Abstract
Constitutive expression of Wnt1 and Wnt5a in HC11 mammary cells led to elevated TCF transcriptional activity. Intriguingly, Wnt-expressing cells also displayed activation of ErbB1 and mitogen-activated protein kinase (MAPK), in contrast to control HC11 cells, which did not. Furthermore, conditioned media harvested from Wnt-expressing cells stimulated ErbB1 and the MAPK cascade when added to control cells. This process was rapid and could be blocked by an ErbB1 antibody that interferes with ligand binding and by matrix metalloproteinase (MMP) inhibitors. These results suggest that in mammary cells Wnt binding to its receptor, Frizzled (Fz), transactivates ErbB1, probably by MMP-mediated release of soluble ErbB1 ligands. Importantly, Wnt-transactivated ErbB1 was responsible for MAPK activation and the increased levels of cyclin D1 present in the Wnt-expressing HC11 cells. Our finding that Wnts transactivate ErbB1 in addition to stimulating the prototypic β-catenin/TCF pathway may help to explain why wnt1 is a potent oncogene in the mammary gland.
Introduction
Wnts are a family of secreted glycoproteins that play important roles in normal development. The mammary gland expresses multiple Wnts (Gavin & McMahon, 1992), and some, like Wnt4, are known to have specific developmental roles (Brisken et al., 2000). Members of the Frizzled (Fz) family of seven-pass transmembrane proteins are receptors for Wnts. Wnt binding to Fz initiates a pathway that prevents glycogen synthase kinase-3β (GSK-3β) from phosphorylating β-catenin, one of its important substrates. This leads to β-catenin stabilization and translocation to the nucleus, where it binds transcription factors of the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family (Nusse, 1999; van Noort & Clevers, 2002). This pathway is a driving force in the development of various human cancers such as colonic cancer and melanomas (Bienz & Clevers, 2000; Polakis, 2000).
The ErbB family of receptor tyrosine kinases (RTKs) and their activating ligands, the epidermal growth factor (EGF)-related peptides, have important functions in the normal mammary gland (Troyer & Lee, 2001) and in the development of breast cancer (Olayioye et al., 2000). Furthermore, ErbB1 has emerged as an important mediator of signalling from other classes of transmembrane receptors including G protein-coupled receptors (GPCRs), other RTKs, and cytokine and integrin receptors (Carpenter, 1999; Gschwind et al., 2001). Considering the central role of ErbB1 in signal transduction, we have explored the possibility that the Wnt pathway interacts with the ErbB receptor/ligand network in the mammary gland.
In these studies we have used the HC11 mammary cell line, an immortalized cell line that has been valuable in characterizing the role of ErbB receptors in proliferation, differentiation and transformation (Taverna et al., 1991; Brandt et al., 2001). We demonstrate here, for the first time, that Wnt peptides transactivate ErbB1, presumably by increasing the availability of ErbB1 ligands, which in turn leads to strong stimulation of the mitogen-activated protein kinase (MAPK) pathway. ErbB1 transactivation has a specific biological effect: stimulation of cyclin D1 expression. These findings highlight the importance of ErbB1 signalling in the mammary gland and may provide a novel means of interfering with the activity of this receptor in breast cancer.
Results and Discussion
Wnt1 and Wnt5a stimulate TCF transcriptional activity
In our studies we used Wnt1, the prototypic oncogene first detected in mouse mammary tumour virus (MMTV)-induced mammary cancer (Nusse & Varmus, 1982) and Wnt5a, one of the Wnt family members normally expressed in the mammary gland (Gavin & McMahon, 1992). HC11 cells lines stably expressing these Wnt proteins were engineered and either studied directly or used as a source of Wnt-containing conditioned media. To do this, HC11 cells were initially transfected with vectors conferring puromycin resistance and containing a TCF luciferase reporter (TopTK) (Brantjes et al., 2001). After antibiotic selection, cells were infected with retroviruses encoding either neomycin resistance and Wnt1/Wnt5a or neomycin resistance only. Cell lines with double drug-resistance, referred to as Wnt1-HC11, Wnt5a-HC11 and C-HC11 (control), were selected. Microarray analyses were performed to determine whether Wnt and Fz genes are expressed in C-HC11 cells and to confirm expression of the introduced Wnt-encoding complementary DNAs in the Wnt1-HC11 and Wnt5a-HC11 cell lines. C-HC11 cells were found to express mRNAs for Fz6, Fz7 and Fz8, and Wnt4 and Wnt7b; high levels of Wnt1 and Wnt5a messenger RNA were detected in the cell lines Wnt1-HC11 and Wnt5a-HC11 (Table 1).
Table 1.
Expression of Wnt and Fz genes in cell lines as assayed by microarray analysis
| Gene expression levels (arbitrary units) |
|||
| C-HC11 |
Wnt1-HC11 |
Wnt5a-HC11 |
|
| Wnt1 | 0 | 20,765 | 0 |
| Wnt4 | 3,066 | 0 | 3,252 |
| Wnt5a | 0 | 675 | 7,630 |
| Wnt7b | 2,891 | 0 | 3,853 |
| Fz6 | 586 | n.t. | n.t. |
| Fz7 | 284 | n.t. | n.t. |
| Fz8 | 295 | n.t. | n.t. |
n.t. indicates that the cell line was not tested for expression of the corresponding gene.
Elevation of cytosolic levels of β-catenin is considered important for its ability to bind and transcriptionally activate TCF (Bienz, 1998). In comparison to C-HC11 cells, cytosolic fractions from Wnt1-HC11 and Wnt5a-HC11 cells contain high levels of β-catenin (Fig. 1A) and display enhanced Top-TK luciferase activity (Fig. 1B). Thus, in HC11 cells, both Wnt1 and Wnt5a activate the classic β-catenin/TCF pathway, presumably through TCF4, which is expressed in these cells (Fig. 1B). To examine short-term Wnt-induced signalling, C-HC11 cells were treated with conditioned media harvested from cultures of either Wnt1-HC11 or Wnt5a-HC11 cells. Conditioned media from both cultures caused a fivefold increase in luciferase activity, confirming the biological activity of the secreted Wnt proteins (Fig. 1C).
Figure 1.
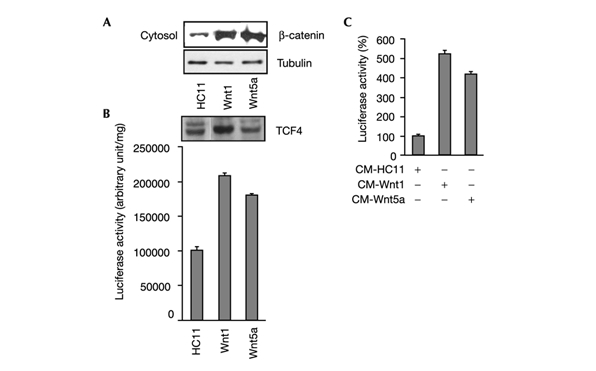
Activity of the Wnt/β-catenin/TCF pathway in HC11 cells. C-HC11 and Wnt-HC11 cells were used in (A) and (B); conditioned medium (CM) from Wnt-HC11 cells was used in (C). (A) Cytosolic fractions were prepared and 50 μg samples used for immunoblots to detect β-catenin and tubulin expression (tubulin was used used as a loading control). (B) (Upper panel) Whole cell lysates were immunoblotted with a TCF4-specific antibody. (Lower panel) TopTK activity was measured using whole cell lysates; luciferase activity was standardized for protein content. (C) TopTK activity was measured 48 h after addition of Wnt-containing conditioned medium.
Wnts activate ErbB1 and ERK
ErbB1 is transactivated by many classes of membrane proteins (Carpenter, 1999; Gschwind et al., 2001); however, Wnt/Fz-mediated ErbB1activation has not been reported. To examine this, ErbB1 was immunoprecipitated and a western blot analysis was performed with phosphotyrosinespecific antiserum. In contrast to ErbB1 from C-HC11 cells, which showed low levels of tyrosine phosphorylation, ErbB1 was highly phosphorylated in Wnt1-HC11 and Wnt5a-HC11 cells (Fig. 2A column 1). In C-HC11 cells, but not Wnt1- or Wnt5a-expressing cells, treatment with EGF led to a large increase in ErbB1 phosphorylation (Fig. 2A, column 2), suggesting that the receptor is fully activated in Wnt1-HC11 and Wnt5a-HC11 cells. Neither ErbB2 nor ErbB3 was phosphorylated in C-HC11, Wnt1-HC11 or Wnt5a-expressing cells (not shown). The MAPK and phosphatidylinositol-3-OH kinase (PI(3)K) pathways are major signalling cascades downstream of activated ErbB receptors (Olayioye et al., 2000). Antisera specific for the active, phosphorylated forms of ERK1 and ERK2 and protein kinase B (PKB), the major kinases in the MAPK and PI(3)K pathways respectively, were used to detect their activity. In Wnt1-HC11 and Wnt5a-HC11 cells, the basal level of phosphorylated ERK1/2 (Fig. 2B, column 1), but not of phosphorylated PKB (not shown), was elevated compared to the level in C-HC11 cells. To examine the role of activated ErbB1 in MAPK signalling we used PKI166, an ErbB1selective kinase inhibitor (Traxler et al., 2001). Pretreatment of C-HC11 cells with PKI166 prevented EGF induction of MAPK activity (Fig. 2B, column 4). Treatment of Wnt-HC11 cells with PKI166 decreased the level of ErbB1 phosphotyrosine residues by approximately 50% (Fig. 2A, column 3). Importantly, PKI166 treatment reduced the constitutive level of tyrosine phosphorylation of ERK1/2 to basal levels in the Wnt-expressing HC11 cells (Fig. 2B, column 3), suggesting that in these cells ErbB1 is responsible for activation of the MAPK pathway.
Figure 2.
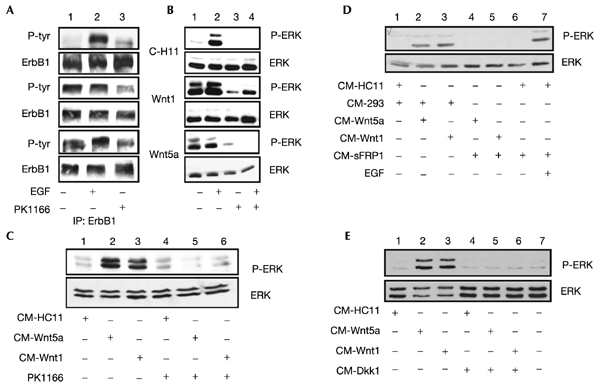
ErbB1 and ERK1/2 are active in Wnt-HC11 cells. (A, B) C-HC11 and Wnt-HC11 cells were either incubated for 10 min with 100 ng ml−1 EGF and/or for 1 h with 5 μM PKI166, or left untreated before preparing whole cell lysates. (A) ErbB1 was immunoprecipitated from 500 μg whole cell lysate and immunoblotted with a phosphotyrosine specific antibody (P-ERK indicates tyrosine-phosphorylated ERK); membranes were stripped and reprobed for ErbB1. (B–E) Samples of whole cell lysate (50 μg) were immunoblotted with a phospho-ERK1/2 antibody; membranes were stripped and reprobed for ERK1/2 or ERK1. (C) C-HC11 cells were incubated for 10 min with conditioned media from C-HC11 and Wnt-HC11 cells; where indicated, cultures were pretreated for 1 h with PKI166. (D) Conditioned media from untransfected (control) or sFRP1-expressing 293 cells was premixed for 1 h with an equal volume of conditioned media from C-HC11 or Wnt-expressing HC11 cells. These mixtures were then added to C-HC11 cultures for 10 min; where indicated, cells were incubated with EGF for 10 min. (E) C-HC11 cells were pretreated 1 h with conditioned media from untransfected or Dkk1-expressing 293 cells, followed by incubation of cultures with conditioned media from C-HC11 or Wnt-HC11 cells for 10 min.
We then tested the ability of Wnt-containing conditioned media to stimulate the MAPK pathway. Treatment of C-HC11 cells for 10 min with conditioned media containng Wnt1 or Wnt5a increased ERK1/2 phosphotyrosine levels fivefold to sixfold (Fig. 2C, columns 2 and 3); conditioned medium from C-HC11 cells had no effect (Fig. 2C; column 1). Furthermore, in the presence of PKI166, the ability of Wnt-containing conditioned media to increase phospho-ERK1/2 levels was blocked (Fig. 2C, columns 5 and 6), suggesting a direct involvement of ErbB1 in Wnt-induced ERK1/2 phosphorylation. The effect of conditioned media on ERK1/2 activity was shown to be due to signalling through the standard Wnt/Fz/low-density-lipoprotein-related protein (Lrp) pathway: in the presence of conditioned medium containing secreted Frizzled-related protein-1 (sFRP-1), which competes with Fz for Wnt binding (Uren et al., 2000), ERK phosphotyrosine levels were decreased (Fig. 2D). The same effect was seen when Dickkopf-1 (Dkk), which antagonizes Lrp, a Wnt co-receptor (Mao et al., 2001), was present in the medium (Fig. 2D). Treatment of cells with EGF induced ERK1/2 phosphorylation in the presence of sFRP1 (Fig. 2D, column 7), showing that sFRP1 specifically blocks Wnts.
Wnt effects require metalloproteinase activity
These data suggest that in HC11 mammary cells Wnts have the ability to transactivate ErbB1 and to stimulate MAPK. A straightforward explanation for these findings would be that Wnt binding to Fz causes an increase in the availability of ErbB1-activating ligands expressed in the cells (Table 2). The ectodomains of these ligands are processed by metalloproteinases, leading to the production of soluble growth factors (Massague & Pandiella, 1993). Activation of ErbB1 by soluble ligands, rather than by membrane-bound forms, is thought to be required for most of its biological functions (Dong et al., 1999). Wnt could increase the activity of specific metalloproteinases, which cleave these ligands. Furthermore, it has been demonstrated that ErbB1 transactivation by the G-protein-coupled receptor ligands endothelin and thrombin results from metalloproteinase-mediated cleavage of the precursor of heparin-binding EGF (HB-EGF) (Prenzel et al., 1999; Gschwind et al., 2001).
Table 2.
Expression levels of TGF-α, HB-EGF and AR in C-HC11 and Wnt-transfected HC11 cells
| Expression levels (arbitrary units) |
|||
| TGF-α |
HB-EGF |
AR |
|
| C-HC11 | 3.66 | 1.48 | 35.8 |
| Wnt1-HC11 | 0 | 2.31 | 1.27 |
| Wnt5a-HC11 | 2.69 | 2.42 | 32.3 |
To test the function of metalloproteinases in Wnt-expressing HC11 cells, two metalloproteinase inhibitors were used: phenanthroline, a metal-ion chelator, and CGS27023A, an enzyme inhibitor (Wang et al., 1999). Treatment of cells for 1 h with each compound led to a strong decrease in the level of phospho-ErbB1 (Fig. 3A, columns 2 and 3). Furthermore, each inhibitor also decreased the level of phospho-ERK1/2 in these cells (Fig. 3B, columns 2 and 3). In C-HC11 cells, ErbB1 and ERK1/2 display basal levels of phosphorylation, which are further reduced in the presence of the MMP inhibitors (Fig. 3A and B), suggesting that ligand-activated ErbB1 is responsible for basal levels of ERK1/2 activation. In Wnt1-HC11 and Wnt5a–HC11 cells, this process appears to be enhanced; the MMP inhibitors were next tested in the presence of Wnt-containing conditioned media, and CGS 27023A (Fig. 3C, columns 4–6) and phenanthroline (Fig. 3C, columns 7–9) both blocked the ability of the Wnt proteins to stimulate ERK1/2 activity in C-HC11 cells (Fig. 3C, columns 1–3).
Figure 3.
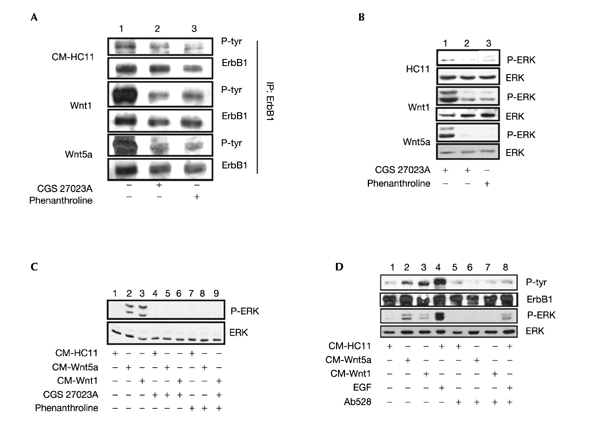
Activity of ErbB1 and ERK1/2 is sensitive to metalloproteinase (MMP) inhibitors. (A, B) C-HC11 and Wnt-expressing HC11 cultures were treated for 1 h with CGS27023A, phenanthroline (both at 50 μM) or carrier (DMSO). (A) ErbB1 was immunoprecipitated (IP) from 500 μg whole cell lysate and immunoblotted with a phosphotyrosine (p-Tyr)-specific antibody; membranes were stripped and reprobed for ErbB1. (B) Samples of whole cell lysate (50 μg) were immunoblotted with a anti-phospho-ERK1/2 antibody; membranes were stripped and reprobed for ERK1. (C) C-HC11 or (D) 293 cultures were treated for 10 min with conditioned media from C-HC11 or Wnt-expressing HC11 cells. Where indicated, cultures were pretreated for 1 h with MMP inhibitors, or 1 h with 10 μg ml−1 Ab528, or treated for 10 min with EGF. Phospho-ERK1/2 and ERK1 levels were determined as in (B); the level of ErbB1 phosphotyrosine was determined as in (A).
These results suggest that Wnt/Fz-mediated activation of ErbB1 and the MAPK pathway occurs through MMP-induced processing of ErbB1 ligands. To test this hypothesis we employed an ErbB1-specific blocking antibody, which interferes with ligand binding to the extracellular domain of the receptor. There are no commercially available blocking antibodies specific for the mouse receptor; however, monoclonal antibody (mAb) 528 intereferes with ligand binding to the human receptor (Badache & Hynes, 2000). Therefore, human embryonic kidney (HEK)-293 cells, which endogenously express ErbB1, were used for this experiment. As seen for HC11 cells, treatment of HEK-293 cells with conditioned media containing Wnt1 or Wnt5a stimulated TCF transcriptional activity (G.C., unpublished results). There was also an increase in the amount of phosphotyrosine on the immunoprecipitated ErbB1 (Fig. 3D, columns 2 and 3), as well as increased phospho-ERK levels (Fig. 3D, columns 2 and 3). In the presence of mAb 528 the ability of EGF, as well as Wnt-containing conditioned media, to stimulate ErbB1 phosphorylation and MAPK activity was blocked (Fig. 3D, columns 6–8).
These results suggest that Wnt binding to Fz increases the availability of ErbB1 ligands. HC11 cells, like the mammary gland (Troyer & Lee, 2001), express transforming growth factor-α (TGF-α), heparin-binding EGF (HB-EGF) and amphiregulin (AR) (Table 2; Hynes et al., 1990). Quantitative real-time polymerase chain reaction (PCR) (Sorensen et al., 2000) revealed that these ligands are expressed at similar levels in C-HC11 and Wnt5a-HC11 cells, wheras Wnt1-HC11 cells express HB-EGF, but express low levels of AR and no TGF-α (Table 2). Thus, HB-EGF appears to be a likely candidate for mediating the effects of Wnts on ErbB1. The fact that the Wnt/Fz/Lrp pathway antagonists sFRP1 and Dkk1 blocked the ability of Wnt-containing conditioned media to activate ErbB1 and the MAPK pathway (Fig. 2D and E) suggests that in the conditioned media the EGF-related ligands are not freely available for ErbB1 binding. These results, which are in agreement with the work of Prenzel et al. (1999), suggest that the ligands might be trapped by the extracellular matrix.
Wnt1 stimulates cyclin D1 through ErbB1
Wnt target genes have have been identified in different biological systems and include cell cycle regulators such as D-type cyclins and c-myc (He et al., 1998; Tetsu & McCormick, 1999; van de Wetering et al., 2002; Willert et al., 2002). Both Wnt1-HC11 and Wnt5a-HC11 cells express higher levels of cyclin D1, in comparison with C-HC11 cells. In addition, Wnt1-HC11 cells, but not C-HC11 or Wnt5a cells, express cyclin D2 (Fig. 4A). C-HC11 cells treated for 6 h with Wnt-containing conditioned media responded in a similar way: both Wnts stimulated cyclin D1 expression, while only Wnt1 enhanced cyclin D2 levels (Fig. 4B). In mammary cells, D-type cyclins are targets of active ErbB receptors (Lane et al., 2000; Neve et al., 2000). Therefore, we used PKI166 and CGS27023A to assess the contribution of Wnt-transactivated ErbB1 on D-type cyclin expression. PKI166 reduced cyclin D1 to control levels in both Wnt1-HC11 and Wnt5a-HC11 cells (Fig. 4C), suggesting that ErbB1 is responsible for increased cyclin D expression in Wnt-expressing cells. Addition of EGF to the medium was also shown to stimulate cyclin D1 expression (Fig. 4C). In contrast, the ErbB1 inhibitor had no effect on cyclin D2 levels (Fig. 4D). Wnt-expressing HC11 cells treated with the MMP inhibitor CGS27023A also showed decreased expression of cyclin D1, but not cyclin D2 (Fig. 4D).
Figure 4.
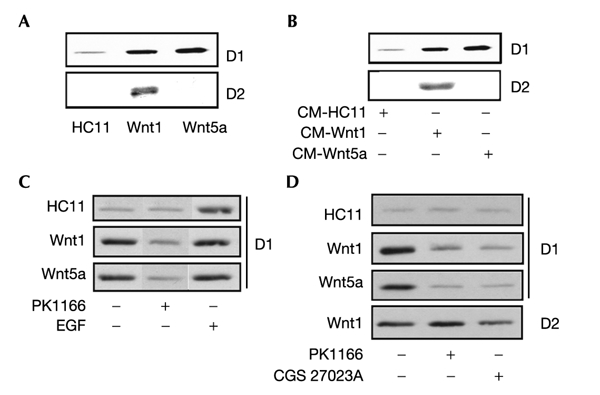
Cyclin D1 and cyclin D2 are differentially controlled by Wnt1 and Wnt5a. Whole cell lysates were prepared and 50 μg samples immunoblotted with antibodies against cyclin D1 or cyclin D2. C-HC11 and Wnt-expressing HC11 cells were treated for 6 h with PKI166 (A), CGS27023A (C) or EGF (D). (B) C-HC11 cells were treated for 6 h with conditioned medium from Wnt-HC11 cells.
In summary, our results show that ErbB1 is transactivated by Wnt1 and Wnt5a in mammary epithelial cells. The ability of Wnt proteins to transactivate this receptor has not been described previously, thus adding the Wnts to the list of receptors that use ErbB1 as a target to stimulate intracellular signalling (Gschwind et al., 2001). Transactivated ErbB1 has a specific effect in mammary cells: it is responsible for increasing cyclin D1 expression. Although it has been reported that cyclin D1 is a direct target of TCF (Tetsu & McCormick, 1999), our results are more consistent with a recent publication showing that expression of a dominant-negative form of TCF in colonic cancer cells had no effect on cyclin D1 levels (van de Wetering et al., 2002). In contrast to cyclin D1, Wnt1 stimulates cyclin D2 expression in an ErbB1-independent manner in HC11 cells. Interestingly, increased levels of cyclin D2 have been found in mammary tumours arising in MMTV-Wnt1 transgenics (Yu et al., 2001), suggesting that cyclin D2 could be a direct target of Wnt1 in mammary cells.
Although wnt1 was the first oncogene detected in MMTV-induced mammary cancer (Nusse & Varmus, 1982), there is still uncertainty concerning the role of the Wnt signalling pathway in human breast cancer. Unlike other types of cancer in which mutations in the genes encoding the adenomatous polyposis coli protein or β-catenin lead to stabilization of the β-catenin/TCF complex and increased activity of the pathway (Polakis, 2000), there is no evidence that these two proteins are consistently affected in breast cancer. However, other mechanisms, leading to constitutive activation of this pathway in breast tumours could exist (see, for example, Ugolini et al., 2001). Our results suggest that aberrant Wnt expression may contribute to breast cancer malignancy by increasing the signalling potential of ErbB1. Therapeutic compounds designed to inhibit the ability of Wnts to interact with their receptor could potentially provide an additional means to lower the level of ErbB signalling, thereby decreasing the malignancy of breast cancers.
Methods
Antibodies and inhibitors.
Antibodies used were: anti-β-catenin and anti-ERK1 (Transduction Laboratories); anti-TCF4 and anti-phosphotyrosine AG10 (Upstate Biotechnology); anti-ErbB1 (mAbs 1005 and 528) (Santa Cruz Biotechnology); anti-phospho-specific ERK1/2 and anti-ERK1/2 (New England Biolabs); anti-ErbB2 (21N) (Lane et al., 2000); anti-cyclin-D1 (NovaCastra); anti-cyclin-D2 (SantaCruz). PKI166 and CGS27023A were donated by Novartis Pharma AG; phenanthroline was purchased from Calbiochem.
Preparation of cells and conditioned media.
HC11 cells were cultured in RPMI medium plus 10% FCS, EGF and insulin (Hynes et al., 1990). Cultures were co-transfected using SuperFect (Qiagen) with vectors carrying a puromycin resistance gene and with a TCF luciferase reporter (TopTK) (Brantjes et al., 2001) (a gift from H. Clevers). After selection for puromycin resistance, cells were infected with retroviruses (gifts of A. Kisper and R. Kemmler) encoding Wnt1, Wnt5a or neomycin resistance; cell lines with resistance to both antibiotics were selected. Wnt-containing conditioned media were collected from cultures of Wnt1-HC11 or Wnt5a-HC11 cells grown overnight in the absence of EGF and insulin, and were added to C-HC11 or HEK-293 cultures. Conditioned media from HEK-293 cells expressing the sFRP1 vector (a gift from J. Rubin) or the Dkk1 vector (a gift from C. Brisken) were used in some experiments.
Microarray analysis of gene expression.
RNA was prepared and analysed on an Affymetrix GeneChip Murine Genome U74A array in accordance with the manufacturer's instructions; hybridization data were analysed using the software provided (MAS4.0).
Luciferase assays.
Luciferase activity was determined using the Promega assay system (no. E1910). Total light emission was measured using a luminometer (Berthold Microlumat LB96) during the first 3 s of the reaction.
Lysate preparation, immunoprecipitations and western blotting.
Whole cell lysates were prepared by solubilizing cultures in Nonidet P40 extraction buffer (Lane et al., 2000). Immunoprecipitations and immunoblotting were performed as described in Lane et al. (2000).
Analysis of gene expression by real-time PCR.
mRNA was isolated from C-HC11 and Wnt-expressing HC11 cells and the levels of TGF-α, HB-EGF and AR were measured by real-time PCR using specific oligonucleotides, as previously described (Sorensen et al., 2000).
Acknowledgments
We thank A. Badache for comments on the manuscript and N. Torring for the RT–PCR analysis. The laboratory of N.E.H. is supported by the Novartis Forschungsstiftung, Zweigniederlassung Friedrich Miescher Institute for Biomedical Research. T.H. was partly supported by stipends from the Novartis Stiftung Für Biomedizinische Forschung and Krebsliga Beider Basel.
References
- Badache A. & Hynes N.E. (2000) Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res., 61, 383–391. [PubMed] [Google Scholar]
- Bienz M. (1998) TCF: transcriptional activator or repressor? Curr. Opin. Cell Biol., 10, 366–372. [DOI] [PubMed] [Google Scholar]
- Bienz M. & Clevers H. (2000) Linking colorectal cancer to Wnt signalling. Cell, 103, 311–320. [DOI] [PubMed] [Google Scholar]
- Brandt R., Wong A.M.L. & Hynes N.E. (2001) Mammary glands reconstituted with Neu/ErbB2 transformed HC11 cells provide a novel orthotopic model for testing anti-cancer agents. Oncogene, 20, 5459–5465. [DOI] [PubMed] [Google Scholar]
- Brantjes H., Roose J., van de Wetering M. & Clevers H. (2001) All Tcf HMG box transcription factors interact with groucho-related co-repressors. Nucleic Acids Res., 29, 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C. et al. (2000) Essential function of Wnt-4 in mammary gland development downstream of progesterone signalling. Genes Dev., 14, 650–654. [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. (1999) Employment of the epidermal growth factor receptor in growth factor- independent signalling pathways. J. Cell Biol., 146, 697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. et al. (1999) Metalloprotease-mediated ligand release regulates autocrine signalling through the epidermal growth factor receptor. Proc. Natl Acad. Sci. USA, 96, 6235–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin B. & McMahon A.P. (1992) Differential regulation of the wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol. Cell. Biol., 12, 2418–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind A., Zwick E., Prenzel N., Leserer M. & Ullrich A. (2001) Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene, 20, 1594–1600. [DOI] [PubMed] [Google Scholar]
- He T.C. et al. (1998) Identification of c-Myc as a target of the APC pathway. Science, 281, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Hynes N.E. et al. (1990) EGF receptor, but not c-erbB2, activation prevents lactogenic hormone induction of the β-casein gene in mouse mammary epithelial cells. Mol. Cell. Biol., 10, 4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H.A. et al. (2000) ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol. Cell. Biol., 20, 3210–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B. et al. (2001) LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature, 411, 321–325. [DOI] [PubMed] [Google Scholar]
- Massague J. & Pandiella A. (1993) Membrane-anchored growth factors. Annu. Rev. Biochem., 62, 515–541. [DOI] [PubMed] [Google Scholar]
- Neve R., et al. (2000) Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumor cells. Oncogene, 19, 1647–1656. [DOI] [PubMed] [Google Scholar]
- van Noort M. & Clevers H. (2002) TCF transcription factors, mediators of Wntsignalling in development and cancer. Dev. Biol., 244, 1–8. [DOI] [PubMed] [Google Scholar]
- Nusse R. (1999) Wnt targets. Repression and activation. Trends Genet., 15, 1–3. [DOI] [PubMed] [Google Scholar]
- Nusse R. & Varmus H.E. (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell, 31, 99–109. [DOI] [PubMed] [Google Scholar]
- Olayioye M.A., Neve R.M., Lane H.A. & Hynes N.E. (2000) The ErbB signalling network: receptor heterodimerization in development and cancer. EMBO J., 19, 3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. (2000) Wnt signalling and cancer. Genes Dev., 14, 1837–1851. [PubMed] [Google Scholar]
- Prenzel N. et al. (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature, 402, 884–888. [DOI] [PubMed] [Google Scholar]
- Sorensen B.S., Torring N., Bor M.V. & Nexo E. (2000) Quantitation of the mRNA expression of the EGF system: selective induction of HB-EGF and AR expression by growth factor stimulation of prostate stromal cells. J. Lab. Clin. Med., 136, 209–217. [DOI] [PubMed] [Google Scholar]
- Taverna D., Groner B. & Hynes N.E. (1991) EGF receptor, PDGR receptor, and c-erbB2 receptor activation all promote growth but have distinctive effects upon mouse mammary epithelial cell differentiation. Cell Growth Differ., 2, 145–154. [PubMed] [Google Scholar]
- Tetsu O. & McCormick F. (1999) Beta-catenin regulates expression of cyclinD1 in colon carcinoma cells. Nature, 398, 422–426. [DOI] [PubMed] [Google Scholar]
- Traxler P., et al., (2001) Tyrosine kinase inhibitors: from rational design to clinical trials. Med. Res. Rev., 21, 499–512. [DOI] [PubMed] [Google Scholar]
- Troyer K.L. & Lee D.C. (2001) Regulation of mouse mammary gland development and tumorigenesis by the ERBB signalling network. J. Mammary Gland Biol. Neoplasia, 6, 7–21. [DOI] [PubMed] [Google Scholar]
- Ugolini F. et al. (2001) WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas of the medullary type. Oncogene, 20, 5810–5817. [DOI] [PubMed] [Google Scholar]
- Uren A. et al. (2000) Secreted frizzled-related protein 1 binds directly to wingless and is a biphasic modulator of Wnt signalling. J. Biol. Chem., 275, 4374–4382. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., et al. (2002) The β-catenin/Tcf-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell, 111, 241–250. [DOI] [PubMed] [Google Scholar]
- Wang Y., Johnson A.R., Ye Q.Z. & Dyer R.D. (1999) Catalytic activities and substrate specificity of the human membrane type 4 matrix metalloproteinase catalytic domain. J. Biol. Chem., 274, 33043–33049. [DOI] [PubMed] [Google Scholar]
- Willert J., Epping M., Pollack J.R., Brown P.O. & Nusse R. (2002) A transcriptional response to Wnt proteins in human embryonic carcinoma cells. BMC Dev. Biol., 2, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Geng Y. & Sicinski P. (2001) Specific protection against breast cancers by cyclin D1 ablation. Nature, 411, 1017–1021. [DOI] [PubMed] [Google Scholar]


