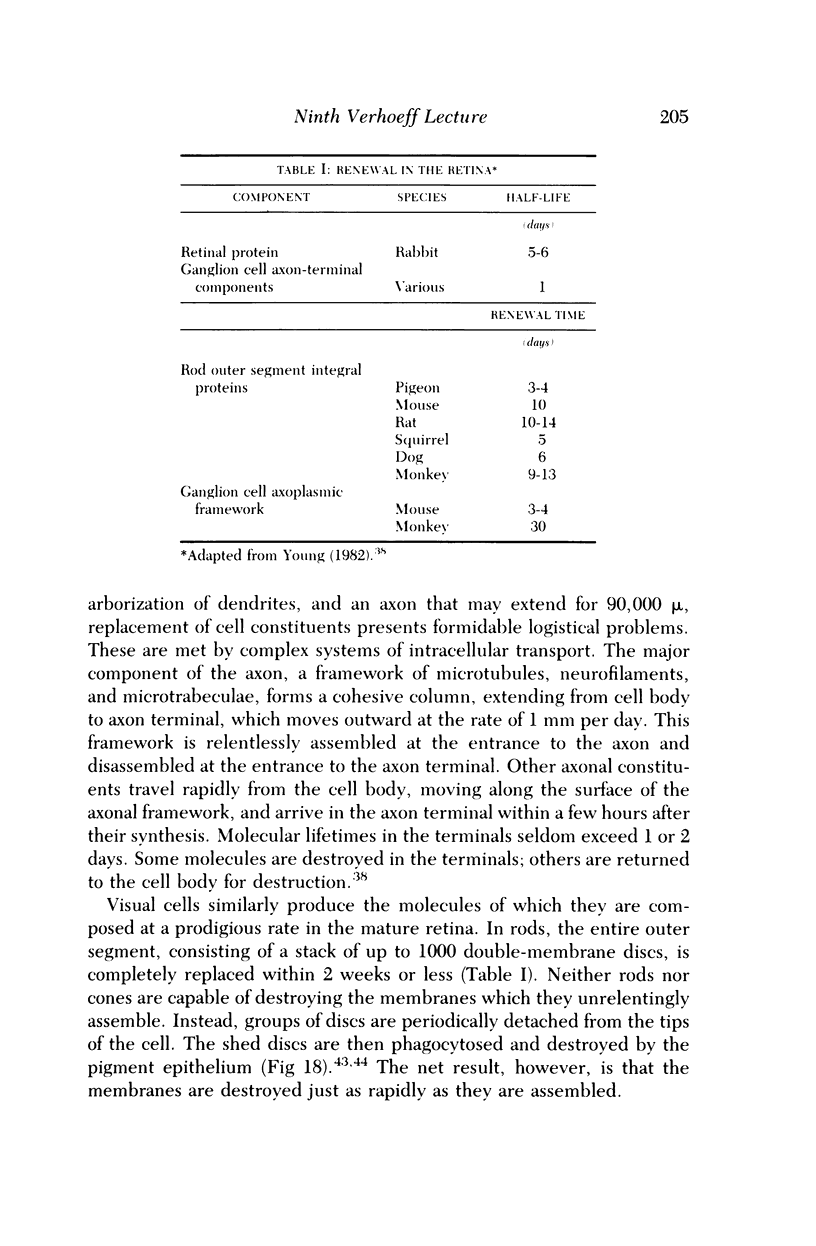
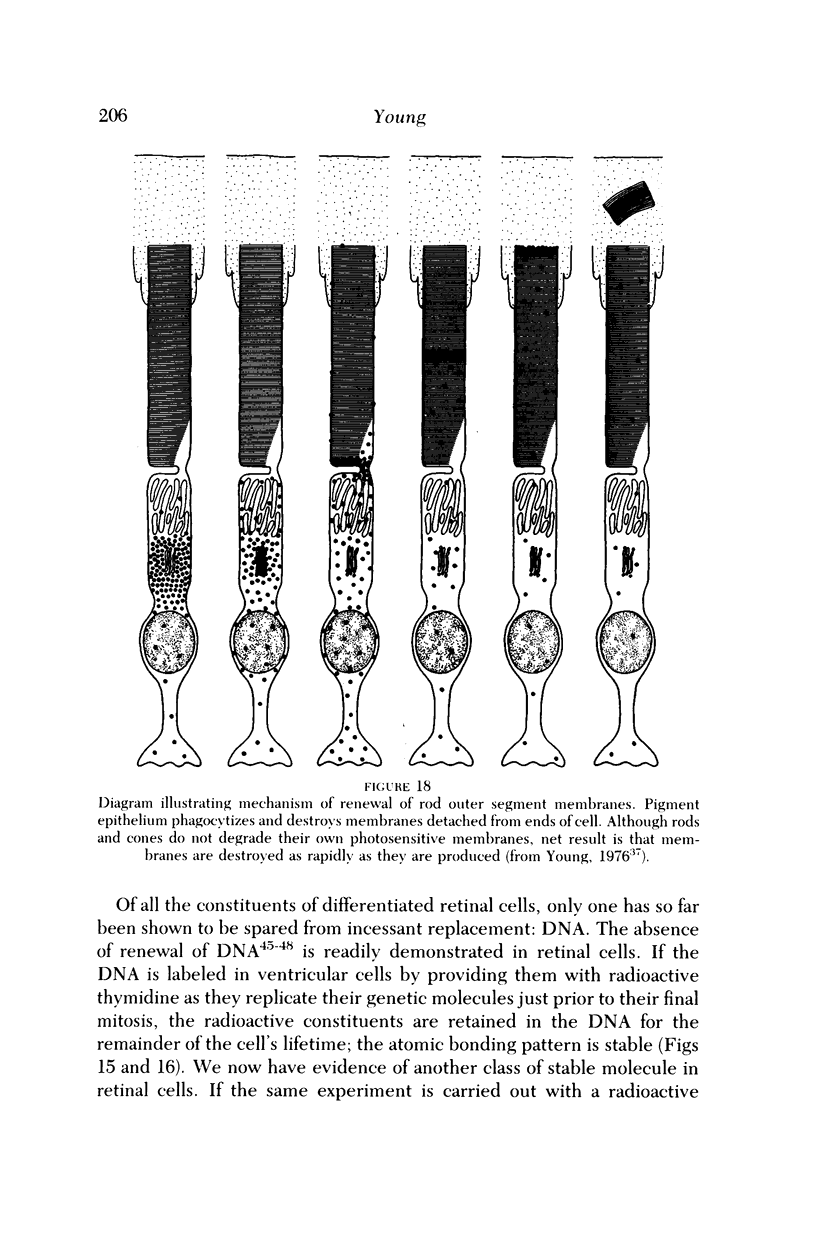
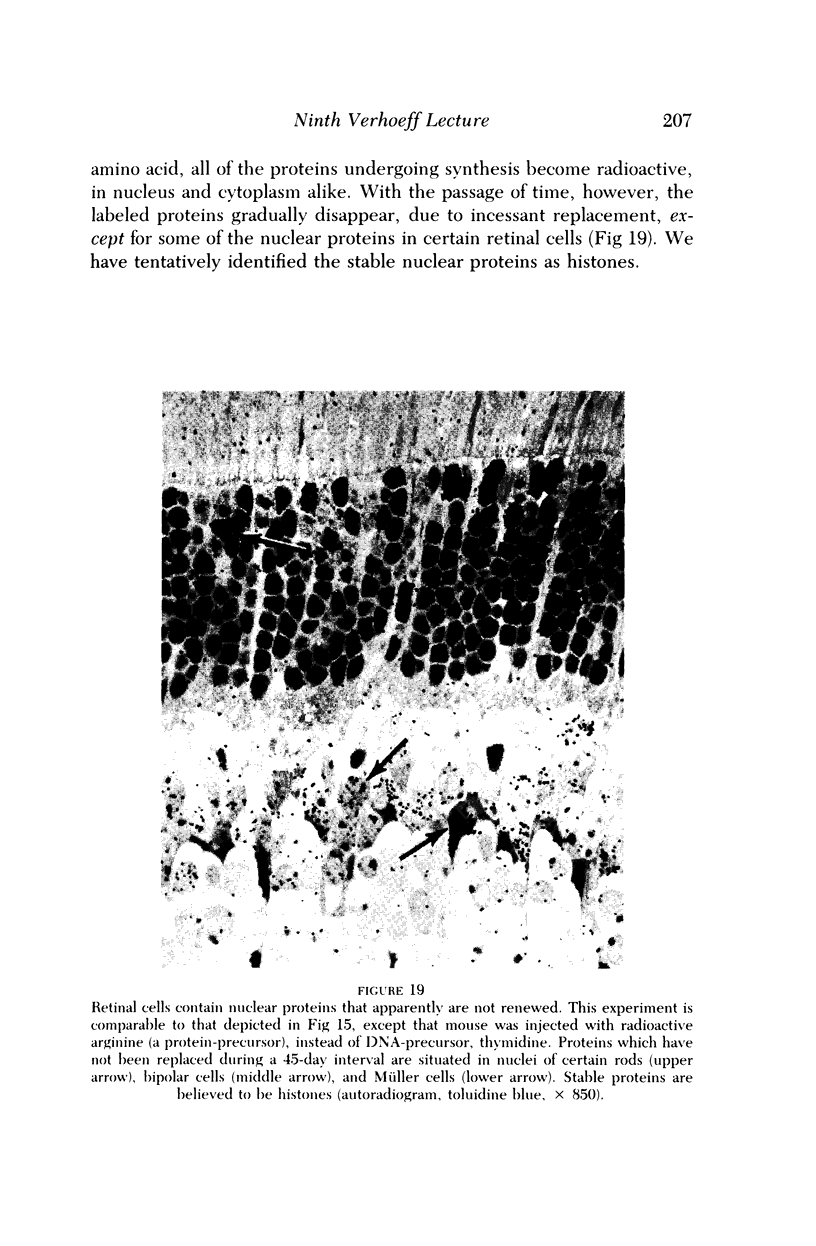

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Ames A., 3rd, Parks J. M., Nesbett F. B. Protein turnover in retina. J Neurochem. 1980 Jul;35(1):131–142. doi: 10.1111/j.1471-4159.1980.tb12498.x. [DOI] [PubMed] [Google Scholar]
- Appels R., Bolund L., Ringertz N. R. Biochemical analysis of reactivated chick erythrocyte nuclei isolated from chick-HeLa heterokaryons. J Mol Biol. 1974 Aug 5;87(2):339–355. doi: 10.1016/0022-2836(74)90154-5. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Oliver D., Hohmann P., Chalkley R., Granner D. Turnover of deoxyribonucleic acid, histones, and lysine-rich histone phosphate in hepatoma tissue culture cells. Biochemistry. 1972 Oct 10;11(21):3915–3921. doi: 10.1021/bi00771a013. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Hahn W. E. Complexity and characterization of polyadenylated RNA in the mouse brain. Cell. 1976 May;8(1):139–150. doi: 10.1016/0092-8674(76)90195-1. [DOI] [PubMed] [Google Scholar]
- Beach D. H., Jacobson M. Patterns of cell proliferation in the retina of the clawed frog during development. J Comp Neurol. 1979 Feb 1;183(3):603–613. doi: 10.1002/cne.901830308. [DOI] [PubMed] [Google Scholar]
- Bedell A. J. The Frederick H. Verhoeff Lecture: Angioid Streaks, a Kodachrome exposition. Trans Am Ophthalmol Soc. 1961;59:111–140. [PMC free article] [PubMed] [Google Scholar]
- Berg W. E., Mertes D. H. Rates of synthesis and degradation of protein in the sea urchin embryo. Exp Cell Res. 1970 May;60(2):218–224. doi: 10.1016/0014-4827(70)90508-2. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. The gene numbers game. Cell. 1974 Jun;2(2):81–86. doi: 10.1016/0092-8674(74)90095-6. [DOI] [PubMed] [Google Scholar]
- Boulikas T., Wiseman J. M., Garrard W. T. Points of contact between histone H1 and the histone octamer. Proc Natl Acad Sci U S A. 1980 Jan;77(1):127–131. doi: 10.1073/pnas.77.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braekevelt C. R., Hollenberg M. J. The development of the retina of the albino rat. Am J Anat. 1970 Mar;127(3):281–301. doi: 10.1002/aja.1001270305. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Wiebold J. L., Brunner S. Protein metabolism in preimplanted mouse ova. Dev Biol. 1976 Jul 15;51(2):215–224. doi: 10.1016/0012-1606(76)90139-1. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brown S. W. Heterochromatin. Science. 1966 Jan 28;151(3709):417–425. doi: 10.1126/science.151.3709.417. [DOI] [PubMed] [Google Scholar]
- Brunner G. Membrane impression and gene expression. Towards a theory of cytodifferentiation. Differentiation. 1977 Aug 11;8(2):123–132. doi: 10.1111/j.1432-0436.1977.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Burdman J. A., Haglid K., Dravid A. R. Protein synthesis in fractions from isolated brain cell nuclei. J Neurochem. 1970 May;17(5):669–676. doi: 10.1111/j.1471-4159.1970.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Byvoet P. Metabolic integrity of deoxyribonucleohistones. J Mol Biol. 1966 Jun;17(2):311–318. doi: 10.1016/s0022-2836(66)80143-2. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L. D., LaVail M. M. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979 Nov 15;188(2):263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- Chambon P. Split genes. Sci Am. 1981 May;244(5):60–71. doi: 10.1038/scientificamerican0581-60. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Dawid I. B. Biogenesis of mitochondria during Xenopus laevis development. Dev Biol. 1972 Apr;27(4):504–518. doi: 10.1016/0012-1606(72)90189-3. [DOI] [PubMed] [Google Scholar]
- Chikaraishi D. M., Deeb S. S., Sueoka N. Sequence complexity of nuclear RNAs in adult rat tissues. Cell. 1978 Jan;13(1):111–120. doi: 10.1016/0092-8674(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Cogan D. G. Frederick Herman Verhoeff--personal recollections. Trans Am Ophthalmol Soc. 1969;67:96–109. [PMC free article] [PubMed] [Google Scholar]
- DUKE-ELDER S. THE FREDERICK H. VERHOEFF LECTURE: THE SAGA OF A CENTURY. Trans Am Ophthalmol Soc. 1964;62:193–202. [PMC free article] [PubMed] [Google Scholar]
- Darnell F. E. mRNA structure and function. Prog Nucleic Acid Res Mol Biol. 1976;19:493–511. doi: 10.1016/s0079-6603(08)60941-1. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Note on the control of gene expression during development. J Theor Biol. 1971 Jul;32(1):123–130. doi: 10.1016/0022-5193(71)90140-8. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Candido E. P. Acetylated histone H4 is preferentially associated with template-active chromatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3574–3577. doi: 10.1073/pnas.75.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. J., Smith S. A. Electrophoretic analysis of proteins synthesized by preimplantation mouse embryos. Dev Biol. 1974 Oct;40(2):233–244. doi: 10.1016/0012-1606(74)90126-2. [DOI] [PubMed] [Google Scholar]
- FRESCO J. R., BENDICH A. The metabolic stability of rat liver deoxyribonucleic acid: a turnover study. J Biol Chem. 1960 Apr;235:1124–1128. [PubMed] [Google Scholar]
- FUJITA H., FUJITA S. Electron microscopic studies on neuroblast differentiation in the central nervous system of domestic fowl. Z Zellforsch Mikrosk Anat. 1963;60:463–478. doi: 10.1007/BF00336619. [DOI] [PubMed] [Google Scholar]
- FUJITA S. Kinetics of cellular proliferation. Exp Cell Res. 1962 Oct;28:52–60. doi: 10.1016/0014-4827(62)90311-7. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Trendelenburg M., Zentgraf H., Spring H. Morphology of transcriptionally active chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):755–772. doi: 10.1101/sqb.1978.042.01.076. [DOI] [PubMed] [Google Scholar]
- GROBSTEIN C. CYTODIFFERENTIATION AND ITS CONTROLS. Science. 1964 Feb 14;143(3607):643–650. doi: 10.1126/science.143.3607.643. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Lipson E. D., Britten R. J., Davidson E. H. Synthesis and turnover of polysomal mRNAs in sea urchin embryos. Cell. 1977 Mar;10(3):415–432. doi: 10.1016/0092-8674(77)90029-0. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev G. P. On the structural organization of operon and the regulation of RNA synthesis in animal cells. J Theor Biol. 1969 Dec;25(3):473–490. doi: 10.1016/s0022-5193(69)80034-2. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Butler P. J. Structure of transcriptionally-active chromatin subunits. Nucleic Acids Res. 1977 Sep;4(9):3155–3173. doi: 10.1093/nar/4.9.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Murphy R. F., Bonner J. Structure of transcriptionally active chromatin. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4404–4408. doi: 10.1073/pnas.72.11.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobstein C. What we do not know about differentiation. Am Zool. 1966 Feb;6(1):89–95. doi: 10.1093/icb/6.1.89. [DOI] [PubMed] [Google Scholar]
- Grouse L., Chilton M. D., McCarthy B. J. Hybridization of ribonucleic acid with unique sequences of mouse deoxyribonucleic acid. Biochemistry. 1972 Feb 29;11(5):798–805. doi: 10.1021/bi00755a019. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Hardin J. M. The metabolism of histone fractions. 3. Synthesis and turnover of histone f1. Arch Biochem Biophys. 1970 Feb;136(2):392–401. doi: 10.1016/0003-9861(70)90210-9. [DOI] [PubMed] [Google Scholar]
- Hancock R. Conservation of histones in chromatin during growth and mitosis in vitro. J Mol Biol. 1969 Mar 28;40(3):457–466. doi: 10.1016/0022-2836(69)90165-x. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Hervé B., Jacquemin E., Courtois Y. Histones biosynthesis and turnover in epithelial lens cells cultured in vitro. Cell Biol Int Rep. 1979 May;3(3):271–281. doi: 10.1016/0309-1651(79)90040-7. [DOI] [PubMed] [Google Scholar]
- Hinds J. W., Hinds P. L. Differentiation of photoreceptors and horizontal cells in the embryonic mouse retina: an electron microscopic, serial section analysis. J Comp Neurol. 1979 Oct 1;187(3):495–511. doi: 10.1002/cne.901870303. [DOI] [PubMed] [Google Scholar]
- Hinds J. W., Hinds P. L. Early development of amacrine cells in the mouse retina: an electron microscopic, serial section analysis. J Comp Neurol. 1978 May 15;179(2):277–300. doi: 10.1002/cne.901790204. [DOI] [PubMed] [Google Scholar]
- Hinds J. W., Hinds P. L. Early ganglion cell differentiation in the mouse retina: an electron microscopic analysis utilizing serial sections. Dev Biol. 1974 Apr;37(2):381–416. doi: 10.1016/0012-1606(74)90156-0. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Rao P. N. Nucleo-cytoplasmic interactions in the acheivement of nuclear synchrony in DNA synthesis and mitosis in multinucleate cells. Biol Rev Camb Philos Soc. 1971 Feb;46(1):97–155. doi: 10.1111/j.1469-185x.1971.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Reich J. Stability of differentiation-specific and nonspecific messenger RNA in insect cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1458–1465. doi: 10.1073/pnas.60.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman S. L. Lengthening of the generation cycle during embryonic differentiation of the mouse neural tube. Exp Cell Res. 1968 Feb;49(2):420–424. doi: 10.1016/0014-4827(68)90191-2. [DOI] [PubMed] [Google Scholar]
- Konyukhov B. V., Sazhina M. V. Genetic control over the duration of G 1 phase. Experientia. 1971 Aug;27(8):970–971. doi: 10.1007/BF02135780. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Klug A. The nucleosome. Sci Am. 1981 Feb;244(2):52–64. doi: 10.1038/scientificamerican0281-52. [DOI] [PubMed] [Google Scholar]
- LAURENCE D. J., BUTLER J. A. METABOLISM OF HISTONES IN MALIGNANT TISSUES AND LIVER OF THE RAT AND MOUSE. Biochem J. 1965 Jul;96:53–62. doi: 10.1042/bj0960053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard D. A., La Marca M. J. In vivo synthesis and turnover of cytoplasmic ribosomal RNA by stage 6 oocytes of Xenopus laevis. Dev Biol. 1975 Jul;45(1):199–202. doi: 10.1016/0012-1606(75)90254-7. [DOI] [PubMed] [Google Scholar]
- Levenson R., Housman D. Commitment: how do cells make the decision to differentiate? Cell. 1981 Jul;25(1):5–6. doi: 10.1016/0092-8674(81)90225-7. [DOI] [PubMed] [Google Scholar]
- Levinson J., Goodfellow P., vadeboncoeur M., McDevitt H. Identification of stage-specific polypeptides synthesized during murine preimplantation development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3332–3336. doi: 10.1073/pnas.75.7.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levner M. H. RNA transcription in mature sea urchin eggs. Exp Cell Res. 1974 Apr;85(2):296–302. doi: 10.1016/0014-4827(74)90130-x. [DOI] [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Ruddle F. H. The status of the gene map of the human chromosomes. Science. 1977 Apr 22;196(4288):390–405. doi: 10.1126/science.850784. [DOI] [PubMed] [Google Scholar]
- McMahon D. Chemical messengers in development: a hypothesis. Science. 1974 Sep 20;185(4156):1012–1021. doi: 10.1126/science.185.4156.1012. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Schull W. J. On some trends in understanding the genetics of man. Perspect Biol Med. 1968 Summer;11(4):565–602. doi: 10.1353/pbm.1968.0011. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Perry W. M., Chalkley R. Sensitivity of regions of chromatin containing hyperacetylated histones to DNAse I. Biochem Biophys Res Commun. 1978 May 15;82(1):365–363. doi: 10.1016/0006-291x(78)90617-4. [DOI] [PubMed] [Google Scholar]
- Nemer M., Dubroff L. M., Graham M. Properties of sea urchin embryo messenger RNA containing and lacking poly(A). Cell. 1975 Oct;6(2):171–178. doi: 10.1016/0092-8674(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Oba Y., Hayashi K., Nakagawa Y., Yamaguchi Z. Metabolic activities of histones in rat liver and spleen. Eur J Biochem. 1975 Aug 15;56(2):343–352. doi: 10.1111/j.1432-1033.1975.tb02239.x. [DOI] [PubMed] [Google Scholar]
- Ohta T., Kimura M. Functional organization of genetic material as a product of molecular evolution. Nature. 1971 Sep 10;233(5315):118–119. doi: 10.1038/233118a0. [DOI] [PubMed] [Google Scholar]
- Oudet P., Germond J. E., Bellard M., Spadafora C., Chambon P. Nucleosome structure. Philos Trans R Soc Lond B Biol Sci. 1978 May 11;283(997):241–258. doi: 10.1098/rstb.1978.0021. [DOI] [PubMed] [Google Scholar]
- Oudet P., Spadafora C., Chambon P. Nucleosome structure II: structure of the SV40 minichromosome and electron microscopic evidence for reversible transitions of the nucleosome structure. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):301–312. doi: 10.1101/sqb.1978.042.01.032. [DOI] [PubMed] [Google Scholar]
- Paul J. The transcriptional unit in eukaryotes. Genetics. 1975 Jun;79 (Suppl):151–157. [PubMed] [Google Scholar]
- Peterson J. L., McConkey E. H. Non-histone chromosomal proteins from HeLa cells. A survey by high resolution, two-dimensional electrophoresis. J Biol Chem. 1976 Jan 25;251(2):548–554. [PubMed] [Google Scholar]
- Piha R. S., Cuénod M., Waelsch H. Metabolism of histones of brain and liver. J Biol Chem. 1966 May 25;241(10):2397–2404. [PubMed] [Google Scholar]
- Richards B. M., Pardon J. F., Lilley D. M., Cotter R. I., Wooley J. C., Worcester D. L. Nucleosome sub-structure during transcription and replication. Philos Trans R Soc Lond B Biol Sci. 1978 May 11;283(997):287–289. doi: 10.1098/rstb.1978.0025. [DOI] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. M. Increase in retinal surface area during infancy and childhood. J Pediatr Ophthalmol Strabismus. 1982 Jul-Aug;19(4):16–20. doi: 10.3928/0191-3913-19820701-06. [DOI] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT R. B., BELL E. PROTEIN SYNTHESIS DURING DEVELOPMENT: CONTROL THROUGH MESSENGER RNA. Science. 1964 Aug 14;145(3633):711–714. doi: 10.1126/science.145.3633.711. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L., MIALE I. L., FEDER N. Cell proliferation and migration in the primitive ependymal zone: an autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1959 Oct;1:322–333. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- SONNEBORN T. M. THE DETERMINANTS AND EVOLUTION OF LIFE. THE DIFFERENTIATION OF CELLS. Proc Natl Acad Sci U S A. 1964 May;51:915–929. doi: 10.1073/pnas.51.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami H., Mitsui Y., Murota S., Yamada M. Effect of growth stage on histone H1 metabolism in human diploid fibroblasts. J Cell Physiol. 1982 Feb;110(2):213–218. doi: 10.1002/jcp.1041100216. [DOI] [PubMed] [Google Scholar]
- Savage M. J., Sala-Trepat J. M., Bonner J. Measurement of the complexity and diversity of poly(adenylic acid) containing messenger RNA from rat liver. Biochemistry. 1978 Feb 7;17(3):462–467. doi: 10.1021/bi00596a014. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Deininger P. L. Sequence organization of the human genome. Cell. 1975 Nov;6(3):345–358. doi: 10.1016/0092-8674(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Letourneau G. E., Wassarman P. M. Program of early development in the mammal: changes in patterns and absolute rates of tubulin and total protein synthesis during oogenesis and early embryogenesis in the mouse. Dev Biol. 1979 Feb;68(2):341–359. doi: 10.1016/0012-1606(79)90209-4. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res. 1978 Jun;5(6):1863–1876. doi: 10.1093/nar/5.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley M. A., Anderson K. M. Electron-microscopic visualization of transcriptionally active and less active chromatin fractions from the rat ventral prostate and their content of histones. Can J Biochem. 1977 Jan;55(1):9–18. doi: 10.1139/o77-002. [DOI] [PubMed] [Google Scholar]
- Sieger M., Pera F., Schwarzacher H. G. Genetic inactivity of heterochromatin and heteropycnosis in Microtus agrestis. Chromosoma. 1970;29(3):349–364. doi: 10.1007/BF00325948. [DOI] [PubMed] [Google Scholar]
- Sinitsina V. F. Sintez DNK i kinetika kletochnykh populiatsii pri 'embrional'nom gistogeneze setchatki u myshei. Arkh Anat Gistol Embriol. 1971 Oct;61(10):58–67. [PubMed] [Google Scholar]
- Solter D., Skreb N., Damjanov I. Cell cycle analysis in the mouse EGG-cylinder. Exp Cell Res. 1971 Feb;64(2):331–334. doi: 10.1016/0014-4827(71)90084-x. [DOI] [PubMed] [Google Scholar]
- Spira A. W., Hollenberg M. J. Human retinal development: ultrastructure of the inner retinal layers. Dev Biol. 1973 Mar;31(1):1–21. [PubMed] [Google Scholar]
- Stein G., Baserga R. Continued synthesis of non-histone chromosomal proteins during mitosis. Biochem Biophys Res Commun. 1970 Nov 9;41(3):715–722. doi: 10.1016/0006-291x(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Tarnowka M. A., Baglioni C., Basilico C. Synthesis of H1 histones by BHK cells in G1. Cell. 1978 Sep;15(1):163–171. doi: 10.1016/0092-8674(78)90092-2. [DOI] [PubMed] [Google Scholar]
- Taylor A. C., Weiss P. Demonstration of axonal flow by the movement of tritium-labeled protein in mature optic nerve fibers. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1521–1527. doi: 10.1073/pnas.54.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. RNA metabolism in previtellogenic oocytes of Xenopus laevis. Dev Biol. 1974 Aug;39(2):191–197. doi: 10.1016/0012-1606(74)90234-6. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Tolstoshev P., Crystal R. G. Secondary structures for splice junctions in eukaryotic and viral messenger RNA precursors. Nucleic Acids Res. 1980 Aug 25;8(16):3659–3672. doi: 10.1093/nar/8.16.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J., Barton S. C., Johnson M. H. Molecular differentiation in the preimplantation mouse embryo. Nature. 1976 Jan 29;259(5541):319–321. doi: 10.1038/259319a0. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953 Apr 25;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- WATTS J. W., HARRIS H. Turnover of nucleic acids in a non-multiplying animal cell. Biochem J. 1959 May;72(1):147–153. doi: 10.1042/bj0720147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Rutter W. J. Phases in cell differentiation. Sci Am. 1969 Mar;220(3):36–44. doi: 10.1038/scientificamerican0369-36. [DOI] [PubMed] [Google Scholar]
- Wolpert L., Lewis J. H. Towards a theory of development. Fed Proc. 1975 Jan;34(1):14–20. [PubMed] [Google Scholar]
- Young R. W., Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969 Aug;42(2):392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967 Apr;33(1):61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976 Sep;15(9):700–725. [PubMed] [Google Scholar]
- van Blerkom J., Brockway G. O. Qualitative patterns of protein synthesis in the preimplantation mouse embryo. I. Normal pregnancy. Dev Biol. 1975 May;44(1):148–157. doi: 10.1016/0012-1606(75)90382-6. [DOI] [PubMed] [Google Scholar]