Abstract
The Matα2 (α2) protein is a transcriptional repressor necessary for the proper expression of cell type-specific genes in Saccharomyces cerevisiae. Like many transcription factors, α2 is rapidly degraded in vivo by the ubiquitin-proteasome pathway. At least two different ubiquitin-dependent pathways target α2 for destruction, one of which recognizes the well-characterized Deg1 degradation determinant near the N terminus of the protein. Here we report that the α2 corepressors Tup1 and Ssn6 modify the in vivo degradation rate of α2. Tup1 modulates the metabolic stability of α2 by directly binding to the Deg1-containing region of the protein. TUP1 overexpression specifically stabilizes Deg1-containing proteins but not other substrates of the same ubiquitination enzymes that recognize Deg1. Point mutations in both α2 and Tup1 that compromise the α2-Tup1 binding interaction disrupt the ability of Tup1 to stabilize Deg1 proteins. The physical association between Tup1 and α2 competes with the ubiquitination machinery for access to the Deg1 signal. Finally, we observe that overproduction of both Tup1 and Ssn6, but not either alone, strongly stabilizes the endogenous α2 protein. From these results, we propose that the fraction of α2 found in active regulatory complexes with Tup1 and Ssn6 is spared from rapid proteolytic destruction and is stabilized relative to the uncomplexed pool of the protein.
The determination of different cell types in Saccharomyces cerevisiae provides a simple model for understanding the transcriptional regulatory mechanisms that specify distinct cellular identities (10, 11, 17). Haploid yeast cells exist as either of two types, a or α, that can conjugate with each other to produce a third kind of cell, the a/α diploid. These three cell types are phenotypically distinct because of the expression of cell type-specific genes: cells of the α type exclusively activate α-specific genes, while the a-specific genes are transcribed only in a cells. In addition, a set of haploid-specific genes are expressed in both a and α cells but are repressed in a/α diploids. These unique patterns of gene expression are regulated by a small number of transcription factors that function in various combinations to create this complex transcriptional circuit (7). For example, the a-specific genes are activated in a cells by the DNA-binding protein Mcm1 but are strongly repressed in α and a/α cells through the combinatorial action of Mcm1 and the homeodomain protein Matα2 (α2). The binding of these two proteins to sequences in the upstream region of a-specific genes tethers the Tup1-Ssn6 general repression complex in the vicinity of a-specific gene promoters (19, 20), where it potently represses transcription by a variety of mechanisms (35). This repression complex is recruited to target promoters by α2 through several distinct protein-protein interactions: Tup1 binds to the N-terminal domain of α2, while Ssn6 directly contacts the homeodomain in the C terminus of the protein (22, 34, 36).
Although α2 directs the extremely robust and stable repression of a-specific genes in α haploid cells, the α2 protein itself is very short lived in vivo (half-life, <5 min) (16). This rapid degradation is carried out by the ubiquitin-proteasome system (4, 14), which plays an essential role in a wide array of diverse cellular processes (9, 13, 43). In addition to degrading naturally short-lived regulators like α2, the ubiquitin system is also responsible for recognizing aberrant, nonnative proteins and tagging them for destruction. For efficient degradation by the 26S proteasome, nearly all substrates are modified by polymers of the small protein ubiquitin (9, 30). The conjugation of ubiquitin to target proteins requires a series of enzymes that include the E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin-protein ligase. Although some E3 proteins directly participate in the catalytic transfer of ubiquitin to substrates by forming a ubiquitin-E3 thiolester intermediate, most E3s facilitate E2-dependent protein ubiquitination through direct interactions with both the E2 and the substrate that bring them in close proximity and activate ubiquitin transfer (29, 43).
For the α2 protein, normal rates of degradation in α haploid cells depend on at least two different ubiquitination pathways that each require distinct E2 and E3 enzymes (5, 24, 38). One of these pathways recognizes an undefined degradation signal and utilizes the closely related E2 enzymes Ubc4 and Ubc5. The other uses a ubiquitination complex composed of a RING-CH domain E3 called Doa10 and the E2s Ubc6 and Ubc7 to recognize the Deg1 degradation signal found in the N terminus of α2 (5, 38). Mutagenesis experiments have implicated the hydrophobic face of a predicted amphipathic helix as the critical determinant of the Deg1 signal, suggesting that this surface serves as the primary element that is discriminated by the Ubc6/Ubc7/Doa10 complex (18).
Interestingly, both of these degradation signals in α2 are concealed by the formation of a heterodimer between α2 and its partner protein Mata1 (a1). In a/α diploid cells where both proteins are normally expressed, the exposed hydrophobic surface of the N-terminal amphipathic helix in α2 is buried within the interface of a coiled-coil interaction with a1, effectively masking Deg1 from the ubiquitin system and stabilizing the α2 repressor (18). In addition to a1, other proteins interact directly with α2 and potentially modify the degradation of this protein. These include the corepressors Ssn6 and Tup1, the latter of which binds to the N-terminal domain of α2 that contains the Deg1 signal. In the studies presented here, we identify Tup1 as an inhibitor of Deg1-dependent proteolysis, which upon binding to the N terminus of α2 blocks the ubiquitination machinery from accessing the Deg1 degradation signal. Moreover, we show that Tup1, in conjunction with Ssn6, can strongly stabilize the α2 protein, suggesting that the transcriptionally active form of α2 bound by its corepressors represents a distinct pool of α2 with increased metabolic stability. These observations imply a mechanism by which a constitutively unstable transcription factor is able to exert stringent transcriptional control and highlight the important role played by functionally relevant protein-protein interactions in modulating the stability of regulatory proteins.
MATERIALS AND METHODS
Yeast methods and strains.
Standard methods were used for the growth and genetic analysis of yeast. Experiments involving galactose-dependent regulation used media containing 3% raffinose and 3% galactose. Quantitative assays of β-galactosidase (β-gal) activity were performed on cells grown in liquid media using o-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate essentially as described previously (26).
Table 1 lists the strains utilized in this study. JY100 is an Ade+ revertant of YPH500 (33) in which Deg1-lacZ was integrated into the LEU2 locus (using YIp33-Deg1-lacZ [16]). JY102 and JY103 were derived from a cross of JY100 and YPH499. Strain JY104 was derived from JY100 by integrating YDp-K/Deg1-URA3 (α1) into the LYS2 locus and JY112 from JY100 after integration of YDp-K/Deg1-URA3 (note that the Deg1-URA3 fragment integrated in JY104 also contained other MATα sequences, including the matα1 [α1] gene, while the fragment integrated in JY112 did not contain α1). JY115 was derived from a cross of JY112 and JY103. JY172 was generated from JY112 in several steps by disrupting UBC4 and UBC6 with restriction fragments containing ubc4Δ::HIS3 and ubc6Δ::LEU2, respectively. JY187 was constructed by disrupting UBC6 in JY115 with a ubc6Δ::HIS3 PCR fragment and crossing the resulting strain to JY103. To generate JY204, the prc1-1 allele from YTX140 was introduced into the JY102 background by repeated (four times) backcrossing. Strain JY253 was derived from JY102 by disrupting the MATα2 (α2) gene with an α2Δ::HIS3MX PCR fragment. The strain JY383 was generated from a cross of JY381 (a lys− mutant derivative of JY172) and JY352, which was produced from JY172 in a series of steps by disrupting MATα2 with an α2Δ::kanMX PCR fragment and swapping the LEU2 marker with a TRP1 marker. Proper integration of all gene disruptions or integrations was determined by genomic PCR or Southern analysis, and single-site integration was verified by segregation analysis.
TABLE 1.
Yeast strains
| Strain | Genotype | Reference or source |
|---|---|---|
| YPH499 | MATaura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 | 33 |
| YPH500 | MATα ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 | 33 |
| JY100 | MATα ura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1::LEU2-Deg1-lacZ | This study |
| JY102 | MATα ura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 | This study |
| JY103 | MATaura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 | This study |
| JY104 | MATα ura3-52 lys2-801::LYS2-Deg1-URA3(α1) ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1::LEU2-Deg1-lacZ | This study |
| JY112 | MATα ura3-52 lys2-801::LYS2-Deg1-URA3 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1::LEU2-Deg1-lacZ | This study |
| JY115 | MATα ura3-52 lys2-801::LYS2-Deg1-URA3 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 | This study |
| JY172 | MATα ura3-52 lys2-801::LYS2-Deg1-URA3 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 ubc4Δ::HIS3 ubc6Δ::LEU2 | This study |
| JY187 | MATα ura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 ubc6Δ::HIS3 | This study |
| JY204 | MATα ura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 prc1-1 | This study |
| JY253 | matα2Δ::HIS3MX ura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 | This study |
| JY352 | matα2Δ::kanMX ura3-52 lys2-801::LYS2-Deg1-URA3 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 ubc4Δ::HIS3 ubc6Δ::leu2::TRP1 | This study |
| JY381 | MATaura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 ubc4Δ::HIS3 ubc6Δ::LEU2 | This study |
| JY383 | matα2Δ::kanMX ura3-52 lys2-801 ADE2 trp1-Δ63 his3-Δ200 leu2-Δ1 ubc4Δ::HIS3 ubc6Δ::leu2::TRP1 | This study |
| YTX140 | MATaura3-52 lys2-801 trp1-1 his3-Δ200 leu2-3,112 prc1-1 | 3 |
Isolation of cDNAs that inhibit Deg1-mediated degradation.
The pTRP plasmid expression library used in this study was generated by cre-mediated recombination from lambdaTRP (ATCC number 87277). In this library, the yeast cDNAs are cloned between the GAL1 promoter and the CYC1 terminator in the pTRP vector, which contains a TRP1 marker and the 2μm origin of replication. Two different approaches were utilized to isolate pTRP transformants in which Deg1-dependent turnover was disrupted. Approximately 60,000 Trp+ transformants of JY100 were screened for increased blue color development on 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) plates, while ∼350,000 Trp+ transformants of JY104 were plated on media lacking uracil to select for Ura+ colonies. Transformant colonies that turned blue on X-gal media or were Ura+ were rescreened by plating again onto the same media. Potential positives were then subjected to quantitative β-galactosidase activity assays using ONPG as a substrate. To identify plasmids that increased β-gal activity in a galactose-dependent manner, β-galactosidase assays were performed on cultures grown in raffinose or raffinose plus galactose. Plasmid DNA was isolated from these transformants and reintroduced into JY100 to confirm that the increase in Deg1-β-gal activity was plasmid dependent. The ends of the isolated cDNAs were sequenced using primers that hybridize to the GAL1 promoter or CYC1 terminator.
Plasmids.
To construct an integrating vector containing Deg1-URA3, the 5.2-kb HindIII fragment from YCp-Deg1-URA3 (5) was subcloned into YDp-K (2), generating YDp-K/Deg1-Ura3 (α1). This vector also contains other MATα sequences, including the α1 gene. A Deg1-URA3 integrating vector that lacks α1 (YDp-K/Deg1-Ura3) was constructed by subcloning a 3-kb EcoRV fragment from YCp-Deg1-URA3 into the SmaI site in YDp-K.
The plasmid YCplac111-Deg1-URA3 (L10S) was constructed from YCp-Deg1-URA3 by replacing the NdeI-BamHI fragment containing the wild-type Deg1 sequence with that carrying a L10S mutant version of Deg1, which was amplified from pKK99 (22) by PCR.
The L10S and R173A changes in α2 were constructed by the PCR-based QuikChange site-directed mutagenesis method (Stratagene) in pAV115, a CEN LEU2 MATα plasmid. The construction of plasmids pAV115 and pAV115/H3-3A have been described previously (41).
The pTRP-tup1-C348R and pTRP-tup1-S448P plasmids are derived from pTRP-TUP1 (3.2.2), which was isolated from the pTRP library as described above. These mutants were constructed by PCR in two steps. Segments of TUP1 from Val codon 228 to the site of the mutation were amplified, with the downstream primers introducing the Arg-348 or Pro-448 mutations. A second set of primer pairs was used to amplify other segments of TUP1 from the site of the mutation to Tyr codon 541, with the upstream primers introducing the Arg-348 or Pro-448 mutation. The two TUP1 segments for each mutation were combined by a second PCR step, and the resulting fragments were digested with BamHI and BstEII and ligated to similarly digested pTRP-TUP1 (3.2.2). The resulting plasmids were sequenced to confirm that no mutations other than the one desired were introduced.
A plasmid to express SSN6 from the GAL1 promoter was constructed by isolating the SSN6 coding sequence from pLN113-3 (32) on a SpeI/XbaI fragment and ligating it into SpeI-digested p426GAL1 (27).
Expression plasmids for Leu-β-gal and Ub-Pro-β-gal were constructed by amplifying the respective coding sequences and subcloning the resulting PCR fragments in p415GPD (28). A plasmid that expresses β-gal-SL17 has been described previously (8).
Pulse-chase analysis and ubiquitination assays.
Pulse-chase experiments were performed as described previously (5). Degradation rates were determined after quantitation on a PhosphorImager. Proteins containing β-galactosidase were immunoprecipitated with anti-β-gal antibodies (ICN), while Deg1-containing proteins and α2 were immunoprecipitated with antibodies raised against α2 (16).
The ubiquitination of Deg1-β-gal was monitored as described previously (23). Proteins were precipitated from cell lysates with anti-β-gal and immunoblotted with an anti-ubiquitin monoclonal antibody (a gift of Dan Gottschling, Fred Hutchinson Cancer Research Center).
RESULTS AND DISCUSSION
A genetic selection for negative regulators of α2 degradation.
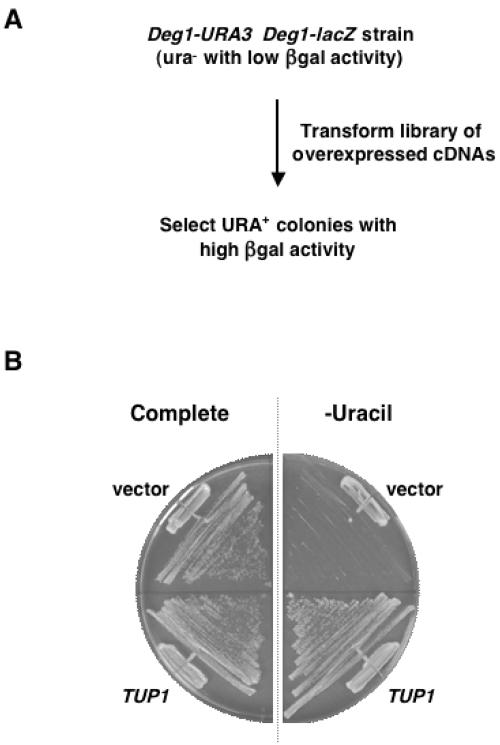
To identify genes that affect the turnover of the short-lived α2 protein, we constructed S. cerevisiae strains in which the degradation of unstable reporter proteins can be monitored readily. The well-characterized Deg1 degradation signal from α2 was fused to the normally stable yeast Ura3 and bacterial β-galactosidase enzymes to create short-lived versions of these proteins. Since the steady-state level of these proteins is a function of their intracellular half-life, the activity of the fusion proteins in cells reflects their degradation rate. Rapid turnover of Deg1-Ura3 strongly impairs the growth of cells on media lacking uracil when the fusion protein is the only source of Ura3 activity (5, 38). Similarly, yeast cells expressing a Deg1-β-galactosidase (Deg1-β-gal) fusion have low levels of β-galactosidase activity and produce very pale blue colonies on plates containing the chromogenic substrate X-gal (5, 16). Variants that fail to rapidly degrade these Deg1-containing substrates can be identified by their increased β-gal activity and enhanced growth on medium that does not contain uracil.
Strains expressing these short-lived reporter constructs were transformed with a high-expression yeast cDNA library to identify genes or gene fragments whose overexpression inhibited Deg1-mediated protein degradation (Fig. 1A). The cDNAs in this library were expressed from the GAL1 promoter, which is strongly induced in the presence of galactose (6, 31). We obtained over 400,000 library transformants and isolated a total of 54 clones that exhibited a plasmid- and galactose-dependent increase in the activity of the Deg1-containing proteins. DNA sequencing of the cDNA inserts identified 15 different genes, 8 of which were represented by full-length clones inserted in the correct orientation (Table 2).
FIG. 1.
Genetic selection for negative regulators of α2 proteolysis. (A) Selection scheme. The screen exploits the portability of the well-characterized Deg1 degradation signal of α2 (16). Yeast cells, whose only functional copy of URA3 is the Deg1-URA3 construct, require uracil for growth, since the Deg1-Ura3 protein is rapidly degraded. Overexpressed cDNAs that stabilize Deg1-Ura3 and Deg1-β-gal were isolated by their ability to allow cell growth in the absence of uracil and by an increase in β-gal activity. (B). Overexpression of TUP1 confers uracil prototrophy. Yeast cells expressing the Deg1-URA3 construct (JY104) were transformed with the empty GAL1 expression vector or with TUP1 expressed from this vector and grown on minimal complete media or minimal media lacking uracil.
TABLE 2.
Overexpressed cDNA clones impair deg1-mediated degradation
| Gene | No. of isolated clones/no. of different cDNAs | Increase in Deg1-β-gal activity (fold) | Comment(s) |
|---|---|---|---|
| Full-length cDNAs | |||
| GLC7 | 1/1 | 2 | |
| SKP1 | 1/1 | 2 | |
| SMI1 | 1/1 | 2 | |
| SRL1 | 1/1 | 2 | |
| TUP1 | 2/2 | 7 | |
| UBC4 | 1/1 | 2 | |
| UBC6 | 20/6 | 2-4 | |
| YDR132c | 1/1 | 2 | |
| cDNA fragments or inverted cDNAs | |||
| “EFB1” | 2/1 | 2 | cDNA in inverted orientation |
| “HEX3” | 1/1 | 2 | Fragment from 5′ end of gene; predicted to encode the first 269 amino acids of the 619-residue wild-type protein |
| “MNR2” | 7/1 | 3 | Fragment from 3′ end of gene; 5′ end of cDNA starts at +2258; predicted to encode a 22-amino-acid peptide from the +1 reading frame |
| “SAS3” | 3/1 | 2 | cDNA in inverted orientation |
| “SIS2” | 2/1 | 3 | cDNA in inverted orientation; predicted to encode an 118-amino-acid polypeptide |
| “STE12” | 8/1 | 6 | Fragment from 3′ end of gene; 5′ end of cDNA starts at +825; predicted to encode a 63-amino-acid polypeptide from the +1 reading frame |
| “YHR054c” | 3/1 | 3 | Fragment from 3′ end of gene; 5′ end of cDNA starts at +467 |
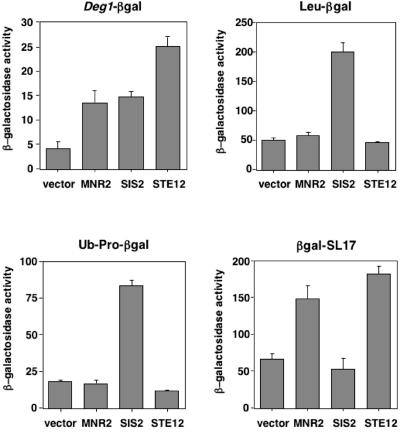
Interestingly, seven of the isolated plasmids contained fragments from the 5′ or 3′ ends of genes or were full-length cDNAs inserted in the reverse (antisense) orientation (Table 2). Among this group were cDNAs that, when overexpressed, produced some of the largest increases in Deg1-β-gal protein activity. To determine if expression of the nonphysiological products of these cDNAs affected the turnover of Deg1-containing proteins specifically or instead led to general defects in ubiquitin-mediated proteolysis, representatives of this class (“MNR2,” “SIS2,” and “STE12;” quotation marks are used to emphasize that these DNAs are not the named full-length genes) were tested with other short-lived substrates of the ubiquitin-proteasome pathway (Leu-β-gal, Ub-Pro-β-gal, and β-gal-SL17). Although all of these proteins are targeted to the 26S proteasome for destruction, each is recognized by a different ubiquitination pathway (8, 40).
Expression of the “SIS2” cDNA from the GAL1 promoter increased Leu-β-gal and Ub-Pro-β-gal activity approximately fourfold, similar to its effects on Deg1-β-gal (Fig. 2) and, based on pulse-chase analysis, also slowed the degradation rate of Ub-Pro-β-gal (data not shown). These results imply that the product of this cDNA insert, which is predicted to be an extremely hydrophobic polypeptide of 118 amino acids (residues 11 to 70 are exclusively leucine, valine, isoleucine, or phenylalanine), impairs a common step in the ubiquitin pathway utilized by each of these protein substrates, such as binding to the 26S proteasome.
FIG. 2.
Overexpression of specific protein fragments can impair the degradation of various substrates at different steps along the ubiquitin-proteasome pathway. Turnover of the short-lived β-gal fusion proteins was monitored by assaying β-galactosidase activity in JY104 cells expressing Deg1-β-gal or in JY102 cells transformed with expression plasmids for Leu-β-gal, Ub-Pro-β-gal, or β-gal-SL17; these cells also contained the empty GAL1 expression vector or this vector expressing the indicated cDNAs. The bars represent the averages of β-galactosidase activity measurements (in Miller units), and the error bars indicate the standard deviations.
In contrast to the effects of the cDNA from the “SIS2” gene, overexpression of the other two cDNAs (fragments of “MNR2” and “STE12”) did not alter the degradation kinetics of Ub-Pro-β-gal or increase Leu-β-gal or Ub-Pro-β-gal activity. Instead, increased expression of the “MNR2” or “STE12” gene fragments selectively enhanced Deg1-β-gal and β-gal-SL17 activity (Fig. 2). Both of these proteins are degraded in a DOA10/UBC6/UBC7-dependent manner (38; R. Swanson and M. Hochstrasser, unpublished observations), suggesting that the overexpression of these cDNA products inhibited an early step in the degradation of Deg1-β-gal and β-gal-SL17, perhaps when the proteins are recognized by the Ubc6/Ubc7/Doa10 enzymes. The products of the “MNR2” and “STE12” gene fragments may inhibit one or more of these ubiquitination enzymes noncompetitively or could be substrates that, when present in excessive amounts, titrate limiting quantities of Ubc6, Ubc7, and/or Doa10. In support of this latter hypothesis, we have found that the 63-amino-acid missense polypeptide presumably expressed from the “STE12” cDNA fragment has a predicted α-helical region that conforms to a 3,4-hydrophobic heptad repeat, consistent with it folding into an amphipathic helix. Since the hydrophobic face of an amphipathic helix is the critical determinant of the Deg1 signal recognized by the Ubc6/Ubc7/Doa10 ubiquitination complex, this finding suggests that the product of the “STE12” cDNA fragment mimics the Deg1 degradation signal and interferes with Deg1-mediated degradation in a competitive manner.
Of the properly oriented full-length genes identified by our genetic methods, UBC4 and UBC6 were known previously to be involved in α2 degradation (5). Although Ubc4 functions in another α2 ubiquitination pathway that does not utilize the Deg1 degradation signal (15), overexpression of this protein was known to stabilize both α2- and Deg1-containing substrates (M. Hochstrasser, unpublished observations). In fact, the overexpression of UBC4 was used as a positive control during our screen. UBC6 was the gene identified most frequently in our screen (Table 2); overexpression of this gene has been observed previously to inhibit the turnover of Ubc6-dependent proteolytic substrates (25, 37). Consistent with this, the overexpression of UBC6 only increased the activity of substrates (Deg1-β-gal and β-gal-SL17) that are known to be degraded in a UBC6-dependent manner and did not alter the levels of Ub-Pro-β-gal or Leu-β-gal, proteins that are destroyed by UBC6-independent pathways (data not shown; see also reference 25). Since Ubc6 itself is a relatively short-lived proteasomal substrate that requires its own catalytic activity for turnover (42), excess amounts of this unstable protein may outcompete other UBC6-dependent proteolytic substrates, like Deg1-containing proteins, for ubiquitination and degradation. Alternatively, an overabundance of Ubc6 may compete with Ubc7 for binding to the Doa10 ubiquitin ligase, which requires both of these E2s for activity.
To recognize the Deg1 degradation signal, Ubc6 functions with Ubc7 and the Ubc7 cofactor Cue1 (3, 5). Since all three of these proteins work together in Deg1-mediated proteolysis and only UBC6 was isolated in our screens, we determined if UBC7 or CUE1 overexpression had a similar effect on the steady-state level of Deg1-β-gal. Expression of CUE1 from the GAL1 promoter increased Deg1-β-gal activity to a degree comparable to that observed with UBC6 overexpression, whereas GAL1-driven expression of UBC7 did not (data not shown). Levels of Cue1, which is required for Ubc7 function, might be limiting in the latter case. It is unclear why CUE1 was not identified in the overexpression screen, but one likely explanation is that the screen was not saturating.
Five of the identified genes (GLC7, SKP1, SMI1, SRL1, and YDR132c) were each isolated only once, and their overexpression only weakly increased Deg1-β-gal activity (Table 2). Pulse-chase analyses of Deg1-β-gal degradation were performed to confirm that this enhanced β-gal activity was caused by the stabilization of the Deg1-β-gal protein. In wild-type cells, Deg1-β-gal has a 15-min half-life, and its stability was increased ∼1.5-fold (GLC7) to ∼2-fold (SKP1, SMI1, and YDR132c) in cells overexpressing the indicated cDNAs (SRL1 was not assayed). The degradation of Deg1-containing substrates was also determined in strains carrying loss-of-function mutant alleles of these genes. Pulse-chase experiments indicated little, if any, alteration in protein stability (data not shown). Therefore, the analysis of these genes in Deg1-mediated turnover was not pursued further.
The final full-length gene identified was TUP1, which encodes a corepressor protein recruited to α2-target genes by direct contact with the N terminus of α2 (20, 22, 35). TUP1 overexpression confers strong uracil-independent growth on ura3 cells carrying the Deg1-URA3 fusion (Fig. 1B) and increased Deg1-β-gal activity sevenfold (Table 2). Because of these strong effects on the activity of Deg1-containing proteins and the fact that Tup1 is involved in the transcriptional repression activity of the α2 protein, we concentrated on characterizing its role in the degradation of α2.
Tup1 stabilizes Deg1-containing proteins through a direct physical interaction.
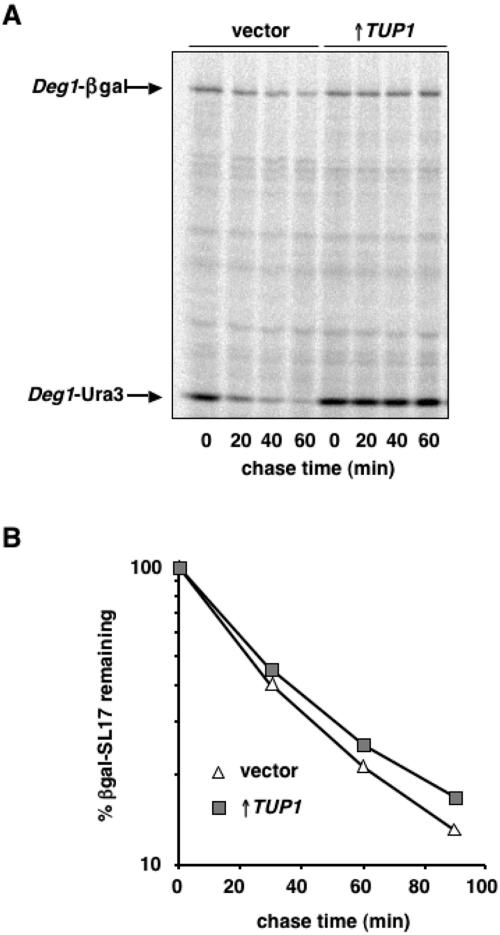
To confirm that the enhanced β-gal activity and increased growth on media lacking uracil observed in cells overexpressing TUP1 was due to the stabilization of Deg1-β-gal and Deg1-Ura3, we determined the degradation rate of these proteins by pulse-chase experiments (Fig. 3A). In cells carrying the empty GAL1 expression vector, the half-lives of Deg1-β-gal and Deg1-Ura3 were ∼20 min and ∼10 min, respectively. In contrast, in cells overexpressing TUP1, very little degradation of these proteins was apparent after the 60-min chase. Cells expressing increased amounts of TUP1 therefore have strong defects in Deg1-mediated proteolysis.
FIG. 3.
Overexpression of TUP1 specifically stabilizes Deg1-containing proteins. (A) Pulse-chase analysis of Deg1-β-gal and Deg1-Ura3 degradation in JY104 cells transformed with the empty GAL1 expression vector (vector) or with TUP1 expressed from this vector (↑TUP1). Deg1-containing proteins were immunoprecipitated from lysates with antibodies to α2 and quantitated by PhosphorImager analysis. (B) The degradation kinetics of β-gal-SL17 in vector-containing JY102 cells and in cells overexpressing TUP1. Radiolabeled β-gal-SL17 was immunoprecipitated from lysates with antibodies to β-gal and quantitated with a PhosphorImager.
We envisioned two different models to explain how the increased expression of TUP1 could alter the stability of Deg1-containing proteins. Since the Tup1 protein is targeted to a wide variety of genes and potently represses transcription (35), TUP1 overexpression could indirectly regulate the turnover of Deg1 substrates by repressing the expression of a positive regulator of Deg1-mediated proteolysis. Alternatively, Tup1 may directly interfere with the ability of the degradation machinery to recognize and/or degrade Deg1-containing proteins, e.g., by competing with the Doa10 E3 ligase for binding to the Deg1-encoded region of α2.
To discriminate between these two hypotheses, we monitored the specificity of the TUP1 overexpression effect by assessing the degradation of a number of other ubiquitin-proteasome pathway substrates. As measured with pulse-chase assays, extra TUP1 did not alter the degradation kinetics of Leu-β-gal or Ub-Pro-β-gal proteins (data not shown). It also did not change the degradation rate of CPY* (data not shown). CPY* is a misfolded endoplasmic reticulum protein that is degraded by a Ubc7- and Cue1-dependent mechanism but requires the Hrd1/Der3 ubiquitin ligase rather than Doa10 (1, 12). Most tellingly, overexpression of TUP1 did not stabilize β-gal-SL17 (Fig. 3B), a substrate whose degradation requires precisely the same set of E2 and E3 enzymes implicated in the destruction of Deg1-containing proteins (8, 38, and R. Swanson and M. Hochstrasser, unpublished observations). This clear specificity for α2-derived Deg1-encoded proteins suggests that Tup1 directly modulates the metabolic stability of these substrates rather than indirectly repressing a positive regulator of Deg1-mediated turnover.
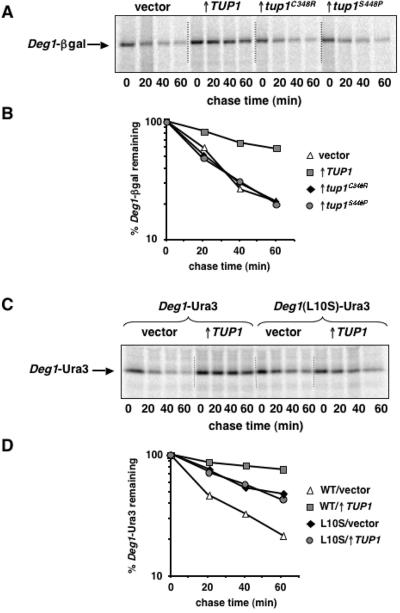
If Tup1 binding at or near Deg1 sterically occludes this degradation signal from the ubiquitination machinery, as suggested above, then mutations that impair Tup1-α2 binding should relieve the inhibitory effect of overexpressed TUP1 on the degradation of Deg1-containing substrates. Amino acid substitutions in the WD repeats of Tup1 (C348R and S448P) had been isolated previously and shown to disrupt the direct interaction between Tup1 and α2 (21). These tup1 mutant alleles were expressed from the GAL1 promoter, and the degradation of Deg1-β-gal was examined by pulse-chase analyses. While the overexpression of wild-type TUP1 inhibited Deg1-β-gal turnover, neither of the mutant proteins affected the stability of this substrate (Fig. 4A and B, compare TUP1 to vector). A trivial explanation for this result is that the mutant Tup1 proteins accumulate to lower levels than the wild-type protein. However, immunoblotting experiments using Tup1-specific antibodies performed in parallel demonstrated that all three Tup1 proteins are overproduced to a similar extent (data not shown). Thus, point mutations in Tup1 that impair its binding to the Deg1 portion of α2 abrogate TUP1-mediated stabilization of Deg1-β-gal.
FIG. 4.
Point mutations that disrupt α2/Tup1 binding prevent TUP1-mediated stabilization. (A) Pulse-chase analysis of Deg1-β-gal degradation in JY112 cells transformed with the empty GAL1 expression vector, the GAL1-TUP1 plasmid, or the same vector expressing the tup1C348R and tup1S448P mutant alleles. Radiolabeled Deg1-β-gal was immunoprecipitated from lysates with antibodies to β-gal and analyzed as described in the legend to Fig. 3. (B) Quantitation of the pulse-chase data shown in panel A. (C) Pulse-chase analysis of Deg1-Ura3 or Deg1(L10S)-Ura3 degradation in JY102 cells transformed with the empty GAL1 expression vector (vector) or with TUP1 expressed from this vector (↑TUP1). The Deg1-Ura3 proteins were expressed from YCplac111-Deg1-URA3. Radiolabeled Deg1-Ura3 was immunoprecipitated from lysates with antibodies to α2 and analyzed as described in the legend to Fig. 3. (D) Quantitation of the pulse-chase data shown in panel C. WT, wild type.
In complementary experiments, the stability of a Deg1 mutant protein defective for interaction with Tup1 was examined. An L10S mutation in α2, which was previously shown to severely compromise binding to Tup1 (22), was constructed in the context of the Deg1-Ura3 fusion protein, and the degradation of this protein was assayed by pulse-chase experiments. In contrast to the impaired turnover of wild-type Deg1-Ura3 in cells expressing increased amounts of TUP1, the degradation kinetics of the Deg1(L10S)-Ura3 protein were identical in cells carrying the empty GAL1 expression vector and in cells overexpressing TUP1 (Fig. 4C and D, compare TUP1 to vector). Although the Deg1(L10S)-Ura3 protein is moderately stabilized relative to wild-type Deg1-Ura3 (probably because the L10S mutation slightly impairs Deg1-mediated degradation), Tup1 overproduction did not further stabilize this protein. Importantly, Deg1(L10S)-Ura3 was strongly stabilized in ubc6Δ cells (data not shown). This UBC6-dependent turnover demonstrates that the same degradation pathway that recognizes wild-type Deg1-containing substrates also targets this mutant Deg1-containing protein. Taken together with the analysis of interaction-defective tup1 alleles, these mutational studies demonstrate that direct binding of Tup1 to Deg1 blocks the degradation of Deg1-containing substrates.
Binding of Tup1 to the Deg1 region of α2 blocks Deg1-mediated ubiquitination.
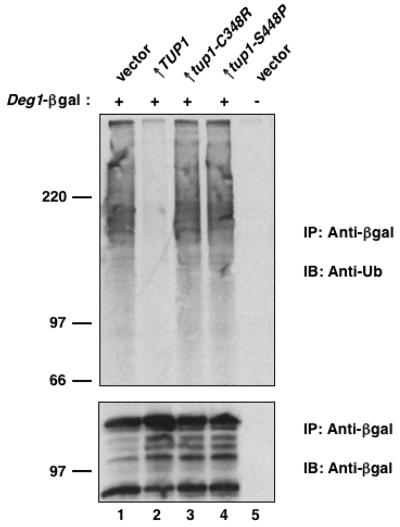
One mechanism by which the direct interaction of Tup1 with Deg1-containing proteins could inhibit their degradation is that the ubiquitin-conjugating machinery does not recognize the Deg1 degradation signal in the presence of bound Tup1. To test this, the ubiquitination state of Deg1-β-gal was analyzed. Deg1-β-gal was immunoprecipitated from cell lysates, and its ubiquitinated forms were visualized by anti-ubiquitin immunoblotting. Ubiquitin conjugates of Deg1-β-gal were observed in cells expressing this protein but were not detected in cells that lack Deg1-β-gal (Fig. 5, compare lanes 1 and 5) or in cells that express a mutant Deg1-β-gal with an amino acid substitution that inactivates the Deg1 degradation signal (23). In cells overproducing wild-type Tup1, the levels of ubiquitinated Deg1-β-gal were dramatically reduced (Fig. 5, lane 2). Moreover, in agreement with the pulse-chase experiments described above, overproduction of the Tup1 mutants defective in binding to α2 did not inhibit Deg1-mediated ubiquitination (Fig. 5, lanes 3 and 4). These results indicate that the binding of Tup1 to the N terminus of α2 blocks the ability of the ubiquitin-conjugating machinery to utilize the Deg1 degradation signal.
FIG. 5.
Overproduction of Tup1 inhibits Deg1-mediated ubiquitination. (Top) The level of polyubiquitin-Deg1-β-gal conjugates in lysates of JY102 cells carrying YEplac195-Deg1-lacZ or the empty vector. The cells also contained plasmids for overexpressing TUP1, the tup1 mutants, or the empty vector. Proteins were immunoprecipitated with anti-β-gal antibodies and then analyzed by antiubiquitin immunoblotting. (Bottom) The immunoblot was reprobed with anti-β-gal antibodies as a control for loading. IP, immunoprecipitation; IB, immunoblot; Ub, ubiquitin.
Increasing the expression of both TUP1 and SSN6 strongly stabilizes α2.
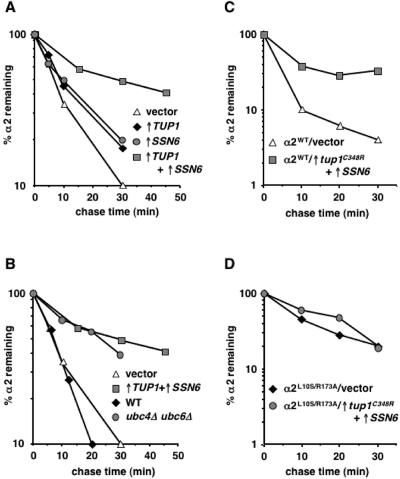
Since the overexpression of TUP1 impaired Deg1-dependent turnover, we also examined whether overproduction of Tup1 could stabilize the endogenous α2 protein. In addition to the Deg1-mediated pathway, a second ubiquitination pathway is required for normal rates of α2 proteolysis. Blocking either the Ubc4/Ubc5 or the Ubc6/Ubc7 pathway results in only a modest increase (∼2-fold) in the half-life of α2. However, when these ubiquitination pathways are disrupted simultaneously, the degradation of α2 is strongly impaired (5, 24, 38). Pulse-chase experiments with cells expressing extra TUP1 demonstrated that the degradation of α2 was weakly inhibited, consistent with the isolation of Tup1 as an inhibitor of the deg1-dependent pathway (Fig. 6A, ↑TUP1). Interestingly, TUP1 overexpression appears to impair more than just the Ubc6/Ubc7 pathway, since overproduction of Tup1 in ubc6Δ mutant cells stabilized α2 by an additional twofold (data not shown).
FIG. 6.
Co-overexpression of TUP1 and SSN6 strongly stabilizes α2. (A) The degradation kinetics of α2 in JY102 cells carrying the empty GAL1 expression vectors (vector) or plasmids for overexpressing TUP1 alone (↑TUP1), overexpressing SSN6 alone (↑SSN6), or overexpressing both TUP1 and SSN6 simultaneously (↑TUP1+ ↑SSN6). (B) The degradation kinetics of α2 in wild-type cells and ubc4Δ ubc6Δ mutants. For comparison, the pulse-chase data of vector-containing cells and TUP1 plus SSN6-overexpressing cells from panel A are added. (C) The kinetics of α2 turnover in JY253 cells carrying the MATα-containing plasmid pAV115 and the empty GAL1 expression vectors (vector) or plasmids for overexpressing tup1C348R and SSN6 (↑tup1C348R+↑SSN6). (D) The degradation of α2L10S/R173A was monitored in JY253 cells containing pAV115-L10S/R173A and the empty GAL1 expression vectors (vector) or plasmids for overexpressing tup1C348R and SSN6 (↑tup1C348R+↑SSN6). In all of these pulse-chase experiments, radiolabeled α2 was immunoprecipitated from lysates with antibodies to α2 and analyzed as described in the legend to Fig. 3. WT, wild type.
Tup1 functions as a transcriptional regulator as part of a complex with the corepressor Ssn6. In α2-mediated repression of a-specific genes, both Tup1 and Ssn6 make independent physical contacts with the α2 protein (22, 35, 36). Because of these observations, we tested whether overexpression of SSN6, like that of TUP1, also affected α2 turnover. Similar to the results observed with TUP1, expressing SSN6 from the GAL1 promoter modestly increased the stability of α2 (Fig. 6A, ↑SSN6). In striking contrast to the weak effects of increasing the expression of either TUP1 or SSN6 alone, the simultaneous overexpression of both these genes strongly impaired the degradation of α2 (Fig. 6A, ↑TUP1+↑SSN6). The degree of α2 stabilization in cells overexpressing both TUP1 and SSN6 is identical to that seen in a ubc4Δ ubc6Δ double mutant (Fig. 6B), which has the most severe α2 degradation defect known (5, 38). This result highlights the potency of overproducing Tup1 and Ssn6 on α2 stability.
The most straightforward interpretation of these Tup1 and Ssn6 overexpression results is that the physical association of these corepressors with α2 precludes the rapid ubiquitin-dependent turnover of α2. This conclusion is based on (i) the demonstration that the binding of Tup1 to the N terminus of α2 is required for Tup1 to block the ubiquitination and turnover of proteins containing the Deg1 signal from α2 (Fig. 4 and 5), (ii) the finding that Tup1 and Ssn6 are associated together in a protein complex (39, 44), and (iii) the observation, noted above, that Ssn6 binds directly to the homeodomain in the C-terminal domain of α2 (34, 36). Furthermore, we have observed that the stabilization of the α2 protein by TUP1 and SSN6 overexpression is abolished by mutations that impair α2-corepressor binding (Fig. 6C and D). These experiments utilized a mutant form of α2 containing the L10S and R173A amino acid substitutions; these single mutations have been isolated previously and shown to disrupt the α2-Tup1 and α2-Ssn6 interactions, respectively (22, 34). The α2L10S/R173A double mutant was expressed in cells, and the degradation of this protein was examined by pulse-chase assays. Overexpression of wild-type TUP1 and SSN6 stabilized this doubly mutant form of α2 (data not shown), suggesting that any deficit in affinity of the α2 mutant for the corepressors could be overcome by the overproduction of Tup1 and Ssn6. In contrast, the co-overexpression of SSN6 and the tup1C348R mutant, which is expected to further compromise the interaction between α2 and its corepressors, did not strongly impair the degradation of the α2L10S/R173A protein (Fig. 6D). The stability of wild-type α2, however, was increased by the overexpression of tup1C348R and SSN6 (Fig. 6C).
To verify that the same ubiquitination pathway that operates on wild-type α2 also targets the α2L10S/R173A protein, we determined the degradation kinetics of α2L10S/R173A in wild-type and ubc4Δ ubc6Δ cells. This mutant protein, like wild-type α2, was degraded in a UBC4/UBC6-dependent manner (data not shown). Thus, the degradation of endogenous α2 is markedly influenced by protein-protein interactions between α2 and its corepressors.
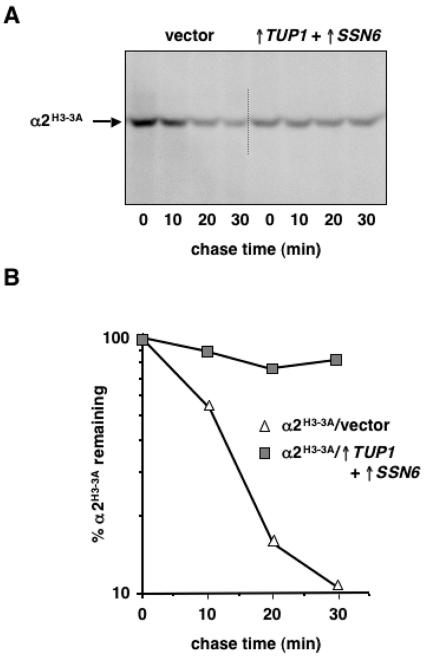
Interestingly, the stabilization of α2 by overproduced Tup1-Ssn6 does not require complex formation at α2 DNA-binding sites. The turnover of the α2H3-3A mutant, which is severely compromised in its ability to bind DNA-target sites because of three substitutions of amino acid residues that make direct contacts with DNA (41), was strongly impaired in the presence of increased TUP1 and SSN6 (Fig. 7). This finding suggests that under conditions where Tup1-Ssn6 is expressed in excess, Tup1-Ssn6 can interact with α2 in solution and thereby inhibit the rapid turnover of α2. Such a mechanism for stabilizing α2 recalls previous data demonstrating that the physical association of a1 with α2, which normally occurs in a/α diploid cells, blocks the ubiquitination and degradation of full-length α2 as well as a truncated form consisting of the N-terminal globular domain of α2 that does not bind DNA (18). An important distinction from this earlier study is that α2 is being stabilized in cells that must at some point degrade the entire active pool of the repressor because efficient cell type switching demands its elimination. These results emphasize the importance of physiologically relevant, alternative protein complexes in regulating the degradation of α2.
FIG. 7.
Mutations in α2 that disrupt DNA binding do not alter its stabilization by Tup1-Ssn6. (A) Pulse-chase analysis of α2H3-3A degradation in JY253 cells carrying pAV115/H3-3A and the empty GAL1 expression vectors (vector) or plasmids for overexpressing TUP1 and SSN6 (↑TUP1+↑SSN6). Radiolabeled α2H3-3A was immunoprecipitated from lysates with antibodies to α2 and analyzed as described in the legend to Fig. 3. (B) Quantitation of the pulse-chase data shown in panel A.
α2 is protected from degradation by interactions with its corepressors Tup1 and Ssn6.
Taken together, the results presented here suggest that the endogenous α2 protein is relatively stable when assembled into complexes with its corepressors Tup1 and Ssn6. Distinct pools of the repressor, each with different protein stability, can therefore exist in α haploid cells: “bulk” α2 with a very short half-life and a much smaller but more stable fraction of the functionally engaged repressor protein.
Current models of α2-mediated repression suggest that, under normal expression levels of α2 and its corepressors, DNA-bound α2 recruits the Tup1-Ssn6 complex to promoters (35), which implies that the functionally engaged, stabilized form of α2 exists primarily, if not exclusively, on DNA. This relatively stable pool of α2 has not been observed previously in haploid cells, as newly synthesized protein is destroyed with first-order kinetics (16), similar to the degradation of the steady-state population (24), suggesting that all detectable α2 molecules are equivalent in their susceptibility to rapid turnover. However, since <10 specific sites for α2 repression complexes exist in the yeast genome (45), we expect that only a small number of repressor molecules at any given time belong to this “privileged” pool of relatively stable α2. Experiments designed to detect the small fraction of α2 that is stabilized and characterize its dynamics under different physiological conditions are under way.
Why might the active fraction of α2 be stabilized? Although rapid degradation of the repressor is necessary to prevent the misregulation of cell-type-specific genes after mating-type switching (24), sufficient amounts of α2 must accumulate in α haploid cells to provide for the very strong repression activity of a-specific genes observed in this cell type. Stabilizing the actively engaged repressor, even partially, may allow for the maintenance of strong and stable a-specific gene repression, even though α2 is otherwise a rapidly degraded protein. Thus, the protection of a small pool of α2 may reflect the opposing requirements that the α2 protein initiate and maintain the stable repression of its a-specific gene targets yet still be sufficiently short lived to allow for cells to change phenotypically from the α to a state after a DNA switch at the MAT locus.
Our data imply that protein degradation rates can be dramatically altered by the dynamics of a protein's association with its physiological partner proteins. Changes in protein-protein interaction may represent a regulated step in the ubiquitination and degradation of a specific pool of a target protein. In the example of α2, this might depend on posttranslational modification of α2 or one of its binding partners or on the process of DNA replication through a DNA sequence bound by α2. Because many proteins, particularly transcription factors, function in the context of multiprotein complexes, we believe the principles illustrated by the present study will have broad relevance.
Acknowledgments
We thank Kris Siriratsivawong for plasmid and strain construction, Drew Vershon, Kelly Komachi, and Sandy Johnson for plasmids, Dan Gottschling for providing the antiubiquitin monoclonal antibody, and Tricia Serio, Rich Freiman, Alec DeSimone, and Christina Sowards for suggestions and critiques of the manuscript.
This work was supported by grants from the National Institutes of Health (GM46904), the American Cancer Society (PF4397), and the March of Dimes Birth Defects Foundation (5-FY05-28).
REFERENCES
- 1.Bays, N. W., R. G. Gardner, L. P. Seelig, C. A. Joazeiro, and R. Y. Hampton. 2001. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat. Cell Biol. 3:24-29. [DOI] [PubMed] [Google Scholar]
- 2.Berben, G., J. Dumont, V. Gilliquet, P. A. Bolle, and F. Hilger. 1991. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast 7:475-477. [DOI] [PubMed] [Google Scholar]
- 3.Biederer, T., C. Volkwein, and T. Sommer. 1997. Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278:1806-1809. [DOI] [PubMed] [Google Scholar]
- 4.Chen, P., and M. Hochstrasser. 1995. Biogenesis, structure, and function of the yeast 20S proteasome. EMBO J. 14:2620-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, P., P. Johnson, T. Sommer, S. Jentsch, and M. Hochstrasser. 1993. Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MATα2 repressor. Cell 74:357-369. [DOI] [PubMed] [Google Scholar]
- 6.Elledge, S. J., J. T. Mulligan, S. W. Ramer, M. Spottswood, and R. W. Davis. 1991. Lambda YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc. Natl. Acad. Sci. USA 88:1731-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galgoczy, D. J., A. Cassidy-Stone, M. Llinas, S. M. O'Rourke, I. Herskowitz, J. L. DeRisi, and A. D. Johnson. 2004. Genomic dissection of the cell-type-specification circuit in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 101:18069-18074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilon, T., O. Chomsky, and R. G. Kulka. 1998. Degradation signals for ubiquitin system proteolysis in Saccharomyces cerevisiae. EMBO J. 17:2759-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 10.Herskowitz, I. 1989. A regulatory hierarchy for cell specialization in yeast. Nature 342:749-757. [DOI] [PubMed] [Google Scholar]
- 11.Herskowitz, I., J. Rine, and J. Strathern. 1992. Mating-type determination and mating-type interconversion in Saccharomyces cerevisiae, p. 583-656. In E. W. Jones, J. R. Pringle, and J. R. Broach (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 2. Cold Spring Harbor Laboratory Press, Woodbury, N.Y. [Google Scholar]
- 12.Hiller, M. M., A. Finger, M. Schweiger, and D. H. Wolf. 1996. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273:1725-1728. [DOI] [PubMed] [Google Scholar]
- 13.Hochstrasser, M. 1996. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30:405-439. [DOI] [PubMed] [Google Scholar]
- 14.Hochstrasser, M., M. J. Ellison, V. Chau, and A. Varshavsky. 1991. The short-lived MATα2 transcriptional regulator is ubiquitinated in vivo. Proc. Natl. Acad. Sci. USA 88:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochstrasser, M., F. R. Papa, P. Chen, S. Swaminathan, P. Johnson, L. Stillman, A. Amerik, and S.-J. Li. 1995. The DOA pathway: studies on the functions and mechanisms of ubiquitin-dependent protein degradation in the yeast Saccharomyces cerevisiae. Cold Spring Harbor Symp. Quant. Biol. 60:503-513. [DOI] [PubMed] [Google Scholar]
- 16.Hochstrasser, M., and A. Varshavsky. 1990. In vivo degradation of a transcriptional regulator: the yeast α2 repressor. Cell 61:697-708. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, A. D. 1995. Molecular mechanisms of cell-type determination in budding yeast. Curr. Opin. Genet. Dev. 5:552-558. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, P. R., R. Swanson, L. Rakhilina, and M. Hochstrasser. 1998. Degradation signal masking by heterodimerization of MATalpha2 and MATa1 blocks their mutual destruction by the ubiquitin-proteasome pathway. Cell 94:217-227. [DOI] [PubMed] [Google Scholar]
- 19.Keleher, C. A., C. Goutte, and A. D. Johnson. 1988. The yeast cell-type-specific repressor alpha 2 acts cooperatively with a non-cell-type-specific protein. Cell 53:927-936. [DOI] [PubMed] [Google Scholar]
- 20.Keleher, C. A., M. J. Redd, J. Schultz, M. Carlson, and A. D. Johnson. 1992. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell 68:709-719. [DOI] [PubMed] [Google Scholar]
- 21.Komachi, K., and A. D. Johnson. 1997. Residues in the WD repeats of Tup1 required for interaction with α2. Mol. Cell. Biol. 17:6023-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komachi, K., M. J. Redd, and A. D. Johnson. 1994. The WD repeats of Tup1 interact with the homeo domain protein alpha 2. Genes Dev. 8:2857-2867. [DOI] [PubMed] [Google Scholar]
- 23.Laney, J. D., and M. Hochstrasser. 2002. Assaying protein ubiquitination in Saccharomyces cerevisiae. Methods Enzymol. 351:248-257. [DOI] [PubMed] [Google Scholar]
- 24.Laney, J. D., and M. Hochstrasser. 2003. Ubiquitin-dependent degradation of the yeast Matα2 repressor enables a switch in developmental state. Genes Dev. 17:2259-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenk, U., H. Yu, J. Walter, M. S. Gelman, E. Hartmann, R. R. Kopito, and T. Sommer. 2002. A role for mammalian Ubc6 homologues in ER-associated protein degradation. J. Cell Sci. 115:3007-3014.12082160 [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Mumberg, D., R. Muller, and M. Funk. 1994. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22:5767-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mumberg, D., R. Muller, and M. Funk. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119-122. [DOI] [PubMed] [Google Scholar]
- 29.Petroski, M. D., and R. J. Deshaies. 2005. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 30.Pickart, C. M., and R. E. Cohen. 2004. Proteasomes and their kin: proteases in the machine age. Nat. Rev. Mol. Cell Biol. 5:177-187. [DOI] [PubMed] [Google Scholar]
- 31.Ramer, S. W., S. J. Elledge, and R. W. Davis. 1992. Dominant genetics using a yeast genomic library under the control of a strong inducible promoter. Proc. Natl. Acad. Sci. USA 89:11589-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz, J., and M. Carlson. 1987. Molecular analysis of SSN6, a gene functionally related to the SNF1 protein kinase of Saccharomyces cerevisiae. Mol. Cell. Biol. 7:3637-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, R. L., and A. D. Johnson. 2000. A sequence resembling a peroxisomal targeting sequence directs the interaction between the tetratricopeptide repeats of Ssn6 and the homeodomain of alpha 2. Proc. Natl. Acad. Sci. USA 97:3901-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, R. L., and A. D. Johnson. 2000. Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem. Sci. 25:325-330. [DOI] [PubMed] [Google Scholar]
- 36.Smith, R. L., M. J. Redd, and A. D. Johnson. 1995. The tetratricopeptide repeats of Ssn6 interact with the homeo domain of alpha 2. Genes Dev. 9:2903-2910. [DOI] [PubMed] [Google Scholar]
- 37.Sommer, T., and S. Jentsch. 1993. A protein translocation defect linked to ubiquitin conjugation at the endoplasmic reticulum. Nature 365:176-179. [DOI] [PubMed] [Google Scholar]
- 38.Swanson, R., M. Locher, and M. Hochstrasser. 2001. A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 15:2660-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varanasi, U. S., M. Klis, P. B. Mikesell, and R. J. Trumbly. 1996. The Cyc8 (Ssn6)-Tup1 corepressor complex is composed of one Cyc8 and four Tup1 subunits. Mol. Cell. Biol. 16:6707-6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varshavsky, A. 1997. The ubiquitin system. Trends Biochem. Sci. 22:383-387. [DOI] [PubMed] [Google Scholar]
- 41.Vershon, A. K., Y. Jin, and A. D. Johnson. 1995. A homeo domain protein lacking specific side chains of helix 3 can still bind DNA and direct transcriptional repression. Genes Dev. 9:182-192. [DOI] [PubMed] [Google Scholar]
- 42.Walter, J., J. Urban, C. Volkwein, and T. Sommer. 2001. Sec61p-independent degradation of the tail-anchored ER membrane protein Ubc6p. EMBO J. 20:3124-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissman, A. M. 2001. Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2:169-178. [DOI] [PubMed] [Google Scholar]
- 44.Williams, F. E., U. Varanasi, and R. J. Trumbly. 1991. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex. Mol. Cell. Biol. 11:3307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong, H., R. McCord, and A. K. Vershon. 1999. Identification of target sites of the alpha2-Mcm1 repressor complex in the yeast genome. Genome Res. 9:1040-1047. [DOI] [PubMed] [Google Scholar]