Abstract
The Reelin signaling pathway operates in migrating neurons and is indispensable for their correct positioning during embryonic brain development. Many biochemical and cell biological studies to dissect the Reelin pathway at the molecular level are hampered by the lack of a cell line harboring a functional Reelin signaling pathway. Here we present fibroblast cell lines in which all required functional components of the pathway have been reconstituted. These cells react upon Reelin treatment in the same way as primary neurons. We have subsequently used these cell lines to study the subcellular localization of ApoER2 and the VLDL receptor and could demonstrate that receptor-mediated Dab1 phosphorylation does not depend on lipid rafts and that phosphorylated Dab1 remains bound to the receptor tail when the pathway is activated by Reelin.
During embryonic brain development, the proper positioning of newly generated neurons in the cortex, the cerebellum, and the hippocampus depends on Reelin signaling. The initial steps of the Reelin signaling pathway are already well characterized. Reelin is a glycoprotein secreted by specialized cells (15) which forms homodimers (26) and binds to ApoER2 and the VLDL receptor (VLDLR) expressed by target neurons (13, 18). As a consequence, intracellular Dab1, which binds to the cytoplasmic domains of ApoER2 and VLDLR (21, 48), becomes tyrosine phosphorylated (18, 24). This seems to be a linear pathway, since disruption of any of the corresponding genes causes the same phenotype in mice. Mutations in the Reelin gene (reeler mouse) (14), the dab1 gene (scrambler and yotari mouse) (19, 41, 50), and targeted deletion of the genes for both ApoER2 and VLDLR (49) result in the same cortical layering defect. Phosphorylation of Dab1 is critical for the effect of Reelin, since mice expressing a mutant Dab1 lacking the critical tyrosine phosphorylation sites have the same phenotype as the dab1 null mutant (20). Although the existence of coreceptors for Reelin such as members of the CNR family (40) and α3β1 integrin (17) cannot be ruled out, clustering of ApoER2 and/or VLDLR is sufficient to induce Dab1 phosphorylation in primary neurons (45). Phosphorylation of Dab1 in response to Reelin is mediated by members of the Src family of nonreceptor tyrosine kinases (3, 7). The PTB domain of Dab1 mediates not only binding to the intracellular domains of ApoER2 and VLDLR (21) but also binding to phosphoinositide head groups, which results in membrane localization of Dab1 (8, 44). Recent publications have demonstrated that the PTB domain-mediated membrane localization of Dab1 is indispensable for proper Reelin signaling in primary neurons (23, 43). In contrast to this initial part of the pathway, our knowledge on events downstream of Dab1 phosphorylation is still scarce. Activation of phosphatidylinositol 3 kinase and subsequent phosphorylation of protein kinase B (PKB)/Akt are involved (8). Phosphorylation of critical tyrosine residues of Dab1 regulates α3 integrin levels in migrating neurons and their timely detachment from the radial glial fibers (38). In addition, direct interaction of phosphorylated Dab1 with other intracellular proteins was shown; for instance, interaction with members of the Crk family might connect the Reelin pathway to integrin-mediated adhesion and migration of neurons (5, 11). Reelin-induced remodeling of the actin cytoskeleton might be mediated by binding of Nckβ (35) and association with N-WASP (46). Lis1 appears to interact with phosphorylated Dab1 and might link the Reelin pathway to cellular structures directly involved in neuronal migration (4). A major problem in delineating the molecular details of the Reelin signaling pathway is, besides primary neurons derived from embryonic brains, the lack of an appropriate cell line.
Here we demonstrate for the first time that the Reelin signaling pathway can be reconstituted in mouse fibroblasts by expressing Dab1 and either ApoER2 or VLDLR. Using these fibroblasts we show that ApoER2 and VLDLR reside in distinct subdomains of the plasma membrane and that signaling activity of the Reelin receptors is not related to a localization of the receptors to lipid rafts and/or caveolae. In addition, we could demonstrate that phosphorylated Dab1 stays bound to ApoER2, thus suggesting that any higher order protein complex recruited to phosphorylated Dab1 assembles on the receptor tails.
MATERIALS AND METHODS
Antibodies.
Antibodies (Ab) against the entire ligand-binding domains of ApoER2 (Ab 186) and VLDLR (Ab 74) were described previously (45). The antibody against the first ligand-binding repeat of ApoER2 (Ab 220) was also described previously (25). The antibody against the LDL receptor (Ab 197) has already been described (39). Polyclonal anti-Dab1 was raised in rabbits against a glutathione S-transferase (GST) fusion protein containing the first 180 amino acids of the short splice variant of murine Dab1 (277). Monoclonal mouse anti-Dab1 (D4) and mouse anti-Reelin (G10) were obtained from Andre Goffinet (University of Louvain, Brussels, Belgium). The following antibodies were purchased from the indicated sources: mouse antiphosphotyrosine (PY99), Santa Cruz; phospho-Akt (Ser473; catalog no. 9271S), Cell Signaling Technology.
VLDLR splice variants in murine brain.
Poly(A)+ RNA from murine cerebrum and cerebellum was prepared using the Micro-FastTrack mRNA isolation kit (Invitrogen). First-strand cDNA synthesis was performed using an oligo(dT) primer and SuperScript II Reverse Transcriptase (Invitrogen) in accordance with the manufacturer's protocol. The subsequent PCR was performed using the oligonucleotides VLDLR-O/S 5′GCCCAAGACATCATTGTCTA-3′ and VLDLR-O/AS 5′-ACCTACTGCTGCCATCACTA-3′. PCR products were analyzed by agarose gel electrophoresis.
Plasmids.
Plasmids used for retroviral transduction of murine fibroblasts are based either on the pMSCVpuro vector (Becton-Dickinson and Co.) or the bicistronic retroviral vector pMSCV-IRES-GFP in which the puromycin resistance cassette was replaced by an internal ribosome entry site and the gene coding for green fluorescent protein (GFP). pMSCV-IRES-GFP was a kind gift of Florian Grebien (Medical University of Vienna, Vienna, Austria). The cDNA for full-length murine Dab1 (555) (GenBank accession no. Y08379) was amplified by PCR from a plasmid (Dab wt-1FKBP; 45) using the oligonucleotides Dab1/S, 5′-TTGAATTCATGTCAACTGAGACAGAACTTC-3′ (introduced EcoRI sites are underlined), and Dab1/AS, 5′-TTGAATTCCTAGCTACCGTCTTGTGGAC-3′. The resulting fragment was cloned into the EcoRI restriction site of pMSCV-IRES-GFP, yielding the plasmid pMSCV-Dab1. The cDNAs for full-length murine ApoER2 harboring LA repeats 1 to 3, 7, and 8, containing (+) or lacking (−) the proline-rich cytoplasmic insert (GenBank accession no. NM_053073), were amplified from the corresponding plasmids (ApoER2Δ4-6; 9) by PCR using the oligonucleotides ApoER2/S, 5′-ATGAATTCATGGGCCGCCCAGAACTGG-3′, and ApoER2/AS, 5′-ATGAATTCTCAGGGCAGTCCATCATCTTC-3′. The resulting fragments were cloned into the EcoRI restriction site of pMSCVpuro. The cDNA for VLDLR lacking the “O-linked sugar domain” (GenBank accession no. XM_123381) was amplified by PCR from a cDNA pool derived from mouse brain using the oligonucleotides VLDLR/A, 5′-ATGAATTCTCAGGGCAGTCCATCATCTTC-3′, and VLDLR/AS, 5′-ATGAATTCAAGCCAGATCATCATCTGTGCTTAC-3′. The resulting fragment was cloned into the EcoRI restriction site of pMSCVpuro. pCMV5-c-src (murine neuronal) was a kind gift from Joachim Herz (University of Texas Southwestern Medical Center, Dallas, Tex.).
Production of stable NIH 3T3 cell lines.
For producing retroviral particles, the Phoenix Retrovirus Expression System (Orbigen) was used according to the manufacturer's protocol. The murine fibroblast cell line NIH 3T3 was transduced with the pMSCV-Dab1 plasmid, and positive cells (termed 3T3 D) were obtained by fluorescence-activated cell sorting. Cells were checked for expression of Dab1 by Western blotting and were subjected to a second infection procedure using cDNAs coding for ApoER2 containing (A+) or lacking (A−) the proline-rich cytoplasmic insert or VLDLR (V−) cloned into the pMSCVpuro backbone. Transduced cells were cultivated in selection medium (Dulbecco's modified Eagle medium [DMEM] supplemented with 10% fetal calf serum, antibiotics, and 1.5 μg/ml puromycin) and kept under a high puromycin concentration (1.5 μg/ml) for 7 days before reducing the concentration of puromycin to 0.75 μg/ml. Puromycin-resistant cells (termed 3T3 A+/D, A−/D, and V−/D) were grown in growth medium lacking puromycin for 24 h before use.
Expression of recombinant proteins, preparation of cell extracts, electrophoresis, and Western blotting.
Reelin was expressed in stably transfected 293 cells, and conditioned medium was prepared as described previously (9). Reelin-conditioned medium was analyzed by Western blotting using the anti-Reelin antibody G10. Total cell extracts from 3T3 or 293-HEK cells were obtained after washing the cells twice with phosphate-buffered saline (PBS; pH 7.4), scraping them into Hunt buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% NP-40, and Complete protease inhibitor cocktail [Roche]), and centrifugation for 30 min at 20,000 × g. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (27), and proteins were transferred onto nitrocellulose membranes by semidry blotting. For Western blotting with antibodies 220 and 74, nitrocellulose membranes were blocked for 1 h in PBS-0.1% Tween 20 containing 5% milk powder. For Western blotting using PY99, D4, G10, and anti-phospho-PKB/Akt, 5% bovine serum albumin instead of milk powder was used. Appropriate horseradish peroxidase-conjugated antibodies (1:20,000; Jackson ImmunoResearch) were used for detection with enhanced chemiluminescence (Pierce).
Reelin binding assay.
NIH 3T3 cells were seeded into 6-well dishes (300,000 cells/well) and cultivated for 24 h. Cells were put on ice for 30 min, and the growth medium was removed and replaced by Reelin-conditioned medium. After incubation for 30 min at 4°C, cells were extensively washed with Tris-buffered saline (TBS; pH 7.4) and lysed in Hunt buffer for 30 min on ice. The lysates were centrifuged at 20,000 × g for 30 min, and the supernatants were boiled in reducing Laemmli buffer prior to SDS-PAGE and Western blotting. The presence of Reelin was tested using G10 as primary antibody for Western blotting. Horseradish peroxidase-conjugated goat anti-mouse antibody (1:20,000; Jackson ImmunoResearch) was used for detection with enhanced chemiluminescence (Pierce).
Purification of Reelin from conditioned medium.
Reelin was purified as described previously (29). Briefly, Reelin-conditioned medium from 293 cells was centrifuged for 2 h at 200,000 × g. The resulting pellet was washed with TBS and resuspended in TBS.
Dab1 phosphorylation assay.
Dab1 phosphorylation was measured essentially as described previously (18). Briefly, cells stably expressing the indicated proteins (see the figures) were grown on 10-cm-diameter dishes, washed once with PBS, and incubated with different media containing the indicated ligands (see the figures). After 20 min at 37°C, cells were washed again, scraped into 1 ml of Hunt buffer containing 50 mM NaF and 2 mM Na3VO4, and lysed for 30 min on ice. The lysates were centrifuged at 20,000 × g for 30 min, and the supernatants were immediately used for immunoprecipitation of Dab1 using 5 μl of anti-Dab1 antiserum. After 2 h at 4°C, 20 μl of a suspension containing protein A beads (Amersham) was added, and the mixture was incubated for 2 h at 4°C. The beads were washed with Hunt buffer and boiled in reducing Laemmli buffer prior to SDS-PAGE and Western blotting.
Dab1 degradation assay.
Dab1 degradation was measured essentially as described previously (2). Briefly, 1.6 × 106 3T3 cells stably expressing one of the Reelin receptors and Dab1 were seeded into 10-cm dishes. Cells were starved in plain DMEM containing 20 μg cycloheximide/ml for 60 min and subsequently treated with Reelin or control medium containing 20 μg cycloheximide/ml for the indicated time period. Cells were washed with TBS, lysed in Hunt buffer containing 50 mM NaF and 2 mM Na3VO4, and lysed for 30 min on ice. The lysates were centrifuged at 20,000 × g for 30 min, and the supernatants were immediately used for immunoprecipitation of Dab1 as described for the Dab1 phosphorylation assay.
Phosphorylation of PKB/Akt.
Phosphorylation of PKB/Akt was measured directly in total cell extracts derived from stimulated NIH 3T3 fibroblasts starved for 6 h using plain DMEM to reduce background phosphorylation of PKB/Akt. Briefly, equal amounts of protein (10 μg) from cell lysates were separated by SDS-PAGE and immunoblotted using an antibody directed against phospho-PKB/Akt.
Dab1 pulldown.
293-HEK cells were transiently transfected with plasmids coding for Dab1 or Dab1 and src using PolyFect (QIAGEN) according to the manufacturer's protocol. Forty-eight hours after transfection, cells were washed twice with PBS, lysed in Hunt buffer for 30 min on ice, and centrifuged at 20,000 × g for 30 min. The lysate was incubated with 25 μl of purified GST-ApoER2-tail containing the proline-rich insert coupled to glutathione Sepharose for 2 h at 4°C as described previously (42). The beads were collected by centrifugation (500 × g, 2 min), washed twice with Hunt buffer, and boiled in reducing Laemmli sample buffer before SDS-PAGE and Western blotting with the indicated antibodies.
Sodium carbonate-based isolation of caveolin-rich light membranes (CLM).
NIH 3T3 fibroblasts were grown to confluence in 10-cm dishes. CLM were prepared using a detergent-free method as described previously (51). All procedures were carried out at 4°C. Briefly, cells from a 10-cm dish were washed with TBS, scraped into 2 ml of MBS buffer (25 mM morpholinethanesulfonic acid, 150 mM NaCl, pH 6.5) containing 500 mM sodium carbonate and Complete protease inhibitors (Roche), and lysed by sonication (6 times for 15 s with 100% sonication cycle time and 70% power using a Bandelin Sonopuls HD70 sonicator). Cell homogenates were mixed with 2 ml of 90% (wt/vol) sucrose in MBS and transferred to a 12-ml ultracentrifuge tube. A discontinuous sucrose gradient was formed above the homogenate by layering on 4 ml of 35% (wt/vol) sucrose in MBS, followed by 4 ml of 5% (wt/vol) sucrose in MBS. After centrifugation at 160,000 × g for 18 h in a Beckman SW40Ti rotor at 4°C, 1-ml fractions were collected from the top of the tube. Fraction 4 at the interface between the 5% and 35% sucrose boundary was designated the CLM fraction. Fraction 12 at the bottom of the tube (45% sucrose) was designated the noncaveolar membrane fraction (NCM). Fractions 4 and 12 were used for Western blotting and immunoprecipitation of ApoER2.
Detergent-based isolation of caveolin-rich light membranes (CLM).
CLM were prepared from stable NIH 3T3 fibroblasts grown to confluence in 15-cm dishes. All procedures were carried out at 4°C. Briefly, cells were washed with TBS, scraped, and pelleted by centrifugation (5 min, 1,400 × g). The supernatant was removed, and cells were solubilized in TBS containing 2% Brij 78P (Fluka) and Complete protease inhibitors (Roche) by passaging the cells 10 times through a 23-gauge needle. Cell debris was removed by centrifugation (10 min, 21,000 × g), and the lysate (0.6 ml) was mixed with 0.6 ml of 90% (wt/vol) sucrose in MBS and transferred to a 4.3-ml ultracentrifuge tube. A discontinuous sucrose gradient was formed above the homogenate by layering on 2.5 ml of 35% (wt/vol) sucrose in MBS, followed by 0.6 ml of 5% (wt/vol) sucrose in MBS. After centrifugation at 160,000 × g for 20 h in a Beckman SW60Ti rotor at 4°C, 0.44-ml fractions were collected from the top of the tube. Fraction 2 at the interface between the 5% and 35% sucrose boundaries was designated the CLM fraction.
Immunoprecipitation of ApoER2.
ApoER2 was immunoprecipitated from fractions 4 and 12 derived from a sodium carbonate-based isolation of CLM. Briefly, 500 μl of each fraction was diluted with 1 ml Hunt buffer, and 5 μl antiserum (Ab 186) was added to precipitate ApoER2. After 2 h at 4°C, 20 μl of a suspension containing protein A beads was added, and the mixture was incubated for 2 h at 4°C. The beads were washed with Hunt buffer and boiled in reducing Laemmli buffer prior to SDS-PAGE and Western blotting.
Wound healing assay.
Cell migration was determined as described previously (37). Briefly, 6-cm dishes were coated overnight with 10 μg/ml fibronectin, washed, and blocked with 2 mg bovine serum albumin/ml for 60 min. 3T3 A−/D cells (1 × 106) were seeded into the coated dishes and allowed to adhere for 4 h at 37°C in a 7.5% CO2 incubator. A wound was created relative to a marking on the culture dish as a reference point by scraping the cell monolayer with a pipette tip, the cells were washed with DMEM containing antibiotics and 2% fetal calf serum, and 5 ml of the same medium was added. Images were acquired using a Zeiss Axiovert 135 microscope immediately after creating the wound and after an incubation time of 15 h. The pictures of the wounded area were overlaid with a grid, and cells within this area were counted.
RESULTS
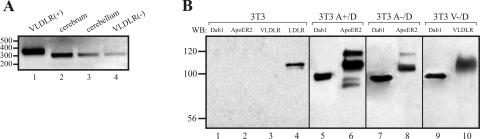
To reconstitute the Reelin signaling pathway, we used mouse 3T3 fibroblasts and stably expressed Dab1 and either ApoER2 or VLDLR. As demonstrated in Fig. 1B, these cells express LDL receptor (lane 4) but no detectable amounts of Dab1 (lane 1), ApoER2 (lane 2), or VLDLR (lane 3). The mammalian VLDLR exists in variant forms arising from differential splicing of exon 16 (31). The variant lacking exon 16 codes for a protein which lacks the “O-linked sugar domain.” In order to decide which variant of the VLDLR should be expressed in our cell model, we tested by reverse transcription-PCR which variant is present in embryonic mouse brain. Primers flanking exon 16 were used, and, as demonstrated in Fig. 1A, the predominant variant expressed in mouse cerebrum (lane 2) and cerebellum (lane 3) has the same length (297 bp) as the product derived from a plasmid containing the cDNA for mouse VLDLR lacking exon 16 (lane 4). As a control for the presence of exon 16, we used a corresponding cDNA as template for the PCR, which resulted in a product of 381 bp (lane 1). We have cloned the product amplified from mouse brain cDNA, and sequencing confirmed that it is indeed derived from the corresponding VLDLR transcript lacking exon 16.
FIG. 1.
Analysis of VLDLR splice variants in murine brain and expression of Reelin receptors and Dab1 in NIH 3T3 cells. (A) cDNA coding for murine VLDLR containing (lane 1) or lacking (lane 4) exon 16 coding for the “O-linked sugar domain” and cDNA derived by reverse transcription of mRNA from murine cerebrum (lane 2) and cerebellum (lane 3) was used for PCR amplification using a primer pair flanking exon 16. Amplified products were separated by DNA electrophoresis on a 1.8% agarose gel. Molecular size markers are shown (in base pairs). (B) Total cell extracts derived from NIH 3T3 cells (lanes 1 to 4) and NIH 3T3 cells stably expressing Dab1 and ApoER2 containing the proline-rich cytoplasmic insert (lanes 5 and 6) (3T3 A+/D), Dab1, ApoER2 lacking the proline-rich cytoplasmic insert (lanes 7 and 8) (3T3 A−/D), and Dab1 and VLDLR lacking the “O-linked sugar domain” (lanes 9 and 10) (3T3 V−/D) were separated on an SDS-8% PAGE gel and immunoblotted. Primary antibodies used for detection were D4 for Dab1, 220 for ApoER2, and 74 for VLDLR. Molecular size markers are shown (in kilodaltons). WB, Western blot.
Due to differential splicing, ApoER2 also exists in variant forms (9, 10). Since it is not clear yet whether the facultative proline-rich insert in the cytoplasmic domain is involved in the initial steps of the Reelin pathway, we produced cells expressing either variant. As the starting cell line, mouse 3T3 fibroblasts expressing murine Dab1 (555) (designated 3T3 D) were produced using the Phoenix Retrovirus Expression System. This cell line was subsequently stably transfected with plasmids coding for murine VLDLR without the “O-linked sugar domain” (V−) or murine ApoER2 with (A+) or without (A−) the proline-rich insert of the cytoplasmic tail. The resulting cell lines (3T3 A+/D, 3T3 A−/D, and 3T3 V−/D) were tested by Western blotting for the expression of the corresponding proteins (Fig. 1B). All three cell lines express comparable amounts of Dab1 (lanes 5, 7, and 9). The two prominent bands for ApoER2 seen in the cell lines expressing the corresponding cDNAs (lanes 6 and 8) arise from differential glycosylation of the precursor and the mature form of the receptor (28). In contrast, murine VLDLR lacking the “O-linked sugar domain” migrates as a single band with a relative molecular mass of about 110 kDa (lane 10). To test whether the cell lines expressing ApoER2 or VLDLR bind Reelin, we performed a cell binding assay. The cells were incubated with Reelin-conditioned medium for 30 min at 4°C. After the incubation, the medium was removed and the cells were washed with TBS and lysed, and bound Reelin was detected by Western blotting. As demonstrated in Fig. 2, all three cell lines expressing either A+, A−, or V− bind Reelin. Mock-transfected cells used as controls did not bind detectable amounts of Reelin (lane 4).
FIG. 2.
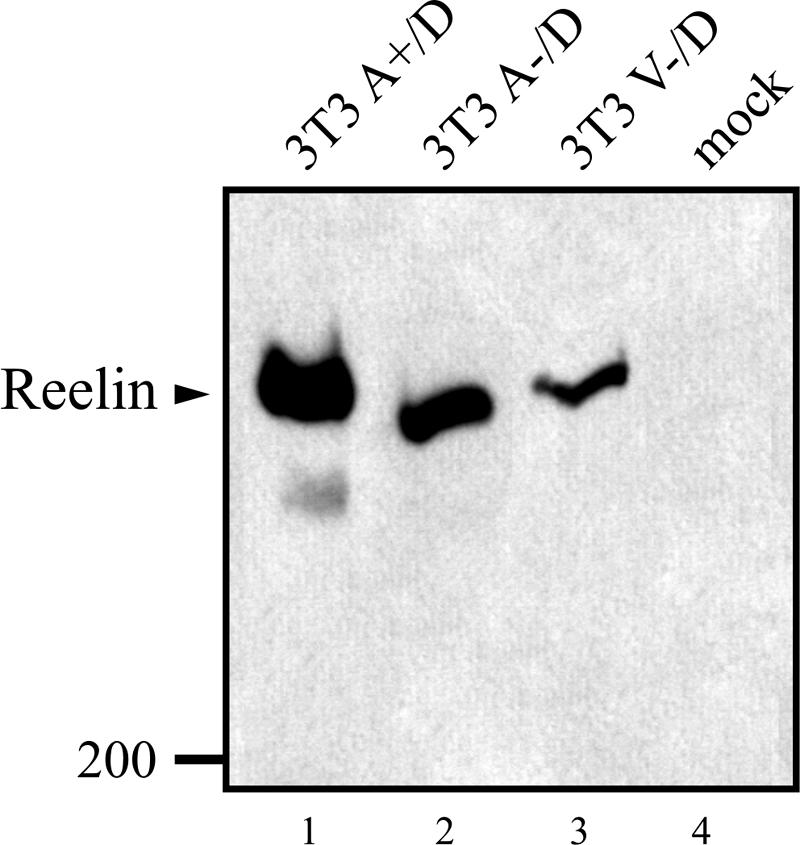
3T3 cells expressing ApoER2 or VLDLR bind Reelin. Cells expressing the Reelin receptors and Dab1 (3T3 A+/D, lane 1; 3T3 A−/D, lane 2; 3T3 V−/D, lane 3; 3T3, lane 4) were chilled on ice and subsequently incubated with Reelin-conditioned medium at 4°C. Unbound Reelin was removed by extensive washing, and cells were lysed. Extracts were separated by SDS-PAGE on a 4% gel and immunoblotted using the Reelin-specific antibody G10. Molecular size markers are shown (in kilodaltons).
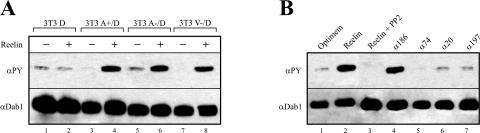
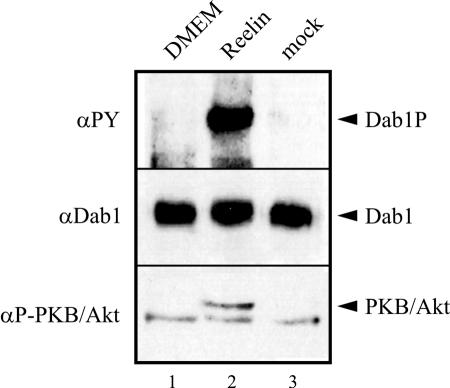
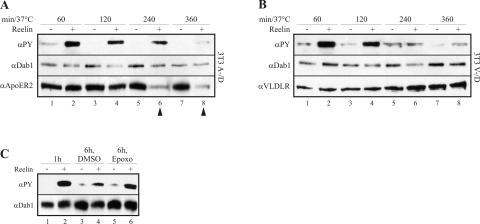
Having established that our 3T3 cell lines properly express the Reelin receptors and indeed bind Reelin, we tested whether incubation with Reelin triggers Dab1 phosphorylation. As shown in Fig. 3A, cells expressing only Dab1 do not respond to Reelin stimulation. However, in these cells a low but detectable level of phosphorylated Dab1 is present (lanes 1 and 2). This effect has been described previously (22) and shown to be triggered by phosphatidylinositol-mediated membrane localization of Dab1 independently of the presence of ApoER2 or VLDLR (23). Thus, the background phosphorylation indicates that Dab1 is correctly localized in 3T3 cells. 3T3 cells expressing Dab1 and one of the Reelin receptors respond to Reelin stimulation with a robust induction of Dab1 phosphorylation (lanes 4, 6, and 8). This effect is indistinguishable whether ApoER2 (lanes 4 and 6) or VLDLR (lane 8) is expressed or whether the proline-rich cytosolic insert is present in ApoER2 (lane 4). To test if Reelin-induced Dab1 phosphorylation in 3T3 cells is qualitatively similar to that in neurons, we stimulated the cell line 3T3 A+/D with Reelin in the presence of PP2, which specifically inhibits Dab1 phosphorylation by src family kinases (3). As demonstrated in Fig. 3B (lane 3), PP2 completely blocks Reelin-induced Dab1 phosphorylation in 3T3 cells. In addition, incubation with an antibody against the extracellular domain of ApoER2 (Ab 186) triggered Dab1 phosphorylation (lane 4) in the same way that Reelin does. This effect is specific, since antibodies against VLDLR (Ab 74; lane 5), the intracellular domain of ApoER2 (Ab 20; lane 6), or against LDLR (Ab 197; lane 7) had no effect. This experiment demonstrates that stimulation of the Reelin pathway in these cells is induced by receptor clustering exactly as described for primary neurons (45). As recently shown, activation of PKB/Akt is a downstream event of Reelin stimulation in primary neurons (6). To test whether Reelin also stimulates PKB/Akt phosphorylation in 3T3 A+/D cells, we had to maintain the cells for 6 h in serum-free medium prior to the addition of Reelin (Fig. 4, lane 1), since PKB/Akt is heavily phosphorylated in 3T3 cells when cultured in serum-containing media (data not shown) (1). Reelin-conditioned medium is usually produced by culturing Reelin-expressing 293 cells in Optimem (see Materials and Methods). However, addition of fresh Optimem to 3T3 cells already induced phosphorylation of PKB/Akt (data not shown). The same medium conditioned with mock-transfected 293 cells induced PKB/Akt phosphorylation even more (most likely due to the presence of secreted growth factors). Thus, we purified Reelin from the conditioned medium by ultracentrifugation. Due to its high molecular mass and the fact that it exists at least as a dimer in its native state, it can be sedimented from the medium by centrifugation for 2 h at 200,000 × g (29). As demonstrated in Fig. 4 (lane 2), Reelin purified by ultracentrifugation is as potent to induce Dab1 phosphorylation as Reelin-conditioned medium and is able to induce PKB/Akt phosphorylation in 3T3 A+/D cells. This effect is specific for Reelin, since the same medium from mock-transfected 293 cells subjected to the same procedure as that for the Reelin purification had no effect (lane 3). Finally, we tested whether Reelin stimulation of 3T3 A−/D and V−/D cells also leads to degradation of phosphorylated Dab1 as described for primary neurons (2). Cells were stimulated with Reelin or mock-conditioned medium in the presence of cycloheximide over a period of 6 h at 37°C, and the amounts of phosphorylated and total Dab1, ApoER2, and VLDLR were measured (Fig. 5). In both cell lines the amount of phosphorylated Dab1 started to decline after 1 h of Reelin incubation and dropped to very low levels after 6 h. In 3T3 V−/D cells, however, the decrease of phosphorylated Dab1 is slower than that in 3T3 A−/D cells. Levels of total Dab1 also dropped in both cell lines, although the decline was less pronounced than that in primary neurons (2). Addition of epoxomicin completely inhibited Reelin-induced Dab1 degradation, demonstrating that this effect is due to proteosomal degradation. Furthermore, epoxomicin treatment increased the amount of detectable total Dab1 after 6 h. Interestingly, the level of ApoER2 also decreased dramatically upon Reelin stimulation (arrows in Fig. 5A, lanes 6 and 8), whereas the level of VLDLR did not change under these conditions (Fig. 5B). In summary, we have established cell lines based on mouse 3T3 fibroblasts that express Dab1 and, in addition, ApoER2 or VLDLR. These cells react to Reelin stimulation in the same way as primary neurons. Dab1 becomes phosphorylated, and this process is dependent on src family kinases, can be mimicked by agents clustering the receptors, and leads to degradation of phosphorylated Dab1 and activation of PKB/Akt. To our knowledge, this is the first heterologous cell system in which the Reelin pathway has been reconstituted.
FIG. 3.
Reelin-induced Dab1 phosphorylation in 3T3 cells is qualitatively indistinguishable from that in primary neurons. (A) Reelin-induced Dab1 phosphorylation was measured after immunoprecipitation of Dab1 from cell lysates derived from 3T3 cells expressing Dab1 or Dab1 and the Reelin receptors as indicated. The immunoprecipitates were separated on an SDS-8% PAGE gel, and Western blotting was performed using antiphosphotyrosine (αPY) and anti-Dab1 antibodies as described in Materials and Methods. 3T3 cells expressing Dab1 or Dab1 and one of the Reelin receptors were stimulated with mock- (−) or Reelin-conditioned (+) medium. (B) 3T3 A+/D cells were stimulated with mock-conditioned medium (lane 1), Reelin-conditioned medium (lane 2), Reelin-conditioned medium plus PP2 [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo(3,4-d)pyramidine; 10 μM] (lane 3), antibody 186 (lane 4), antibody 74 (lane 5), antibody 20 (lane 6), or antibody 197 (lane 7). Dab1 phosphorylation was measured as described for panel A.
FIG. 4.
Reelin induces PKB/Akt phosphorylation in 3T3 A+/D cells. 3T3 A+/D cells were starved in serum-free medium for 6 h to reduce background phosphorylation of PKB/Akt and subsequently stimulated with plain DMEM (lane 1), purified Reelin (lane 2), or control medium (see the text) (lane 3). Dab1 was immunoprecipitated from cell lysates and analyzed by Western blotting using the indicated antibodies. Phosphorylation of PKB/Akt was analyzed by Western blotting of total cell lysates using an anti-phospho-Akt antibody. The lower band in the anti-phospho-Akt blot is unspecific and serves as a loading control. αPY, phosphotyrosine antibody.
FIG. 5.
Reelin stimulation induces proteasome-dependent degradation of phosphorylated Dab1 in 3T3 cells. (A) 3T3 A−/D cells were starved for 60 min using DMEM supplemented with 20 μg cycloheximide/ml and subsequently treated with Reelin (lanes 2, 4, 6, and 8)- or mock (lanes 1, 3, 5, and 7)-conditioned medium for the indicated time period in the presence of 20 μg cycloheximide/ml. Dab1 was precipitated from total cell lysates and analyzed by Western blotting using an antiphosphotyrosine antibody (αPY). Total Dab1 and ApoER2 were detected from total lysates by Western blotting. Arrowheads emphasize the decrease of receptor levels. (B) 3T3 V−/D cells were treated with Reelin (lanes 2, 4, 6, and 8)- or mock (lanes 1, 3, 5, and 7)-conditioned medium for the indicated time period in the presence of 20 μg cycloheximide/ml. Dab1 was precipitated from total cell lysates and analyzed by Western blotting using an antiphosphotyrosine antibody (αPY). Total Dab1 and VLDLR were detected as described for panel A. (C) 3T3 A−/D cells were treated for 1 h (lanes 1 and 2) or 6 h (lanes 3 to 6) with mock (lanes 1, 3, and 5)- or Reelin (lanes 2, 4, and 6)-conditioned medium in the presence of dimethyl sulfoxide (DMSO) (lanes 3 and 4) or 10 μM epoxomicin (Epoxo) (lanes 5 and 6). Total and phosphorylated Dab1 were detected after immunoprecipitation of Dab1.
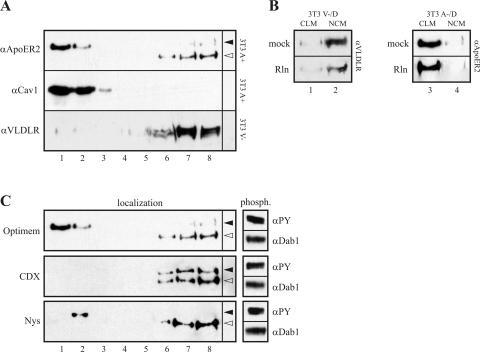
It was suggested that the function of ApoER2 in signaling is related to its localization to lipid rafts and/or caveolae (36, 47). To test this hypothesis we used the 3T3 cell system established here. These cell lines now allow us to prepare caveolin-rich light membrane fractions (CLM), characterized by the presence of caveolin, by density gradient centrifugation, test for the presence of ApoER2 and VLDLR, and relate the subcellular localization of both receptors to their ability to phosphorylate Dab1 upon Reelin stimulation. As demonstrated in Fig. 6A, mature ApoER2 is present in CLM and colocalizes with caveolin as described for CHO (36) and N2a cells (12). The immature form of ApoER2 with a relative molecular mass of 120 kDa, which resides in the endoplasmic reticulum (28), is nicely separated from the mature form of the receptor and is present in the heavy membrane fraction, creating a nice internal control for the separation of CLM from other membranes. In sharp contrast to ApoER2, VLDLR is strictly excluded from the CLM and is found at the bottom of the gradient containing the NCM fraction of the cell. As demonstrated in Fig. 3, in both cell lines, 3T3 A−/D and 3T3 V−/D, Dab1 becomes phosphorylated equally well upon Reelin stimulation, suggesting that the localization of the receptors does not play a role for Reelin-induced Dab1 phosphorylation. To test whether Reelin binding induces a shift of VLDLR to the CLM as described for the ligand-induced shift of the B-cell receptor (33), we incubated 3T3 V−/D cells with Reelin for 45 min at 37°C and tested the membrane distribution of the receptor. As demonstrated in Fig. 6B, VLDLR remained at the bottom fractions of the gradient, demonstrating that Reelin-binding does not induce a shift of VLDLR into the CLM fraction. Incubation of 3T3 A−/D cells with Reelin under the same conditions also did not change the localization of ApoER2, which stays associated with the CLM. Next we incubated the cells with caveola-disrupting agents such as methyl-β-cyclodextrin (CDX) and nystatin and examined the distribution of the receptors and Reelin-induced Dab1 phosphorylation. Titrating the amount of CDX demonstrated that, at concentrations of 10 mM or higher, overall protein phosphorylation was dramatically reduced (data not shown). Thus, we used a concentration of 5 mM, and, as demonstrated in Fig. 6C, treatment with CDX completely shifted ApoER2 out of the CLM fraction without significantly reducing Reelin-induced Dab1 phosphorylation. Next, we incubated the cells with nystatin, which was reported to inhibit caveolar function (16). However, we had to use concentrations below 20 μg/ml, since higher concentrations caused significant detachment of the cells and cell death. At 15 μg/ml, nystatin caused only a slight reduction of CLM-associated ApoER2 and no change of Reelin-induced Dab1 phosphorylation. As a control, the same experiments were carried out with 3T3 V−/D cells, and, as expected, CDX and nystatin did not influence VLDLR-mediated Dab1 phosphorylation. These experiments unambiguously demonstrate that Reelin-induced Dab1 phosphorylation occurs independently of the localization of the receptors and is not restricted to CLM.
FIG. 6.
ApoER2 but not VLDLR is localized to lipid rafts. (A) Lipid rafts from 3T3 cells expressing ApoER2 (3T3 A+) or VLDLR (3T3 A−) were isolated by a detergent-based procedure as described in Materials and Methods. Fractions were analyzed by Western blotting using the indicated antibodies. Closed and open arrowheads correspond to the mature and precursor forms of ApoER2, respectively. (B) 3T3 A−/D or 3T3 V−/D cells were incubated with mock- or Reelin-conditioned medium, and caveolin-rich light membranes (CLM) (lanes 1 and 3) were separated from NCM (lanes 2 and 4) as described for panel A. (C) 3T3 A−/D cells were incubated with Optimem or Optimem containing 5 mM methyl-β-cyclodextrin (CDX) or 15 μg nystatin (Nys)/ml, and lipid rafts were isolated as described for panel A. Fractions were analyzed by immunoblotting using an ApoER2-specific antibody. Additionally, 3T3 A−/D cells were incubated for 60 min with DMEM, DMEM containing 5 mM CDX, or DMEM containing 15 μg Nys/ml and subsequently stimulated for 60 min with Reelin-conditioned medium (upper panel), Reelin-conditioned medium containing 5 mM CDX (middle panel), or 15 μg Nys/ml (lower panel). Cells were lysed, Dab1 was immunoprecipitated, and the immunoprecipitate was analyzed for Dab1 phosphorylation (phosph.) by Western blotting using the indicated antibodies.
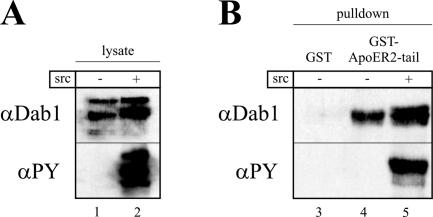
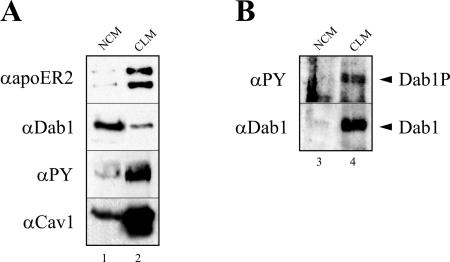
Next, we used the reconstituted cell system to answer the question of whether phosphorylated Dab1 still binds to the receptor tails or dissociates from them. First, we performed pull-down assays using a GST fusion protein containing the intracellular domain of ApoER2 with the proline-rich insert. 293 Cells were transiently transfected with a plasmid coding for Dab1 or doubly transfected with plasmids coding for Dab1 and src, respectively, and the resulting cell extracts were mixed with the fusion protein. Subsequently, the fusion protein was precipitated using glutathione Sepharose, and the precipitate was examined by Western blotting. As demonstrated in Fig. 7A, the transfected 293 cells express Dab1 (lanes 1 and 2), which in the presence of overexpressed src becomes strongly phosphorylated (lane 2). As expected, the GST-receptor tail fusion protein is able to pull down Dab1 from the cell extracts (Fig. 7B, lane 4), while GST alone does not (lane 3). Most importantly, the fusion protein also binds phosphorylated Dab1, as detected with an antiphosphotyrosine antibody in Western blot analyses of the precipitate (Fig. 7B, lane 5). This result shows that phosphorylated Dab1 binds to the receptor tail. To demonstrate that Dab1 phosphorylated via the Reelin signal indeed stays on the receptor, coimmunoprecipitation of Dab1 with the receptor would be necessary. A general problem of such experiments is that the antibodies against ApoER2 do not precipitate efficiently, and the amount of Dab1 which can be coimmunoprecipitated from primary neurons is not sufficient to produce reliable results. Thus, we turned to our fibroblast cell model, which allows enrichment of ApoER2 by preparing the CLM fraction prior to immunoprecipitation of ApoER2. 3T3 A+/D cells were stimulated with Reelin for 30 min, the cells were harvested, the CLM fraction was prepared, ApoER2 was immunoprecipitated using antibody 186, and the precipitate was tested for the presence of phosphorylated Dab1. As demonstrated in Fig. 8A (lane 2), the CLM fraction is enriched in ApoER2 and caveolin. The major part of Dab1, however, remains in the bottom fraction of the gradient, which contains cytosolic proteins and NCM fractions (lane 1). This indicates that a large fraction of Dab1 in this cell system is not associated with either the receptor or caveola-like membranes. Western blotting of total lysates using an antiphosphotyrosine antibody shows that the vast majority of phosphorylated Dab1 is present in the caveolar fraction. The caveolar fraction was then used to coimmunoprecipitate Dab1 with the antibody against ApoER2 (186). As demonstrated in Fig. 8B (lane 4), Dab1 is coprecipitated with the receptor, and, more importantly, it is phosphorylated. As control for the specificity of the antibody, we used the bottom fraction from the gradient, which contains only residual amounts of ApoER2. In this case no detectable amounts of Dab1 were precipitated. This experiment clearly demonstrates that Dab1 phosphorylated by the action of Reelin stays associated with the cytoplasmic tail of ApoER2.
FIG. 7.
Phosphorylated Dab1 binds to a recombinant ApoER2 tail fragment. (A) 293-HEK cells were transiently transfected with plasmids coding for Dab1 (lane 1) or Dab1 and src (lane 2). Forty-eight hours after transfection, total cell lysates were analyzed by Western blotting for the presence of Dab1 and phosphorylated Dab1 using the indicated antibodies. (B) Cell extracts shown in panel A were incubated with Sepharose beads coupled to GST (lane 3) or to a fusion protein containing GST and the ApoER2 tail plus insert (lanes 4 and 5), and the precipitated material was analyzed by Western blotting using the indicated antibodies. PY, phosphotyrosine.
FIG. 8.
Dab1 phosphorylated by Reelin is bound to the receptor tail. (A) 3T3 A+/D cells were stimulated with Reelin for 20 min at 37°C, and the caveolin-containing light membrane fraction was prepared as described in Materials and Methods. The caveolin-enriched fraction (lane 2) and the fraction at the bottom of the tube (lane 1) were analyzed by Western blotting using the indicated antibodies. (B) ApoER2 from these fractions was immunoprecipitated, and the precipitates were analyzed by Western blotting for the presence of total Dab1 and phosphorylated Dab1 (lanes 3 and 4). PY, phosphotyrosine.
Finally, we used A−/D and V−/D cells to test whether Reelin signaling modulates basic cell migration. We used a simple wound-healing assay which reflects effects of cell-matrix and cell-cell interactions on cell migration (37). The presence of Reelin did not change the migration behavior of these cells (data not shown).
DISCUSSION
Detailed mechanistic analyses of the Reelin signaling pathway were hampered in the past, since a cell line which would have allowed studying this pathway was not available. Primary neurons are the only cells described so far which express all necessary components of the pathway. These components are the Reelin receptors ApoER2 and VLDLR and the intracellular adapter protein Dab1. The crucial event in this pathway seems to be the phosphorylation of Dab1, since mice expressing a mutant version of Dab1 which cannot be phosphorylated have the same phenotype as mice lacking Reelin, the receptors, or Dab1 (20). Apparently, receptor clustering is sufficient to induce Dab1 phosphorylation, and a coreceptor for Reelin is not required to induce this event (45). Whether phosphorylation of Dab1 is sufficient to induce a “full-blown” Reelin signal in the context of neuronal migration is still unclear. Thus, we reasoned that stable expression of one of the Reelin receptors together with Dab1, in a cell line which is not designed for high expression rates of heterologous cDNAs, should generate a cell line in which the crucial event(s) of the Reelin signaling pathway can be recapitulated. We decided to express the VLDLR variant without exon 16 which codes for the “O-linked sugar domain” (VLDLR−) since, as demonstrated here, this variant is the one expressed in embryonic mouse brain. This expression pattern is surprising, since previous studies in bovine cells using this variant demonstrated that the lack of the “O-linked sugar domain” destabilizes the protein, and a soluble receptor fragment is produced by the action of extracellular proteolytic enzymes (30). Whether this is also true for the murine protein and whether it happens in the brain is not known yet. We created three stable cell lines derived from mouse 3T3 fibroblasts expressing (i) VLDLR without the “O-linked sugar domain” and Dab1 (3T3 V−/D); (ii) ApoER2 with the proline-rich insert in the cytosolic domain and Dab1 (3T3 A+/D); and (iii) ApoER2 without the proline-rich insert and Dab1 (3T3 A−/D). All three cell lines express comparable levels of Dab1 and of the respective receptors and bind Reelin, demonstrating that the receptors are indeed expressed in a ligand-binding-competent conformation and correctly sorted to the cell surface. As demonstrated in this paper, all three cell lines exhibit a functional Reelin pathway indistinguishable from primary neurons: they respond to Reelin stimulation with a robust phosphorylation of Dab1. This event is inhibited by PP2 (3), can be mimicked by receptor clustering independently of Reelin (45), and also leads to the activation PKB/Akt (6). Another feature of the Reelin signaling pathway is that its activation leads to degradation of phosphorylated Dab1, which might ensure a transient response of the neuron to Reelin stimulation (2). In primary neurons, proteasomal degradation of phosphorylated Dab1 results in a significant loss of total Dab1. In our fibroblast cell model targeting of phosphorylated Dab1 to proteosomal degradation did not result in a total loss of Dab1. This can be explained by the fact that the relative expression level of Dab1 and either ApoER2 or VLDLR in the cell lines is not the same as in primary neurons. Apparently, due to the high level of Dab1 expression, a significant amount of Dab1 is expected to be present in the cytoplasm not associated with either the membrane or the receptor. Thus, only a subfraction of total Dab1 becomes phosphorylated upon Reelin stimulation, and degradation of this fraction does not lead to a complete loss of Dab1.
As already demonstrated in primary neurons from mice lacking either VLDLR or ApoER2, the presence of one of the Reelin receptors is sufficient to activate the pathway (45). This system has allowed us to evaluate whether the proline-rich insert facultatively present in receptor variants in the mouse brain is involved in Reelin-induced Dab1 phosphorylation. As demonstrated here, both variants (with and without the insert) support this event equally well.
Having established and characterized these cell lines, we used them to study the membrane localization of the receptors and whether the localization has functional implications for the signaling pathway. In addition, we tackled the question of whether phosphorylated Dab1 is released from the receptor. Prior attempts to solve these problems were unsuccessful, since meaningful preparations of caveolin-rich membranes from primary neurons failed because of the lack of sufficient cell mass, and coimmunoprecipitation of Dab1 from primary neurons with antibodies against one of the receptors turned out to be very inefficient. As demonstrated here, this is now possible using the fibroblast-based cell system.
Preparations of CLM from these cells confirmed previous findings that mature ApoER2 is strictly localized to this cholesterol-rich subdomain of the plasma membrane (36). VLDLR, however, is strictly excluded from this domain, and, even more importantly, VLDLR is not shifted into the CLM fraction by Reelin binding. Translocation of the B-cell receptor, which is excluded from the lipid raft fraction in resting B cells, into this subdomain of the membrane is a prerequisite of B-cell activation (for a review see reference 34). On cross-linking by antigen binding, a significant portion of the amount of B-cell receptors becomes transiently associated with lipid rafts where the receptor is phosphorylated and the signaling cascade is initiated. Apparently, clustering of VLDLR by Reelin (45) does not increase the affinity of the receptor for CLM and still leads to Dab1 phosphorylation indistinguishable from that induced by Reelin binding to ApoER2. In addition, disruption of rafts by CDX which results in a complete loss of ApoER2 from this fraction and blocking the function of raft-associated processes by nystatin does not inhibit ApoER2-mediated Dab1 phosphorylation. This is in contrast to previous results with CDX, which was reported to inhibit Reelin-induced Dab1 phosphorylation in neurons (8). However, overall phosphorylation was also significantly reduced in these experiments, whereas in our experiments the CDX concentration was adjusted to a level where overall phosphorylation was only minimally inhibited but ApoER2 was completely lost from the CLM fraction. These findings demonstrate that lipid rafts or caveolae are not involved in the primary signaling event of the Reelin pathway.
Furthermore, using this cell system we could demonstrate that Dab1 phosphorylated by Reelin activation of ApoER2 indeed stays associated with the receptor. This was shown by pull-down experiments using a recombinant fusion protein containing GST and the cytoplasmic domain of ApoER2 as well as by coimmunoprecipitation with antibodies against ApoER2. In a recent publication it was shown that Dab1 does not associate stably with the receptor in the resting state; rather, it associates with the receptor upon Reelin binding and dissociates from the receptor upon internalization of Reelin (32). Here, we enriched for the mature form of ApoER2 which resides at the plasma membrane by preparing the CLM fraction of the cells. Using this fraction for immunoprecipitation with antibodies against the receptor resulted in coimmunoprecipitation of phosphorylated Dab1. This demonstrates that initial phosphorylation of Dab1 does not lead to a dissociation of the adapter from the receptor. This is compatible with the possibility that other proteins known to interact with phosphorylated Dab1, such as members of the Crk family (5), Nckβ (35), N-WASP (46), or Lis1 (4), assemble a complex signalosome on the receptor tail which may dissociate from the receptor upon internalization of the receptor.
Finally, using the fibroblast cell model, we could demonstrate that Reelin has no influence on the basic migratory behavior of these cells. This supports the concept that neuronal migration per se is not influenced by Reelin.
Acknowledgments
This work was supported by Austrian Science Foundation Grants P13931-MOB, F606, F608, and the Herzfelder'sche Familienstiftung.
We thank Harald Rumpler for excellent technical assistance. Antibodies against Reelin and Dab1 were a generous gift from Andre Goffinet (University of Louvain Medical School). The expression plasmid for Reelin was generously provided by Tom Curran (Department of Developmental Neurobiology, St. Jude's Children's Research Hospital, Memphis, TN).
REFERENCES
- 1.Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. USA 93:5699-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaud, L., B. A. Ballif, and J. A. Cooper. 2003. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell. Biol. 23:9293-9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaud, L., B. A. Ballif, E. Forster, and J. A. Cooper. 2003. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr. Biol. 13:9-17. [DOI] [PubMed] [Google Scholar]
- 4.Assadi, A. H., G. Zhang, U. Beffert, R. S. McNeil, A. L. Renfro, S. Niu, C. C. Quattrocchi, B. A. Antalffy, M. Sheldon, D. D. Armstrong, A. Wynshaw-Boris, J. Herz, G. D'Arcangelo, and G. D. Clark. 2003. Interaction of reelin signaling and Lis1 in brain development. Nat. Genet. 35:270-276. [DOI] [PubMed] [Google Scholar]
- 5.Ballif, B. A., L. Arnaud, W. T. Arthur, D. Guris, A. Imamoto, and J. A. Cooper. 2004. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr. Biol. 14:606-610. [DOI] [PubMed] [Google Scholar]
- 6.Beffert, U., G. Morfini, H. H. Bock, H. Reyna, S. T. Brady, and J. Herz. 2002. Reelin-mediated signaling locally regulates PKB/Akt and GSK-3β. J. Biol. Chem. 277:49958-49964. [DOI] [PubMed] [Google Scholar]
- 7.Bock, H. H., and J. Herz. 2003. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13:18-26. [DOI] [PubMed] [Google Scholar]
- 8.Bock, H. H., Y. Jossin, P. Liu, E. Forster, P. May, A. M. Goffinet, and J. Herz. 2003. PI3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 278:38772-38779. [DOI] [PubMed] [Google Scholar]
- 9.Brandes, C., L. Kahr, W. Stockinger, T. Hiesberger, W. J. Schneider, and J. Nimpf. 2001. Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha2-macroglobulin. J. Biol. Chem. 276:22160-22169. [DOI] [PubMed] [Google Scholar]
- 10.Brandes, C., S. Novak, W. Stockinger, J. Herz, W. J. Schneider, and J. Nimpf. 1997. Avian and murine LR8B and human apolipoprotein E receptor 2: differentially spliced products from corresponding genes. Genomics 42:185-191. [DOI] [PubMed] [Google Scholar]
- 11.Chen, K., P. G. Ochalski, T. S. Tran, N. Sahir, M. Schubert, A. Pramatarova, and B. W. Howell. 2004. Interaction between Dab1 and CrkII is promoted by Reelin signaling. J. Cell Sci. 117:4527-4536. [DOI] [PubMed] [Google Scholar]
- 12.Cuitino, L., R. Matute, C. Retamal, G. Bu, N. C. Inestrosa, and M. P. Marzolo. 2005. ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its rafts' association. Traffic 6:820-838. [DOI] [PubMed] [Google Scholar]
- 13.D'Arcangelo, G., R. Homayoundi, L. Keshvara, D. S. Rice, M. Sheldon, and T. Curran. 1999. Reelin is a ligand for lipoprotein receptors. Neuron 24:471-479. [DOI] [PubMed] [Google Scholar]
- 14.D'Arcangelo, G., G. G. Miao, S. C. Chen, H. D. Soares, J. I. Morgan, and T. Curran. 1995. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374:719-723. [DOI] [PubMed] [Google Scholar]
- 15.D'Arcangelo, G., K. Nakajima, T. Miyata, M. Ogawa, K. Mikoshiba, and T. Curran. 1997. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 17:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Guglielmo, G. M., C. Le Roy, A. F. Goodfellow, and J. L. Wrana. 2003. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5:410-421. [DOI] [PubMed] [Google Scholar]
- 17.Dulabon, L., E. C. Olson, M. G. Taglienti, S. Eisenhuth, B. McGrath, C. A. Walsh, J. A. Kreidberg, and E. S. Anton. 2000. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron 27:33-44. [DOI] [PubMed] [Google Scholar]
- 18.Hiesberger, T., M. Trommsdorff, B. W. Howell, A. Goffinet, M. C. Mumby, J. A. Cooper, and J. Herz. 1999. Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of the adaptor protein disabled-1 and modulates tau phosphorylation. Neuron 24:481-489. [DOI] [PubMed] [Google Scholar]
- 19.Howell, B. W., R. Hawkes, P. Soriano, and J. A. Cooper. 1997. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 389:733-737. [DOI] [PubMed] [Google Scholar]
- 20.Howell, B. W., T. M. Herrick, J. D. Hildebrand, Y. Zhang, and J. A. Cooper. 2000. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr. Biol. 10:877-885. [DOI] [PubMed] [Google Scholar]
- 21.Howell, B. W., L. M. Lanier, R. Frank, F. B. Gertler, and J. A. Cooper. 1999. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol. 19:5179-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, Y., S. Magdaleno, R. Hopkins, C. Slaughter, T. Curran, and L. Keshvara. 2004. Tyrosine phosphorylated Disabled 1 recruits Crk family adapter proteins. Biochem. Biophys. Res. Commun. 318:204-212. [DOI] [PubMed] [Google Scholar]
- 23.Huang, Y., V. Shah, T. Liu, and L. Keshvara. 2005. Signaling through Disabled 1 requires phosphoinositide binding. Biochem. Biophys. Res. Commun. 331:1460-1468. [DOI] [PubMed] [Google Scholar]
- 24.Keshvara, L., D. Benhayon, S. Magdaleno, and T. Curran. 2001. Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J. Biol. Chem. 276:16008-16014. [DOI] [PubMed] [Google Scholar]
- 25.Koch, S., V. Strasser, C. Hauser, D. Fasching, C. Brandes, T. M. Bajari, W. J. Schneider, and J. Nimpf. 2002. A secreted soluble form of ApoE receptor 2 acts as dominant negative receptor and inhibits reelin signaling. EMBO J. 21:5996-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubo, K., K. Mikoshiba, and K. Nakajima. 2002. Secreted Reelin molecules form homodimers. Neurosci. Res. 43:381-388. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Li, Y., W. Lu, M. P. Marzolo, and G. Bu. 2001. Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J. Biol. Chem. 276:18000-18006. [DOI] [PubMed] [Google Scholar]
- 29.Lugli, G., J. M. Krueger, J. M. Davis, A. M. Persico, F. Keller, and N. R. Smalheiser. 2003. Methodological factors influencing measurement and processing of plasma reelin in humans. BMC Biochem. 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magrane, J., R. P. Casaroli-Marano, M. Reina, M. Gafvels, and S. Vilaro. 1999. The role of O-linked sugars in determining the very low density lipoprotein receptor stability or release from the cell. FEBS Lett. 451:56-62. [DOI] [PubMed] [Google Scholar]
- 31.Magrane, J., M. Reina, R. Pagan, A. Luna, R. P. Casaroli-Marano, B. Angelin, M. Gafvels, and S. Vilaro. 1998. Bovine aortic endothelial cells express a variant of the very low density lipoprotein receptor that lacks the O-linked sugar domain. J. Lipid Res. 39:2172-2181. [PubMed] [Google Scholar]
- 32.Morimura, T., M. Hattori, M. Ogawa, and K. Mikoshiba. 2005. Disabled1 regulates the intracellular trafficking of reelin receptors. J. Biol. Chem. 280:16901-16908. [DOI] [PubMed] [Google Scholar]
- 33.Petrie, R. J., P. P. Schnetkamp, K. D. Patel, M. Awasthi-Kalia, and J. P. Deans. 2000. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol. 165:1220-1227. [DOI] [PubMed] [Google Scholar]
- 34.Pierce, S. K. 2002. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2:96-105. [DOI] [PubMed] [Google Scholar]
- 35.Pramatarova, A., P. G. Ochalski, K. Chen, A. Gropman, S. Myers, K. T. Min, and B. W. Howell. 2003. Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol. Cell. Biol. 23:7210-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riddell, D. R., X. M. Sun, A. K. Stannard, A. K. Soutar, and J. S. Owen. 2001. Localization of apolipoprotein E receptor 2 to caveolae in the plasma membrane. J. Lipid Res. 42:998-1002. [PubMed] [Google Scholar]
- 37.Rodriguez, L. G., X. Wu, and J. L. Guan. 2005. Wound-healing assay. Methods Mol. Biol. 294:23-29. [DOI] [PubMed] [Google Scholar]
- 38.Sanada, K., A. Gupta, and L. H. Tsai. 2004. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron 42:197-211. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, W. J., J. L. Goldstein, and M. S. Brown. 1985. Purification of the LDL receptor. Methods Enzymol. 109:405-417. [DOI] [PubMed] [Google Scholar]
- 40.Senzaki, K., M. Ogawa, and T. Yagi. 1999. Proteins of the CNR family are multiple receptors for Reelin. Cell 99:635-647. [DOI] [PubMed] [Google Scholar]
- 41.Sheldon, M., D. S. Rice, G. D'Arcangelo, H. Yoneshima, K. Nakajima, K. Mikoshiba, B. W. Howell, J. A. Cooper, D. Goldowitz, and T. Curran. 1997. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389:730-733. [DOI] [PubMed] [Google Scholar]
- 42.Stockinger, W., E. Hengstschläger-Ottnad, S. Novak, A. Matus, M. Hüttinger, J. Bauer, H. Lassmann, W. J. Schneider, and J. Nimpf. 1998. The LDL receptor gene family: differential expression of two α2-macroglobulin receptors in the brain. J. Biol. Chem. 273:32213-32221. [DOI] [PubMed] [Google Scholar]
- 43.Stolt, P. C., Y. Chen, P. Liu, H. H. Bock, S. C. Blacklow, and J. Herz. 2005. Phosphoinositide binding by the disabled-1 PTB domain is necessary for membrane localization and Reelin signal transduction. J. Biol. Chem. 280:9671-9677. [DOI] [PubMed] [Google Scholar]
- 44.Stolt, P. C., H. Jeon, H. K. Song, J. Herz, M. J. Eck, and S. C. Blacklow. 2003. Origins of Peptide selectivity and phosphoinositide binding revealed by structures of Disabled-1 PTB domain complexes. Structure (Cambridge) 11:569-579. [DOI] [PubMed] [Google Scholar]
- 45.Strasser, V., D. Fasching, C. Hauser, H. Mayer, H. H. Bock, T. Hiesberger, J. Herz, E. J. Weeber, J. D. Sweatt, A. Pramatarova, B. Howell, W. J. Schneider, and J. Nimpf. 2004. Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 24:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suetsugu, S., T. Tezuka, T. Morimura, M. Hattori, K. Mikoshiba, T. Yamamoto, and T. Takenawa. 2004. Regulation of actin cytoskeleton by mDab1 through N-WASP and ubiquitination of mDab1. Biochem. J. 384:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun, X. M., and A. K. Soutar. 2003. The transmembrane domain and PXXP motifs of ApoE receptor 2 exclude it from carrying out clathrin-mediated endocytosis. J. Biol. Chem. 278:19926-19932. [DOI] [PubMed] [Google Scholar]
- 48.Trommsdorff, M., J.-P. Borg, B. Margolis, and J. Herz. 1998. Interaction of cytosolic adaptor proteins with neuronal apoE receptors and the amyloid presursor proteins. J. Biol. Chem. 273:33556-33565. [DOI] [PubMed] [Google Scholar]
- 49.Trommsdorff, M., M. Gotthardt, T. Hiesberger, J. Shelton, W. Stockinger, J. Nimpf, R. Hammer, J. A. Richardson, and J. Herz. 1999. Reeler/Disabled-like disruption of neuronal migartion in knock out mice lacking the VLDL receptor and apoE receptor-2. Cell 97:689-701. [DOI] [PubMed] [Google Scholar]
- 50.Ware, M. L., J. W. Fox, J. L. Gonzalez, N. M. Davis, C. Lambert de Rouvroit, C. J. Russo, S. C. Chua, Jr., A. M. Goffinet, and C. A. Walsh. 1997. Aberrant splicing of a mouse disabled homolog, mdab1, in the scrambler mouse. Neuron 19:239-249. [DOI] [PubMed] [Google Scholar]
- 51.Waugh, M. G., D. Lawson, S. K. Tan, and J. J. Hsuan. 1998. Phosphatidylinositol 4-phosphate synthesis in immunoisolated caveolae-like vesicles and low buoyant density non-caveolar membranes. J. Biol. Chem. 273:17115-17121. [DOI] [PubMed] [Google Scholar]