G-protein-coupled receptors (GPCR) signaling is an evolutionarily ancient mechanism used by all eukaryotes to sense environmental stimuli and mediate cell-cell communication (7, 28). During evolution, GPCR genes expanded enormously in number and diversity. Whereas only three of the ∼5,900 genes in the yeast Saccharomyces cerevisiae encode GPCRs, at least 55 of the ∼12,000 genes in Dictyostelium encode GPCRs (19), and more than 1,000 of the ∼22,000 genes in humans encode receptors of this class (28). GPCRs are of crucial physiologic importance. In eukaryotic microorganisms, GPCRs regulate cell growth, development, morphogenesis, motility, and life span. In humans they mediate the action of hundreds of peptide hormones, sensory stimuli, autacoids, neurotransmitters, and chemokines. GPCRs also are targets of many clinically important drugs as well as drugs of abuse.
Despite exhibiting striking diversity in primary sequence and biologic function, GPCRs possess the same fundamental architecture, consisting of seven transmembrane (TM) domains and share common mechanisms of signal transduction (85). GPCRs transduce extracellular signals by coupling to heterotrimeric guanine nucleotide binding proteins (G proteins) consisting of α, β, and γ subunits. Activated GPCRs stimulate exchange of GTP for GDP on Gα subunits, dissociating Gα and Gβγ subunits that, in turn, trigger biological responses by binding effector proteins that regulate second messenger production, protein kinase cascades, cytoskeletal organization, gene transcription, and ion channel activity. GPCRs also signal by G protein-independent mechanisms through recruitment of scaffold proteins such as β-arrestins (reviewed in reference 54).
In the budding yeast S. cerevisiae, GPCR signaling regulates two biologic processes: conjugation and nutrient sensing (reviewed in references 15 and 106). During conjugation, a mating type cells secrete a-factor, a 12-residue farnesylated oligopeptide pheromone that binds the G-protein-coupled a-factor receptor (STE3 gene product) expressed only by cells of the α mating type. Conversely, α cells secrete α-factor, an unmodified 13-residue peptide pheromone that binds the G-protein-coupled α-factor receptor (STE2 gene product) expressed only by cells of the a mating type. Although a- and α-factor receptors are unrelated in primary sequence, they trigger similar intracellular responses by activating the same G protein-linked mitogen-activated protein kinase cascade. Many fundamentally important aspects of GPCR signaling were first elucidated in budding yeast (reviewed in reference 16), including cloning of the first nonsensory GPCR, signaling by Gβγ subunits, GPCR ubiquitination during endocytosis, signaling via mitogen-activated protein kinase cascades and scaffolding proteins, and G protein regulation by RGS proteins.
The third GPCR in budding yeast, encoded by the GPR1 gene, is a likely receptor for glucose, sucrose, and possibly other ligands (55). This receptor regulates yeast pseudohyphal differentiation, cell size, and life span (44, 58, 102, 109). The Gpr1 homolog of the pathogenic fungus Candida albicans promotes the yeast-to-hyphae transition (67). Gpr1 in S. cerevisiae signals via a pathway using a classical Gα subunit homolog (113) and novel kelch-repeat proteins (3, 35, 36) but lacking typical Gβγ subunits.
Like budding yeast, the fission yeast Schizosaccharomyces pombe possesses three GPCRs (reviewed in reference 41). The mam2+ and mam3+ genes encode receptors for the peptide mating pheromones p-factor and m-factor, respectively, whereas the git3+ gene encodes a putative glucose receptor.
In this review we highlight current understanding of GPCR oligomerization revealed by studies of the α-factor receptor of budding yeast. With the exception of Mam2 in fission yeast, GPCR oligomerization in other eukaryotic microorganisms has yet to be investigated.
GPCR OLIGOMERIZATION
The hypothesis of GPCR dimerization or oligomerization (for simplicity, unless stated otherwise, the term oligomerization will be used to encompass both terms) was first suggested in the 1970s from cooperative ligand binding and radiation inactivation studies (for a review, see references 79 and 95). However, the concept of GPCR oligomerization gained few adherents because such early evidence was equally consistent with other hypotheses. Furthermore, as GPCRs were characterized molecularly, there was no obvious mechanistic reason why they would need to function as oligomers in order to activate G proteins. Indeed, biochemical and biophysical studies of rhodopsin in native membranes or solubilized in detergent indicated that the monomeric form of this GPCR can activate its cognate G protein, transducin (reviewed in reference 11).
Nevertheless, extensive evidence accumulated over the last few years indicates that many GPCRs oligomerize in living cells, that various types of GPCRs can hetero-oligomerize, and that oligomer formation is critical for receptor biogenesis and function (reviewed in reference 22, 30, 33, 79, 81, and 104). What brought about this dramatic turn of affairs? What role did studies of the yeast α-factor receptor play in this transformation? As discussed below, genetic, cell biological and biophysical analyses of α-factor receptors have provided a powerful model system with which to demonstrate and study GPCR oligomerization in a native in vivo setting.
α-FACTOR RECEPTOR OLIGOMERIZATION: THE “DARK AGES”
Soon after the α-factor receptor was cloned in 1985 (8), in vivo evidence began to accumulate suggesting that this GPCR oligomerizes. A key observation came from studies of cells expressing mutant α-factor receptors lacking their regulatory C-terminal cytoplasmic domains. These cells exhibited a 100-fold increase in agonist potency because mutant receptors fail to desensitize or undergo endocytic down-regulation (88, 89). If α-factor receptors are monomeric, these phenotypes were expected to be observed even when cells coexpressed mutant and wild-type receptors. In striking contrast however, when cells coexpressed mutant and wild-type receptors, the receptor desensitization and down-regulation defects of mutant receptors were rescued nearly completely (88, 89). This finding led Thorner and colleagues to suggest the unconventional hypothesis that mutant α-factor receptors are desensitized and down-regulated by associating with wild-type receptors in hetero-oligomeric complexes (88, 89). Consistent with the oligomerization hypothesis, subsequent studies have shown that agonist-independent signaling by constitutively active mutant α-factor receptors is suppressed when wild-type receptors are coexpressed (17, 48, 101).
In concert with genetic studies, biochemical evidence suggesting that α-factor receptors oligomerize has been obtained (6, 73, 114). When solubilized from membrane fractions with a detergent such as octyl glucoside and then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, α-factor receptors are homodimeric (6, 73). Furthermore, coexpressed α-factor receptors tagged with different epitopes can be coimmunoprecipitated from detergent extracts (114). However, these observations are not definitive because solubilization of yeast membrane fractions with the detergent Zwittergent 3-12 prevents detection of α-factor receptor dimers (73). Indeed, detergent-specific effects on membrane protein monomerization and oligomerization have long been known (38, 103). Accordingly, such limitations motivated the development of noninvasive means of detecting GPCR oligomerization in intact cells and membranes.
LIGHTING THE WAY: α-FACTOR RECEPTOR FRET
Detecting α-factor receptor oligomerization in vivo became possible with the advent of fluorescence resonance energy transfer (FRET) experiments using the green fluorescent protein derivatives cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) (68). FRET has proved to be a powerful tool to examine GPCR oligomerization because energy transfer between donor (CFP) and acceptor (YFP) fluorophores occurs when the two are 25 to 100 Å apart (52), within the expected center-to-center distance of subunits in a GPCR complex based on the structure of the rhodopsin monomer (78).
FRET studies of CFP- and YFP-tagged α-factor receptors coexpressed in yeast were the first, and remain among of the few (discussed in reference 80), that have demonstrated oligomerization of GPCRs expressed at wild-type levels in their native cell type and in membrane fractions (13, 72, 74-76). Another notable example where this has been achieved is rhodopsin. Atomic force microscopy has revealed that native, untagged rhodopsin forms dimers that can assemble into oligomeric arrays in disk membranes (27, 34, 47, 53, 56). In contrast, studies of GPCR oligomerization that use heterologous cell types and protein overexpression must be carefully controlled to exclude that the results are due to nonspecific, collisional interactions that can occur between membrane proteins (46, 64, 115).
FRET studies of α-factor receptors have yielded several conclusions that subsequently have been shown to apply to other GPCRs. First, α-factor receptors form highly specific homo-oligomeric complexes, because their interaction with other plasma membrane proteins is not detectable in FRET experiments (75). Second, α-factor receptor oligomerization is constitutive because FRET efficiency is unaffected by agonist or antagonist binding or G protein activation state (75). Therefore, α-factor receptors probably are oligomeric during signal transduction in vivo. Consistent with this hypothesis, constitutively active mutant α-factor receptors oligomerize (73), as do constitutively active mutant p-factor receptors (Mam2) in fission yeast (51). Third, oligomerization is an early event in the biogenesis of α-factor receptors because FRET efficiency is equivalent in subcellular fractions derived from the plasma membrane and endoplasmic reticulum (74). Fourth, α-factor receptors form oligomeric complexes during endocytic down-regulation because coexpressed wild-type receptors recruit endocytosis-defective receptors into the endocytic pathway (75, 114). Therefore, oligomerization occurs throughout the birth, life, and death of α-factor receptors.
Oligomerization of dozens of GPCRs subsequently has been demonstrated by FRET and bioluminescence resonance energy transfer (BRET) experiments, as well as other approaches (reviewed in references 30, 33, 79, 84, and 104). As observed with α-factor receptors, oligomerization of many GPCRs, including δ-opioid, chemokine (CXCR2), vasopressin, and oxytocin receptors (63, 105, 108), is constitutive and largely unaffected by agonist or antagonist binding. However, studies of some GPCRs, including somatostatin receptor SSTR5, gonadotropin-releasing hormone receptor, and luteinizing hormone receptor (14, 90, 91), have reported agonist-induced changes in FRET or BRET; whether this occurs due to changes in receptor conformation or oligomerization is not clear.
Because mammalian GPCRs can be expressed functionally in yeast (83), FRET experiments using this expression system could be used for several purposes. For example, FRET experiments with yeast cells expressing functional human complement C5a receptors have indicated that these receptors oligomerize in the absence of mammalian cell-specific accessory or chaperone proteins (24). Such a system might also be used to identify proteins or small molecules that promote or inhibit GPCR oligomerization.
α-FACTOR RECEPTOR OLIGOMERS VERSUS DIMERS: DOES SIZE MATTER?
Establishing whether α-factor receptors are dimeric or oligomeric in the absence versus presence of agonist may provide new insight into the mechanisms used by this receptor to activate its G protein or undergo desensitization or endocytosis. Because receptor dimers versus oligomers could not be distinguished by simple FRET experiments used to show that α-factor receptors interact, other approaches have been required to address this question. Among such methods, quantitative FRET imaging and donor dequenching upon acceptor photobleaching have been applied recently to coexpressed CFP- and YFP-tagged α-factor receptors (86). Remarkably, these studies showed that in the absence of agonist, the entire α-factor receptor population on the plasma membrane or the endoplasmic reticulum (ER) apparently is, on average, dimeric. Although the effects of α-factor stimulation were not examined, these findings, coupled with previous investigations showing no change in apparent FRET efficiency upon agonist stimulation (75), indicate that activation of the α-factor receptor does not involve a monomer-to-oligomer transition. Agonist binding may nonetheless regulate dimer-oligomer distribution.
Whether GPCRs are dimeric or are distributed among monomeric, dimeric, and oligomeric states generally is not clear. Nevertheless, quantitative BRET experiments have suggested that melatonin MT1R and MT2R receptors form heterodimeric complexes (1). In contrast, cross-linking studies of complement C5a and dopamine D2 receptors and atomic force microscopy and modeling of rhodopsin have suggested that these molecules are oligomeric (27, 34, 47, 53, 56). Despite such evidence, the functions of GPCR oligomerization versus dimerization have yet to be investigated.
MATCHMAKING α-FACTOR RECEPTOR HOMO-OLIGOMERS
Because α-factor receptors do not hetero-oligomerize with other membrane proteins or GPCRs including yeast Gpr1 and human C5a receptors, molecular “matchmaking” mechanisms have been hypothesized to direct the formation of specific homo-oligomeric complexes (24, 74, 75). Such mechanisms could be receptor intrinsic (i.e., specific receptor-receptor contact sites) and/or involve the action of chaperone or accessory proteins.
The existence of specific receptor-receptor contact sites has been suggested by mutagenesis and FRET studies of α-factor receptors. These investigations used a large collection of receptor deletion mutants based on the ability of complementary deletion mutant fragments (e.g., TM1 to TM3 and TM4 to TM7) to interact in FRET experiments (74) and reconstitute receptor function (62). Results indicated, for example, that a fragment containing the N-terminal extracellular domain, TM1, TM2, and TM3, interacts with itself as efficiently as do wild-type receptors (74). In contrast, many mutants lacking these domains did not self-associate. Analysis of other deletion mutants indicated that TM1 is particularly important for oligomerization, whereas the N-terminal domain and TM2 apparently facilitate receptor-receptor interaction (74).
Within TM1 of the α-factor receptor, a GXXXG motif may form the primary homo-oligomerization interface (76). This motif is similar to the well-studied dimerization domain of glycophorin A (59, 60, 94), a protein that spans the membrane once. In the glycophorin A structure (60), two glycine residues of the GXXXG motif form a groove on one face of the transmembrane helix. This groove binds a ridge of aliphatic residues (XXX residues in the GXXXG motif) from the partner subunit in the dimeric complex. A similar mechanism appears to mediate interaction between α-factor receptors, because the types of amino acid substitutions in the GXXXG motif that strongly impair α-factor receptor oligomerization also disrupt glycophorin A dimerization (76). Furthermore, the GXXXG motif in TM1 is predicted to face the lipid bilayer (20, 82) and thus would be available to mediate direct contact between α-factor receptors. However, the GXXXG motif apparently is insufficient to drive the formation of α-factor receptor oligomers, because TM1 expressed alone does not oligomerize with itself, whereas a fragment containing TM1, the N-terminal extracellular domain, and TM2 does (74).
GXXXG-like motifs are present in TM domains of many GPCRs, including the α-factor receptor of other yeast species, the a-factor receptor of various yeast species, class A amine and cannabinoid receptors, class B secretin-like receptors, and class C metabotropic receptors (76). These motifs therefore may prove to be important for oligomerization or for stabilizing helix-helix contacts within a receptor's heptahelical bundle. Indeed, substitutions affecting a GXXXG motif in TM6 of the β2-adrenergic receptor impair oligomerization (96).
However, as would be expected for a large protein family, GPCRs apparently oligomerize by several mechanisms. TM1 and TM4/5 are thought to mediate homo-oligomerization of C5a receptors (47), α1b-adrenergic receptors (10, 66), and rhodopsin (26, 56). Similarly, TM4 mediates oligomerization of D2 dopamine receptors (34, 53), whereas TM5 is critical for interaction between adenosine A2a receptors (107). In contrast, metabotropic glutamate (mGluR1) and calcium-sensing receptors oligomerize at least in part via their extracellular N-terminal ligand-binding domains (32, 50, 87), the mGluR5 glutamate receptor oligomerizes via disulfide and noncovalent bonds (92, 93), and the two subunits of γ-aminobutyric acid [GABA(B)] receptors heterodimerize in part via their cytoplasmic C-terminal domains (111). However, these and their related receptors may also oligomerize via their transmembrane domains, because oligomers persist when disulfide bonds are reduced or C-terminal interaction domains are removed (9, 77, 116). Indeed, putative oligomerization interfaces in GPCR transmembrane domains have been predicted by computational methods (71). Understanding the mechanisms by which GPCRs form specific homo- and hetero-oligomeric assemblies ultimately will require detailed investigation at the structural level.
OLIGOMERIZATION PROMOTES α-FACTOR RECEPTOR BIOGENESIS
Analysis of receptor mutants has indicated that oligomerization is required for α-factor receptor biogenesis. Specifically, amino acid substitutions in the GXXXG motif in TM1 that impaired α-factor receptor oligomerization also reduced expression of the receptor at the plasma membrane without affecting agonist-binding affinity or total receptor protein levels (76). Instead, these mutant receptors were retained in intracellular compartment(s) such as the ER, albeit via unknown mechanisms. Accordingly, it will be important to identify receptor motifs and accessory proteins required for ER retention or export of α-factor receptors.
Consistent with studies of α-factor receptors, receptor biogenesis is perhaps the best understood function for GPCR oligomerization in many instances. Heterodimerization of GABA(B)R1 and GABA(B)R2 subunits was the first and remains the best-understood system where this is the case (43, 45, 61, 77, 111). Here, an RXR-type ER retention motif in the cytoplasmic C-terminal domain of the GABA(B)R1 subunit is masked upon heterodimerization with the GABA(B)R2 subunit, allowing the heterodimeric complex to traffic to the plasma membrane. Other evidence indicating that oligomerization is crucial for GPCR biogenesis includes the following: (i) the ability of ER-retained chemokine receptor (CCR5) mutants to function as dominant-negatives by sequestering wild-type CCR5 in the ER (4, 99), (ii) the retention of oligomerization-defective β2-adrenergic receptor mutants in the ER (96), and (iii) the ER export of functional receptors upon interaction between T1R1 and T1R3 taste receptors (69, 70).
ER export of oligomeric GPCRs can also involve the action of accessory proteins and chaperones. Cell surface targeting of D1 dopamine receptors requires binding to the ER export factor DRIP-78 (5). Other examples include binding of odorant receptors in Caenorhabditis elegans to the single-TM protein ODR-4 (31), the interaction of calcitonin-receptor-like receptor with receptor-associated membrane proteins (reviewed in reference 25), binding of mouse pheromone receptors V1R and V2R to the major histocompatibility complex class Ib (57), and the interaction of rhodopsin with the chaperone NinaA in Drosophila (2) and the DnaJ homolog HSJ1 in mammalian cells (12). Accordingly, it would be interesting to determine whether GPCR oligomerization is required for interaction with these accessory proteins and chaperones.
α-FACTOR RECEPTOR OLIGOMERIZATION PROMOTES G PROTEIN SIGNALING
Determining the role of GPCR oligomerization in G protein activation has been a major objective of the field. The emerging concept is that the mechanistic role of GPCR oligomerization in G protein activation may depend upon the type of receptor being studied. Accordingly, the following mechanisms suggested by studies of the α-factor receptor are likely to provide a conceptual model applicable to a subset of GPCRs.
Two lines of genetic evidence have indicated that α-factor receptor oligomerization is critical for G protein signal transduction. First, among various classes of dominant-negative α-factor receptor mutants, one bearing a substitution in TM6 (M250I) interferes with the ability of wild-type receptors to signal by forming mutant wild-type hetero-oligomers that have impaired signaling activity (75). Second, a substitution affecting the GXXXG motif in TM1 (G56A) impairs α-factor receptor oligomerization and signaling without reducing agonist-binding affinity or total receptor protein expression (76). Although this substitution reduces receptor trafficking to the plasma membrane, the magnitude of this targeting defect is insufficient to impair signaling (97). Therefore, the G56A substitution probably blunts agonist-induced signaling at the plasma membrane by interfering with α-factor receptor oligomerization.
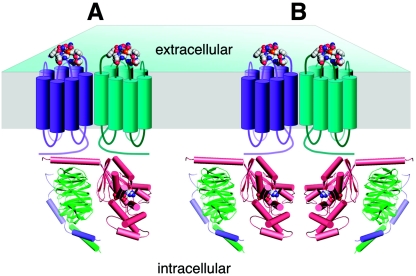
How could α-factor receptor oligomerization promote G protein activation? At least two alternatives, which are not mutually exclusive, can be proposed (Fig. 1). First, each receptor monomer in a presumably dimeric complex could cooperate to activate G protein heterotrimers (Fig. 1A). This could occur, for example, if each receptor subunit contacts different regions of the G protein heterotrimer to induce conformational changes resulting in GDP release and GTP binding to Gα subunits. Second, oligomerization could stabilize the agonist-bound receptor in its activated conformational state such that either or both receptor subunits in a complex could participate in G protein activation (Fig. 1B).
FIG. 1.
Signal transduction by α-factor receptor dimers. In each model, α-factor receptor monomers (cyan and purple) form dimeric complexes in the plasma membrane as indicated by biophysical evidence (86). Each receptor monomer in a dimeric complex binds agonist (α-factor peptide, represented by spacefill), inducing conformational changes required for G protein activation. (A) Model in which two activated receptor subunits cooperate to activate one G protein heterotrimer (Gα subunit, Gpa1 in salmon; Gβγ heterodimer, Ste4 and Ste18 in green and light purple, respectively), possibly by contacting different regions of the G protein. (B) Model in which each receptor monomer in a dimeric complex activates a G protein heterotrimer. See text for further details.
Genetic evidence supporting the receptor subunit cooperation model of G protein activation has been obtained (13). These investigations used two classes of signaling-defective α-factor receptor mutants: one bearing a substitution in the third intracellular loop, which specifically impairs α-factor receptor-G protein coupling (110), and a second mutant that binds agonist normally but is signaling-impaired because it lacks its C-terminal cytoplasmic domain and bears substitutions in the first intracellular loop. When these mutant receptors were coexpressed in trans, the efficiency of α-factor signaling was increased severalfold relative to cells expressing either mutant receptor alone. In contrast, expressing these two classes of mutations in cis within a single receptor reduced signaling efficiency below that observed with either single mutant receptor expressed alone. Thus, when two mutant receptors are coexpressed in trans, one mutant subunit of the receptor dimer may provide a functional form of a G protein contact site that is defective in the other, thereby reconstituting function.
Does agonist binding to one α-factor receptor subunit in a dimer trigger a conformational change in the other receptor subunit that leads to G protein activation? Although a similar hypothesis has been suggested by studies of GABA(B) receptors (18, 29, 37, 112) and chemokine receptors (21, 100), evidence arguing against this hypothesis in yeast has been obtained by showing that coexpression of an agonist-binding α-factor receptor mutant (S184R in the exofacial region of TM4) with a G protein-coupling mutant failed to restore signaling (13). Taken together, these genetic lines of evidence suggest that agonist binding independently to each subunit of an α-factor receptor dimer induces conformational changes that allow the two receptor subunits acting in concert to activate one G protein heterotrimer (Fig. 1A).
However, caveat emptor! Genetic data cannot rule out that each α-factor receptor monomer in an oligomeric complex is sufficient to activate a G protein heterotrimer. Whereas genetic data show that α-factor receptor oligomerization is required for efficient G protein signaling, they do not prove whether this occurs at the level of G protein activation or by other means, such as by promoting the clustering of activated G protein subunits or their effectors in order to transmit downstream signals efficiently. Addressing these questions, therefore, is an important goal of future investigations.
The roles of GPCR oligomerization in signal transduction are the subject of intense discussion in the literature, as recent reviews highlight (11, 79). On the one hand, light activation of a single rhodopsin molecule clearly is sufficient for G protein activation (42, 49). Likewise, only one subunit of the mGluR1 homodimer mediates G protein signaling (40), and in GPCRs that function as obligate heterodimers, such as the GABA(B) receptor and the sweet and umami taste receptors, only one receptor subunit plays a pivotal role in G protein signaling (18, 29, 37, 112). In contrast, however, rhodopsin dimers couple more efficiently with G proteins than do rhodopsin monomers (42), and highly purified forms of dimeric but not monomeric leukotriene B4 receptor can activate G proteins in vitro (65). Clearly, intensive studies of several GPCRs, including the α-factor receptor, will be required to determine whether oligomerization is required for G protein activation or other mechanisms leading to efficient G protein signaling.
DOES α-FACTOR RECEPTOR OLIGOMERIZATION PROMOTE ENDOCYTIC TRAFFICKING?
Several observations lead us to suggest that oligomerization may be critical for endocytic down-regulation of the α-factor receptor. First, α-factor receptors oligomerize during endocytosis (75, 88, 114). Second, upon binding agonist, α-factor receptors are monoubiquitinated, internalized, and sorted into the lysosome-like vacuole via the action of proteins such as Ede1, Ent1, and Vps27 that contain ubiquitin-interacting motifs (UIM) and recruit endosomal sorting complex required for transport (39, 98). Third, a UIM domain of Vps27 crystallizes as a tetramer (23), although in solution it is monomeric. Therefore, when UIM domains of Vps27 or other endocytic sorting proteins bind to ubiquitinated α-factor receptor oligomers, they in turn may oligomerize. Alternatively, because many endocytic sorting proteins have two or more UIM domains, they may bind more than one ubiquitinated α-factor receptor to form a network analogous to that formed upon antigen binding to polyclonal antibodies. By either mechanism, coclusters of UIM proteins and ubiquitinated α-factor receptors would be produced that might serve to exclude nonubiquitinated proteins and selectively sort α-factor receptors along the endocytic pathway.
CONCLUDING REMARKS
We have only begun to fathom the mechanistic and biologic roles of GPCR oligomerization. Because of the rich genetics and molecular cell biology offered by single-celled eukaryotes, however, studies using these systems may answer questions that are difficult to address in more complex systems. Two examples are discussed below.
Is GPCR oligomerization crucial for signaling pathways that do not use classical G protein heterotrimers?
Heptahelical GPCRs can signal independently of classical G protein heterotrimers in order to down-regulate receptors or transduce mitogenic or other signals (54). However, whether GPCR oligomerization is required in such nonclassical signaling mechanisms is poorly understood. In mammalian systems, answering this question is difficult because many GPCRs activate several types of G protein-dependent and -independent pathways. Therefore, disrupting GPCR oligomerization may have complex effects on G protein-dependent or -independent pathways. In contrast, the Gpr1 receptor pathway in yeast and pathogenic fungi signals via a simple nonclassical pathway using Gα subunits that lack Gβγ partners (3, 35, 36, 113). In this system it should be straightforward to determine whether Gpr1 oligomerizes in FRET or BRET experiments and whether this interaction is required for Gpr1 signaling as revealed by structure-function studies. If so, then biochemical and cell biological approaches could determine whether Gpr1 oligomerization mediates Gα activation, effector docking, or other processes.
Does GPCR hetero-oligomerization occur in vivo and does it provide an important means of regulating receptor activity or functional diversity?
Hetero-oligomerization between different GPCRs is hypothesized to provide an important means of regulating receptor function (reviewed in references 22, 30, 33, 79, 81, and 104). However, with a few exceptions such as GABA(B) receptors, the occurrence and roles of GPCR hetero-oligomerization in vivo are poorly understood. These questions could be readily addressed in yeast and Dictyostelium. In yeast, α-factor and a-factor receptors could hetero-oligomerize once cells of opposite mating type have fused during conjugation. In principle, heterodimerization might squelch signaling by both receptors so that cells rapidly resume growth following conjugation. By identifying mutant α-factor and a-factor receptors that do not hetero-oligomerize, one could then establish whether resumption of cell growth is delayed. In Dictyostelium, the existence of 55 GPCRs provides an exceptional opportunity to determine the extent to which coexpressed receptors hetero-oligomerize in a simple developmental system (19). With such information, one could then use gene knockout and structure-function studies to determine whether hetero-oligomerization between certain receptors regulates signaling pathways that control migration, differentiation, or development.
Answers to such questions may ultimately benefit human health. Discoveries made through studies of unicellular eukaryotes may reveal novel and fundamentally important principles of GPCR signaling that could shed light on diseases caused by abnormal GPCR signaling or that provide proof of concept needed to develop therapeutic agents targeted to specific complexes of GPCR homo- or hetero-oligomers.
Acknowledgments
Grant support from the National Institutes of Health (GM44592 and HL075632 to K.J.B.) and the American Heart Association (predoctoral fellowship to S.L.C.) is gratefully acknowledged.
REFERENCES
- 1.Ayoub, M. A., C. Couturier, E. Lucas-Meunier, S. Angers, P. Fossier, M. Bouvier, and R. Jockers. 2002. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 277:21522-21528. [DOI] [PubMed] [Google Scholar]
- 2.Baker, E. K., N. J. Colley, and C. S. Zuker. 1994. The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. EMBO J. 13:4886-4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batlle, M., A. Lu, D. A. Green, Y. Xue, and J. P. Hirsch. 2003. Krh1p and Krh2p act downstream of the Gpa2p G(alpha) subunit to negatively regulate haploid invasive growth. J. Cell Sci. 116:701-710. [DOI] [PubMed] [Google Scholar]
- 4.Benkirane, M., D. Y. Jin, R. F. Chun, R. A. Koup, and K. T. Jeang. 1997. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5delta32. J. Biol. Chem. 272:30603-30606. [DOI] [PubMed] [Google Scholar]
- 5.Bermak, J. C., M. Li, C. Bullock, and Q. Y. Zhou. 2001. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat. Cell Biol. 3:492-498. [DOI] [PubMed] [Google Scholar]
- 6.Blumer, K. J., J. E. Reneke, and J. Thorner. 1988. The STE2 gene product is the ligand-binding component of the alpha-factor receptor of Saccharomyces cerevisiae. J. Biol. Chem. 263:10836-10842. [PubMed] [Google Scholar]
- 7.Bockaert, J., and J. P. Pin. 1999. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 18:1723-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkholder, A. C., and L. H. Hartwell. 1985. The yeast alpha-factor receptor: structural properties deduced from the sequence of the STE2 gene. Nucleic Acids Res. 13:8463-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calver, A. R., M. J. Robbins, C. Cosio, S. Q. Rice, A. J. Babbs, W. D. Hirst, I. Boyfield, M. D. Wood, R. B. Russell, G. W. Price, A. Couve, S. J. Moss, and M. N. Pangalos. 2001. The C-terminal domains of the GABA(b) receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J. Neurosci. 21:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillo, J. J., J. F. Lopez-Gimenez, and G. Milligan. 2004. Multiple interactions between transmembrane helices generate the oligomeric alpha1b-adrenoceptor. Mol. Pharmacol. 66:1123-1137. [DOI] [PubMed] [Google Scholar]
- 11.Chabre, M., and M. le Maire. 2005. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry 44:9395-9403. [DOI] [PubMed] [Google Scholar]
- 12.Chapple, J. P., and M. E. Cheetham. 2003. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J. Biol. Chem. 278:19087-19094. [DOI] [PubMed] [Google Scholar]
- 13.Chinault, S. L., M. C. Overton, and K. J. Blumer. 2004. Subunits of a yeast oligomeric G protein-coupled receptor are activated independently by agonist but function in concert to activate G protein heterotrimers. J. Biol. Chem. 279:16091-16100. [DOI] [PubMed] [Google Scholar]
- 14.Cornea, A., J. A. Janovick, G. Maya-Nunez, and P. M. Conn. 2001. Gonadotropin-releasing hormone receptor microaggregation. Rate monitored by fluorescence resonance energy transfer. J. Biol. Chem. 276:2153-2158. [DOI] [PubMed] [Google Scholar]
- 15.Dohlman, H. G. 2002. G proteins and pheromone signaling. Annu. Rev. Physiol. 64:129-152. [DOI] [PubMed] [Google Scholar]
- 16.Dohlman, H. G., and J. W. Thorner. 2001. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 70:703-754. [DOI] [PubMed] [Google Scholar]
- 17.Dosil, M., K. A. Schandel, E. Gupta, D. D. Jenness, and J. B. Konopka. 2000. The C terminus of the Saccharomyces cerevisiae α-factor receptor contributes to the formation of preactivation complexes with its cognate G protein. Mol. Cell. Biol. 20:5321-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duthey, B., S. Caudron, J. Perroy, B. Bettler, L. Fagni, J. P. Pin, and L. Prezeau. 2002. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J. Biol. Chem. 277:3236-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichinger, L., J. A. Pachebat, G. Glockner, M. A. Rajandream, R. Sucgang, M. Berriman, J. Song, R. Olsen, K. Szafranski, Q. Xu, B. Tunggal, S. Kummerfeld, M. Madera, B. A. Konfortov, F. Rivero, A. T. Bankier, R. Lehmann, N. Hamlin, R. Davies, P. Gaudet, P. Fey, K. Pilcher, G. Chen, D. Saunders, E. Sodergren, P. Davis, A. Kerhornou, X. Nie, N. Hall, C. Anjard, L. Hemphill, N. Bason, P. Farbrother, B. Desany, E. Just, T. Morio, R. Rost, C. Churcher, J. Cooper, S. Haydock, N. van Driessche, A. Cronin, I. Goodhead, D. Muzny, T. Mourier, A. Pain, M. Lu, D. Harper, R. Lindsay, H. Hauser, K. James, M. Quiles, M. Madan Babu, T. Saito, C. Buchrieser, A. Wardroper, M. Felder, M. Thangavelu, D. Johnson, A. Knights, H. Loulseged, K. Mungall, K. Oliver, C. Price, M. A. Quail, H. Urushihara, J. Hernandez, E. Rabbinowitsch, D. Steffen, M. Sanders, J. Ma, Y. Kohara, S. Sharp, M. Simmonds, S. Spiegler, A. Tivey, S. Sugano, B. White, D. Walker, J. Woodward, T. Winckler, Y. Tanaka, G. Shaulsky, M. Schleicher, G. Weinstock, A. Rosenthal, E. C. Cox, R. L. Chisholm, R. Gibbs, W. F. Loomis, M. Platzer, R. R. Kay, J. Williams, P. H. Dear, A. A. Noegel, B. Barrell, and A. Kuspa. 2005. The genome of the social amoeba Dictyostelium discoideum. Nature 435:43-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eilers, M., V. Hornak, S. O. Smith, and J. B. Konopka. 2005. Comparison of class A and D G protein-coupled receptors: common features in structure and activation. Biochemistry 44:8959-8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Asmar, L., J. Y. Springael, S. Ballet, E. U. Andrieu, G. Vassart, and M. Parmentier. 2005. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol. Pharmacol. 67:460-469. [DOI] [PubMed] [Google Scholar]
- 22.Filipek, S., K. A. Krzysko, D. Fotiadis, Y. Liang, D. A. Saperstein, A. Engel, and K. Palczewski. 2004. A concept for G protein activation by G protein-coupled receptor dimers: the transducin/rhodopsin interface. Photochem. Photobiol. Sci. 3:628-638. [DOI] [PubMed] [Google Scholar]
- 23.Fisher, R. D., B. Wang, S. L. Alam, D. S. Higginson, H. Robinson, W. I. Sundquist, and C. P. Hill. 2003. Structure and ubiquitin binding of the ubiquitin-interacting motif. J. Biol. Chem. 278:28976-28984. [DOI] [PubMed] [Google Scholar]
- 24.Floyd, D. H., A. Geva, S. P. Bruinsma, M. C. Overton, K. J. Blumer, and T. J. Baranski. 2003. C5a receptor oligomerization. II. Fluorescence resonance energy transfer studies of a human G protein-coupled receptor expressed in yeast. J. Biol. Chem. 278:35354-35361. [DOI] [PubMed] [Google Scholar]
- 25.Foord, S. M., and F. H. Marshall. 1999. RAMPs: accessory proteins for seven transmembrane domain receptors. Trends Pharmacol. Sci. 20:184-187. [DOI] [PubMed] [Google Scholar]
- 26.Fotiadis, D., Y. Liang, S. Filipek, D. Saperstein, A., A. Engel, and K. Palczewski. 2004. The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 564:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fotiadis, D., Y. Liang, S. Filipek, D. A. Saperstein, A. Engel, and K. Palczewski. 2003. Atomic-force microscopy: rhodopsin dimers in native disc membranes. Nature 421:127-128. [DOI] [PubMed] [Google Scholar]
- 28.Fredriksson, R., and H. B. Schioth. 2005. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 67:1414-1425. [DOI] [PubMed] [Google Scholar]
- 29.Galvez, T., B. Duthey, J. Kniazeff, J. Blahos, G. Rovelli, B. Bettler, L. Prezeau, and J. P. Pin. 2001. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 20:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George, S. R., B. F. O'Dowd, and S. P. Lee. 2002. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 1:808-820. [DOI] [PubMed] [Google Scholar]
- 31.Gimelbrant, A. A., S. L. Haley, and T. S. McClintock. 2001. Olfactory receptor trafficking involves conserved regulatory steps. J. Biol. Chem. 276:7285-7290. [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith, P. K., G. F. Fan, K. Ray, J. Shiloach, P. McPhie, K. V. Rogers, and A. M. Spiegel. 1999. Expression, purification, and biochemical characterization of the amino-terminal extracellular domain of the human calcium receptor. J. Biol. Chem. 274:11303-11309. [DOI] [PubMed] [Google Scholar]
- 33.Gomes, I., B. A. Jordan, A. Gupta, C. Rios, N. Trapaidze, and L. A. Devi. 2001. G protein coupled receptor dimerization: implications in modulating receptor function. J. Mol. Med. 79:226-242. [DOI] [PubMed] [Google Scholar]
- 34.Guo, W., L. Shi, and J. A. Javitch. 2003. The forth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J. Biol. Chem. 278:4385-4388. [DOI] [PubMed] [Google Scholar]
- 35.Harashima, T., and J. Heitman. 2002. The Galpha protein Gpa2 controls yeast differentiation by interacting with kelch repeat proteins that mimic Gbeta subunits. Mol. Cell 10:163-173. [DOI] [PubMed] [Google Scholar]
- 36.Harashima, T., and J. Heitman. 2005. G{alpha} subunit Gpa2 recruits kelch repeat subunits that inhibit receptor-G protein coupling during cAMP-induced dimorphic transitions in Saccharomyces cerevisiae. Mol. Biol Cell. 16:4557-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havlickova, M., L. Prezeau, B. Duthey, B. Bettler, J. P. Pin, and J. Blahos. 2002. The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric gamma-aminobutyrate B receptor. Mol. Pharmacol. 62:343-350. [DOI] [PubMed] [Google Scholar]
- 38.Helenius, A., and K. Simons. 1975. Solubilization of membranes by detergents. Biochim. Biophys. Acta 415:29-79. [DOI] [PubMed] [Google Scholar]
- 39.Hicke, L. 1997. Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J. 11:1215-1226. [DOI] [PubMed] [Google Scholar]
- 40.Hlavackova, V., C. Goudet, J. Kniazeff, A. Zikova, D. Maurel, C. Vol, J. Trojanova, L. Prezeau, J. P. Pin, and J. Blahos. 2005. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 24:499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman, C. S. 2005. Except in every detail: comparing and contrasting G-protein signaling in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Eukaryot. Cell 4:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jastrzebska, B., T. Maeda, L. Zhu, D. Fotiadis, S. Filipek, A. Engel, R. E. Stenkamp, and K. Palczewski. 2004. Functional characterization of rhodopsin monomers and dimers in detergents. J. Biol. Chem. 279:54663-54675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones, K. A., B. Borowsky, J. A. Tamm, D. A. Craig, M. M. Durkin, M. Dai, W. J. Yao, M. Johnson, C. Gunwaldsen, L. Y. Huang, C. Tang, Q. Shen, J. A. Salon, K. Morse, T. Laz, K. E. Smith, D. Nagarathnam, S. A. Noble, T. A. Branchek, and C. Gerald. 1998. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396:674-679. [DOI] [PubMed] [Google Scholar]
- 44.Kaeberlein, M., K. T. Kirkland, S. Fields, and B. K. Kennedy. 2005. Genes determining yeast replicative life span in a long-lived genetic background. Mech. Ageing Dev. 126:491-504. [DOI] [PubMed] [Google Scholar]
- 45.Kaupmann, K., B. Malitschek, V. Schuler, J. Heid, W. Froestl, P. Beck, J. Mosbacher, S. Bischoff, A. Kulik, R. Shigemoto, A. Karschin, and B. Bettler. 1998. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396:683-687. [DOI] [PubMed] [Google Scholar]
- 46.Kenworthy, A. K., and M. Edidin. 1998. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 Å using imaging fluorescence resonance energy transfer. J. Cell Biol. 142:69-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klco, J. M., T. B. Lassere, and T. J. Baranski. 2003. C5a receptor oligomerization. I. Disulfide trapping reveals oligomers and potential contact surfaces in a G protein-coupled receptor. J. Biol. Chem. 278:35345-35353. [DOI] [PubMed] [Google Scholar]
- 48.Konopka, J. B., S. M. Margarit, and P. Dube. 1996. Mutation of Pro-258 in transmembrane domain 6 constitutively activates the G protein-coupled alpha-factor receptor. Proc. Natl. Acad. Sci. USA 93:6764-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kühn, H. 1984. Interactions between photoexcited rhodopsin and light-activated enzymes in rods., p. 123-156. In N. Osborne and J. Chader (ed.), Progress in Retinal Research. Pergamon Press, Oxford, United Kingdom.
- 50.Kunishima, N., Y. Shimada, Y. Tsuji, T. Sato, M. Yamamoto, T. Kumasaka, S. Nakanishi, H. Jingami, and K. Morikawa. 2000. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971-977. [DOI] [PubMed] [Google Scholar]
- 51.Ladds, G., D. K., A. Das, and J. Davey. 2005. A constitutively active GPCR retains its G protein specificity and the ability to form dimers. Mol. Microbiol. 55:482-497. [DOI] [PubMed] [Google Scholar]
- 52.Lakowitz, J. R. 1983. Principles of fluorescence spectroscopy. Plenum Press, New York, N.Y.
- 53.Lee, S. P., B. F. O'Dowd, R. D. Rajaram, T. Nguyen, and S. R. George. 2003. D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry 42:11023-11031. [DOI] [PubMed] [Google Scholar]
- 54.Lefkowitz, R. J., and S. K. Shenoy. 2005. Transduction of receptor signals by beta-arrestins. Science 308:512-517. [DOI] [PubMed] [Google Scholar]
- 55.Lemaire, K., S. Van de Velde, P. Van Dijck, and J. M. Thevelein. 2004. Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol. Cell 16:293-299. [DOI] [PubMed] [Google Scholar]
- 56.Liang, Y., D. Fotiadis, S. Filipek, D. A. Saperstein, K. Palczewski, and A. Engel. 2003. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J. Biol. Chem. 278:21655-21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loconto, J., F. Papes, E. Chang, L. Stowers, E. P. Jones, T. Takada, A. Kumanovics, K. Fischer Lindahl, and C. Dulac. 2003. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell 112:607-618. [DOI] [PubMed] [Google Scholar]
- 58.Lorenz, M. C., X. Pan, T. Harashima, M. E. Cardenas, Y. Xue, J. P. Hirsch, and J. Heitman. 2000. The G protein-coupled receptor gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics 154:609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacKenzie, K. R., and D. M. Engelman. 1998. Structure-based prediction of the stability of transmembrane helix-helix interactions: the sequence dependence of glycophorin A dimerization. Proc. Natl. Acad. Sci. USA 95:3583-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacKenzie, K. R., J. H. Prestegard, and D. M. Engelman. 1997. A transmembrane helix dimer: structure and implications. Science 276:131-133. [DOI] [PubMed] [Google Scholar]
- 61.Margeta-Mitrovic, M., Y. N. Jan, and L. Y. Jan. 2000. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27:97-106. [DOI] [PubMed] [Google Scholar]
- 62.Martin, N. P., L. M. Leavitt, C. M. Sommers, and M. E. Dumont. 1999. Assembly of G protein-coupled receptors from fragments: identification of functional receptors with discontinuities in each of the loops connecting transmembrane segments. Biochemistry 38:682-695. [DOI] [PubMed] [Google Scholar]
- 63.McVey, M., D. Ramsay, E. Kellett, S. Rees, S. Wilson, A. J. Pope, and G. Milligan. 2001. Monitoring receptor oligomerization using time-resolved fluorescence resonance energy transfer and bioluminescence resonance energy transfer. The human delta-opioid receptor displays constitutive oligomerization at the cell surface, which is not regulated by receptor occupancy. J. Biol. Chem. 276:14092-14099. [DOI] [PubMed] [Google Scholar]
- 64.Mercier, J. F., A. Salahpour, S. Angers, A. Breit, and M. Bouvier. 2002. Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J. Biol. Chem. 277:44925-44931. [DOI] [PubMed] [Google Scholar]
- 65.Mesnier, D., and J. L. Baneres. 2004. Cooperative conformational changes in a G-protein-coupled receptor dimer, the leukotriene B(4) receptor BLT1. J. Biol. Chem. 279:49664-49670. [DOI] [PubMed] [Google Scholar]
- 66.Milligan, G., S. Wilson, and J. F. Lopez-Gimenez. 2005. The specificity and molecular basis of alpha1-adrenoceptor and CXCR chemokine receptor dimerization. J. Mol. Neurosci. 26:161-168. [DOI] [PubMed] [Google Scholar]
- 67.Miwa, T., Y. Takagi, M. Shinozaki, C. W. Yun, W. A. Schell, J. R. Perfect, H. Kumagai, and H. Tamaki. 2004. Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot. Cell 3:919-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyawaki, A., J. Llopis, R. Heim, J. M. McCaffery, J. A. Adams, M. Ikura, and R. Y. Tsien. 1997. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388:882-887. [DOI] [PubMed] [Google Scholar]
- 69.Nelson, G., J. Chandrashekar, M. A. Hoon, L. Feng, G. Zhao, N. J. Ryba, and C. S. Zuker. 2002. An amino-acid taste receptor. Nature 416:199-202. [DOI] [PubMed] [Google Scholar]
- 70.Nelson, G., M. A. Hoon, J. Chandrashekar, Y. Zhang, N. J. Ryba, and C. S. Zuker. 2001. Mammalian sweet taste receptors. Cell 106:381-390. [DOI] [PubMed] [Google Scholar]
- 71.Nemoto, W., and H. Toh. 2005. Prediction of interfaces for oligomerization of G-protein coupled receptors. Proteins 58:644-660. [DOI] [PubMed] [Google Scholar]
- 72.Overton, M., and K. Blumer. 2002. Use of fluorescence resonance energy transfer to analyze oligomerization of G-protein-coupled receptors expressed in yeast. Methods 27:324-332. [DOI] [PubMed] [Google Scholar]
- 73.Overton, M. C. 2000. GPCR structure and activation. Ph.D. dissertation. Washington University, St. Louis, Mo.
- 74.Overton, M. C., and K. J. Blumer. 2002. The extracellular N-terminal domain and transmembrane domains 1 and 2 mediate oligomerization of a yeast G protein-coupled receptor. J. Biol. Chem. 277:41463-41472. [DOI] [PubMed] [Google Scholar]
- 75.Overton, M. C., and K. J. Blumer. 2000. G-protein-coupled receptors function as oligomers in vivo. Current Biology 10:341-344. [DOI] [PubMed] [Google Scholar]
- 76.Overton, M. C., S. L. Chinault, and K. J. Blumer. 2003. Oligomerization, biogenesis, and signaling is promoted by a glycophorin A-like dimerization motif in transmembrane domain 1 of a yeast G protein-coupled receptor. J. Biol. Chem. 278:49369-49377. [DOI] [PubMed] [Google Scholar]
- 77.Pagano, A., G. Rovelli, J. Mosbacher, T. Lohmann, B. Duthey, D. Stauffer, D. Ristig, V. Schuler, I. Meigel, C. Lampert, T. Stein, L. Prezeau, J. Blahos, J. Pin, W. Froestl, R. Kuhn, J. Heid, K. Kaupmann, and B. Bettler. 2001. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J. Neurosci. 21:1189-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palczewski, K., T. Kumasaka, T. Hori, C. A. Behnke, H. Motoshima, B. A. Fox, I. Le Trong, D. C. Teller, T. Okada, R. E. Stenkamp, M. Yamamoto, and M. Miyano. 2000. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739-745. [DOI] [PubMed] [Google Scholar]
- 79.Park, P. S., S. Filipek, J. W. Wells, and K. Palczewski. 2004. Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry 43:15643-15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park, P. S., and K. Palczewski. 2005. Diversifying the repertoire of G protein-coupled receptors through oligomerization. Proc. Natl. Acad. Sci. USA 102:8793-8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parnot, C., and B. Kobilka. 2004. Toward understanding GPCR dimers. Nat. Struct. Mol. Biol. 11:691-692. [DOI] [PubMed] [Google Scholar]
- 82.Parrish, W., M. Eilers, W. Ying, and J. B. Konopka. 2002. The cytoplasmic end of transmembrane domain 3 regulates the activity of the Saccharomyces cerevisiae G-protein-coupled alpha-factor receptor. Genetics 160:429-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pausch, M. H. 1997. G-protein-coupled receptors in Saccharomyces cerevisiae: high-throughput screening assays for drug discovery. Trends Biotechnol. 15:487-494. [DOI] [PubMed] [Google Scholar]
- 84.Pfleger, K. D., and K. A. Eidne. 2005. Monitoring the formation of dynamic G-protein-coupled receptor-protein complexes in living cells. Biochem. J. 385:625-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierce, K. L., R. T. Premont, and R. J. Lefkowitz. 2002. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3:639-650. [DOI] [PubMed] [Google Scholar]
- 86.Raicu, V., D. B. Jansma, R. J. Miller, and J. D. Friesen. 2005. Protein interaction quantified in vivo by spectrally resolved fluorescence resonance energy transfer. Biochem. J. 385:265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ray, K., and B. C. Hauschild. 2000. Cys-140 is critical for metabotropic glutamate receptor-1 dimerization. J. Biol. Chem. 275:34245-34251. [DOI] [PubMed] [Google Scholar]
- 88.Reneke, J. E. 1989. Genetic and functional analysis of the yeast alpha-factor receptor. Ph.D. dissertation. University of California, Berkeley, Calif.
- 89.Reneke, J. E., K. J. Blumer, W. E. Courchesne, and J. Thorner. 1988. The carboxy-terminal segment of the yeast alpha-factor receptor is a regulatory domain. Cell 55:221-234. [DOI] [PubMed] [Google Scholar]
- 90.Rocheville, M., D. C. Lange, U. Kumar, R. Sasi, R. C. Patel, and Y. C. Patel. 2000. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 275:7862-7869. [DOI] [PubMed] [Google Scholar]
- 91.Roess, D. A., C. J. Brady, and B. G. Barisas. 2000. Biological function of the LH receptor is associated with slow receptor rotational diffusion. Biochim. Biophys. Acta 1464:242-250. [DOI] [PubMed] [Google Scholar]
- 92.Romano, C., J. K. Miller, K. Hyrc, S. Dikranian, S. Mennerick, Y. Takeuchi, M. P. Goldberg, and K. L. O'Malley. 2001. Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol. Pharmacol. 59:46-53. [PubMed] [Google Scholar]
- 93.Romano, C., W. L. Yang, and K. L. O'Malley. 1996. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem. 271:28612-28616. [DOI] [PubMed] [Google Scholar]
- 94.Russ, W. P., and D. M. Engelman. 2000. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 296:911-919. [DOI] [PubMed] [Google Scholar]
- 95.Salahpour, A., S. Angers, and M. Bouvier. 2000. Functional significance of oligomerization of G-protein-coupled receptors. Trends Endocrinol. Metab. 11:163-168. [DOI] [PubMed] [Google Scholar]
- 96.Salahpour, A., S. Angers, J. F. Mercier, M. Lagace, S. Marullo, and M. Bouvier. 2004. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J. Biol. Chem. 279:33390-33397. [DOI] [PubMed] [Google Scholar]
- 97.Shah, A., and L. Marsh. 1996. Role of Sst2 in modulating G protein-coupled receptor signaling. Biochem. Biophys. Res. Commun. 226:242-246. [DOI] [PubMed] [Google Scholar]
- 98.Shih, S. C., D. J. Katzmann, J. D. Schnell, M. Sutanto, S. D. Emr, and L. Hicke. 2002. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4:389-393. [DOI] [PubMed] [Google Scholar]
- 99.Shioda, T., E. E. Nakayama, Y. Tanaka, X. Xin, H. Liu, A. Kawana-Tachikawa, A. Kato, Y. Sakai, Y. Nagai, and A. Iwamoto. 2001. Naturally occurring deletional mutation in the C-terminal cytoplasmic tail of CCR5 affects surface trafficking of CCR5. J. Virol. 75:3462-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Springael, J. Y., E. Urizar, and M. Parmentier. 2005. Dimerization of chemokine receptors and its functional consequences. Cytokine. Growth Factor Rev. [DOI] [PubMed]
- 101.Stefan, C. J., M. C. Overton, and K. J. Blumer. 1998. Mechanisms governing the activation and trafficking of yeast G protein-coupled receptors. Mol. Biol. Cell 9:885-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tamaki, H., C. W. Yun, T. Mizutani, T. Tsuzuki, Y. Takagi, M. Shinozaki, Y. Kodama, K. Shirahige, and H. Kumagai. 2005. Glucose-dependent cell size is regulated by a G protein-coupled receptor system in yeast Saccharomyces cerevisiae. Genes Cells 10:193-206. [DOI] [PubMed] [Google Scholar]
- 103.Tanford, C., and J. A. Reynolds. 1976. Characterization of membrane proteins in detergent solutions. Biochim. Biophys. Acta 457:133-170. [DOI] [PubMed] [Google Scholar]
- 104.Terrillon, S., and M. Bouvier. 2004. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 5:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Terrillon, S., D. T., Mouillac, B., Breit, A., Ayoub, M. A., Taulan, M., Jockers, R., Barberis, C., Bouvier, M. 2003. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol. Endocrinol. 17:677-691. [DOI] [PubMed] [Google Scholar]
- 106.Thevelein, J. M., R. Gelade, I. Holsbeeks, O. Lagatie, Y. Popova, F. Rolland, F. Stolz, S. Van de Velde, P. Van Dijck, P. Vandormael, A. Van Nuland, K. Van Roey, G. Van Zeebroeck, and B. Yan. 2005. Nutrient sensing systems for rapid activation of the protein kinase A pathway in yeast. Biochem. Soc. Trans. 33:253-256. [DOI] [PubMed] [Google Scholar]
- 107.Thevenin, D., T. Lazarova, M. F. Roberts, and C. R. Robinson. 2005. Oligomerization of the fifth transmembrane domain from the adenosine A2A receptor. Protein Sci. 14:2177-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trettel, F., S. Di Bartolomeo, C. Lauro, M. Catalano, M. T. Ciotti, and C. Limatola. 2003. Ligand-independent CXCR2 dimerization. J. Biol. Chem. 278:40980-40988. [DOI] [PubMed] [Google Scholar]
- 109.Vanoni, M., R. L. Rossi, L. Querin, V. Zinzalla, and L. Alberghina. 2005. Glucose modulation of cell size in yeast. Biochem. Soc. Trans. 33:294-296. [DOI] [PubMed] [Google Scholar]
- 110.Weiner, J. L., S. C. Guttierez, and K. J. Blumer. 1993. Disruption of receptor-G protein coupling in yeast promotes the function of an SST2-dependent adaptation pathway. J. Biol. Chem. 268:8070-8077. [PubMed] [Google Scholar]
- 111.White, J. H., A. Wise, M. J. Main, A. Green, N. J. Fraser, G. H. Disney, A. A. Barnes, P. Emson, S. M. Foord, and F. H. Marshall. 1998. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396:679-682. [DOI] [PubMed] [Google Scholar]
- 112.Xu, H., L. Staszewski, H. Tang, E. Adler, M. Zoller, and X. Li. 2004. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl. Acad. Sci. USA 101:14258-14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xue, Y., M. Batlle, and J. P. Hirsch. 1998. GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Galpha subunit and functions in a Ras-independent pathway. EMBO J. 17:1996-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yesilaltay, A., and D. D. Jenness. 2000. Homo-oligomeric complexes of the yeast alpha-factor pheromone receptor are functional units of endocytosis. Mol. Biol. Cell 11:2873-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zacharias, D. A., J. D. Violin, A. C. Newton, and R. Y. Tsien. 2002. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296:913-916. [DOI] [PubMed] [Google Scholar]
- 116.Zhang, Z., S. Sun, S. J. Quinn, E. M. Brown, and M. Bai. 2001. The extracellular calcium-sensing receptor dimerizes through multiple types of intermolecular interactions. J. Biol. Chem. 276:5316-5322. [DOI] [PubMed] [Google Scholar]