Abstract
This is the first report of molecular characterization of a novel cyclic nucleotide PDE (phosphodiesterase), isolated from the human malaria parasite Plasmodium falciparum and designated PfPDE1. PfPDE1 cDNA encodes an 884-amino-acid protein, including six putative transmembrane domains in the N-terminus followed by a catalytic domain. The PfPDE1 gene is a single-copy gene consisting of two exons and a 170 bp intron. PfPDE1 transcripts were abundant in the ring form of the asexual blood stages of the parasite. The C-terminal catalytic domain of PfPDE1, produced in Escherichia coli, specifically hydrolysed cGMP with a Km value of 0.65 μM. Among the PDE inhibitors tested, a PDE5 inhibitor, zaprinast, was the most effective, having an IC50 value of 3.8 μM. The non-specific PDE inhibitors IBMX (3-isobutyl-1-methylxanthine), theophylline and the antimalarial chloroquine had IC50 values of over 100 μM. Membrane fractions prepared from P. falciparum at mixed asexual blood stages showed potent cGMP hydrolytic activity compared with cytosolic fractions. This hydrolytic activity was sensitive to zaprinast with an IC50 value of 4.1 μM, but insensitive to IBMX and theophylline. Furthermore, an in vitro antimalarial activity assay demonstrated that zaprinast inhibited the growth of the asexual blood parasites, with an ED50 value of 35 μM. The impact of cyclic nucleotide signalling on the cellular development of this parasite has previously been discussed. Thus this enzyme is suggested to be a novel potential target for the treatment of the disease malaria.
Keywords: antimalarial drug, cGMP, malaria parasite, phosphodiesterase (PDE), Plasmodium, zaprinast
Abbreviations: BLAST, basic local alignment search tool; EHNA, erythro-9-(2-hydroxy-3-nonyl)adenine; Ht, hematocrit; IBMX, 3-isobutyl-1-methylxanthine; IPTG, isopropyl β-D-thiogalactoside; PDE(s), phosphodiesterase(s); PfPDE, P. falciparum PDE; PfGC, P. falciparum guanylate cyclase; PfPKG, P. falciparum cGMP-dependent protein kinase; RT, reverse transcriptase; Tb, Trypanosoma brucei
INTRODUCTION
Malaria is a parasitic disease caused by four species of the protozoan parasites of the genus Plasmodium: P. falciparum, P. vivax, P. ovale, and P. malariae in humans. P. falciparum is the most virulent form of the four. Several drugs, including chloroquine and quinine, are used for the treatment of malaria; however, P. falciparum has developed resistance to most antimalarial agents, such as chloroquine. Therefore in order to overcome malaria, the genome project of P. falciparum has been completed [1]. Potential drug target molecules are currently being discussed [2].
The life cycle of the malaria parasite is complex, with several stages [3]. Soon after a bite by an infected Anopheles mosquito, sporozoites invade hepatocytes. Intense asexual division, termed exoerythrocytic schizogony, occurs in the liver, and up to 40000 merozoites are generated after schizont rupture. Merozoites invade erythrocytes and additional rounds of asexual replication, a pathogenic phase of malaria, termed erythrocytic schizogony, takes place. Some merozoites arrest their cell cycle to differentiate into male or female gametocytes (gametogenesis), which are infectious to the Anopheles mosquitoes. Thus cellular differentiation is an important process in the life cycle of the malaria parasite. Intracellular cyclic nucleotides cAMP and cGMP play a pivotal role in the growth and differentiation of this organism, acting as second-messenger molecules. For example, cGMP signalling has been shown to be implicated in Plasmodium gametogenesis [4,5]. Consistent with this, expression of PfGC (guanylate cyclase) protein is found in gametocytes [6,7]. The presence of PfPKG (cGMP-dependent protein kinase) in the ring form of asexual blood stages, but not gametocytes, suggests a role of cGMP signalling in this cell form [8].
This cyclic nucleotide signalling is regulated through cyclic nucleotide production by adenylate and guanylate cyclases and hydrolysis by cyclic nucleotide PDEs (phosphodiesterases) [9,10]. In mammals, PDEs have been categorized into 11 families (PDEs 1–11) according to their amino acid sequence similarity, biochemical properties and inhibitor sensitivity [11,12]. Inhibitors of mammalian PDEs are used, or have been developed, for the treatment of diseases such as erectile dysfunction, thrombosis, asthma and chronic obstructive pulmonary disease [13–15]. PDEs from lower single-cell organisms have been reported and revealed to be distinct from the 11 mammalian PDE families. PDEs from a soil amoeba, Dictyostelium, and a protozoan parasite, Trypanosoma, have been isolated using their genome sequences and their characteristics successfully demonstrated. In Trypanosoma brucei, two class I PDEs (TbPDE1 and TbPDE2), which are specific for cAMP, have been cloned [16–19]. Interestingly PDE inhibitors against TbPDE2 block proliferation of bloodstream form trypanosomes [17], and inactivation of TbPDE2 by RNA interference induces growth inhibition of blood-stream-form T. brucei. This suggests that specific PDE inhibitors may be useful for anti-protozoal chemotherapy [19]. Thus cyclic nucleotide signalling in lower organisms is essential for their survival, and disruption of this signalling would be expected to cause cell stress, leading to cell death.
In Plasmodium, the presence, and some physiological roles, of PDEs have been predicted. Nevertheless, the molecular basis of Plasmodium PDEs has not yet been established, in spite of the genome project of P. falciparum having been completed. In the present paper, we report a novel PDE (PfPDE1) in P. falciparum. The PfPDE1 cDNA was cloned and both the enzymatic characteristics and PDE inhibitor sensitivities of the gene product were investigated in detail. Endogenous PDE activities of this organism were also examined. The physiological effect of a PfPDE1 inhibitor on this organism was also demonstrated. The findings reported here provide us with fundamental knowledge of the cyclic nucleotide metabolism in the human parasite P. falciparum. Plasmodium PDE is suggested to be a novel therapeutic target for the disease malaria and discovery of inhibitors would lead to improved treatment of the disease.
EXPERIMENTAL
Materials
[3H]cAMP, [3H]cGMP and [α-32P]dCTP were purchased from Amersham Biosciences. IBMX (3-Isobutyl-1-methylxanthine) was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Vinpocetine, EHNA [erythro-9-(2-hydroxy-3-nonyl)adenine], milrinone, rolipram, zaprinast, dipyridamole, and chloroquine were purchased from Sigma–Aldrich. Theophylline was obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Papaverine, E4021 and sildenafil were synthesized at Tanabe Seiyaku Co. Ltd. (Osaka, Japan).
Parasite culture and isolation of nucleic acids
P. falciparum 3D7 and Honduras-1 strains were cultivated in RPMI 1640 (Invitrogen) with 10% (v/v) inactivated type A human plasma with 5% (v/v) and 3% (v/v) Ht (haematocrit) type A human erythrocytes respectively [20]. The plate was placed in a CO2 incubator [CO2/O2/N2 (5:5:90)] at 37 °C. Erythrocytes infected with P. falciparum 3D7 were harvested and treated with 0.075% (w/v) saponin in PBS to obtain the parasites. Chromosomal DNA was obtained from the parasites using QIAamp DNA Mini Kit (Qiagen). Total RNA was isolated using Isogen (Nippon Gene, Toyama, Japan) and first-strand cDNA was prepared according to the instructions with GeneAmp RNA PCR Kit (Applied Biosystems).
Cloning of chromosomal DNA and cDNA for P. falciparum PDE
The amino acid sequences of mammalian PDEs (PDEs 1–11) were used as queries to search the expressed sequence tag and genome databases. A BLAST (basic local alignment search tool) search [21] of the National Center for Biotechnology Information P. falciparum genome database identified putative PfPDE genes. PCR primers were designed based on the sequences retrieved from the database.
Chromosomal DNA fragments for the PfPDE1 gene were generated by PCR amplification, using P. falciparum chromosomal DNA as a template. The 5′-region of the PfPDE1 gene was amplified using the primer set (Pf1-F1: 5′-ATGGAATATTTTAATTGTGTTAATAATCTATGTTG-3′ and Pf1-R3: 5′-ATTTAATATATCTTCTATGGGCGATGTAGG-3′). To amplify the 3′-region of PfPDE1 gene, the primer set (Pf1-F3: 5′-CCATCCTTTTATGAATATCTTATGTTTACGTTGATG-3′ and Pf1-R1: 5′-TTATTCAAATTTGATGAGCTCAAGTTTGCTTAG-3′) was used. PCR amplification was carried out through 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 3 min. PCR products were cloned into the TA-cloning vector pGEM-T Easy (Promega) and then sequenced.
The cDNA fragments encoding PfPDE1 were obtained by PCR using P. falciparum cDNA as a template. N-terminal (nt 1–1329) and central to C-terminal (nt 367–2650) regions of PfPDE1 were amplified using the above primer sets, Pf1-F1 plus Pf1-R3 and Pf1-F3 plus Pf1-R1 respectively. PCR amplification was carried out using the conditions described above and the amplified products were cloned into pGEM-T Easy. Five independent PCR clones encoding each region were sequenced to verify that a correct whole cDNA sequence had been cloned. One of the clones that included the C-terminal catalytic domain of PfPDE1, pGEM-PfPDE1c, was used for further experiments.
Nucleotide sequences were determined by an automated DNA sequencer ABI PRISM™ 310 using BigDye Terminator Cycle Sequencing Reaction Kit (Applied Biosystems). Nucleotide and amino acid sequence data were analysed using the computer program GENETYX (Software Development Co., Tokyo, Japan). The deduced amino acid sequence was also analysed using the program SMART [22] for finding functional domains. Phylogenetic tree analysis was performed using CLUSTAL W and PHYLIP [23].
Southern blot analysis
P. falciparum chromosomal DNA (2 μg/lane) was digested using the restriction endonucleases EcoRI, SpeI and XbaI, subjected to 0.8% agarose gel electrophoresis and transferred on to Hybond-N+ nylon membrane (Amersham Biosciences). A 661-bp cDNA fragment of PfPDE1 (nts 1147–1807) was 32P-radiolabelled using the Random Primer DNA Labelling Kit (TaKaRa Bio). Hybridization was performed in PerfectHyb™ (Toyobo, Osaka, Japan) containing the 32P-labelled probe at 60 °C for 4 h. The membrane was washed with 2×SSC (1×SSC: 0.15 M NaCl and 15 mM sodium citrate, pH 7.0) and 0.1% (w/v) SDS at 25 °C for 5 min, followed by two 10 min washes with 0.1×SSC and 0.1% (w/v) SDS at 60 °C. X-ray film was exposed to the membrane at −80 °C for 12 h.
Stage-specific expression of PfPDE1 mRNA
To investigate stage-specific expression of P. falciparum cells at asexual stages, cultures were synchronized by sorbitol lysis and treatment with 66% (v/v) Percoll™ (Amersham Biosciences) [24,25]. In brief, infected erythrocytes with late-stage asexual parasites, containing trophozoites and schizonts, were separated using Percoll™. These concentrated late-stage parasites were suspended with uninfected erythrocytes and cultured for 5–7 h. A proportion of the schizonts proceeded to the merozoite stage and invaded the fresh erythrocytes. These infected erythrocytes were treated with 66% (v/v) Percoll™ to prevent minimal contamination with gametocytes and then with sorbitol to remove the remaining late-stage parasites (trophozoites and schizonts). The resultant ring forms were returned to culture and, 21, 32, 38, 43 and 50 h later, each sample was centrifuged at 830 g for 5 min at 4 °C. The parasites were obtained by treating with 0.075% (w/v) saponin in PBS. Total RNA was obtained using Isogen and first-strand cDNA was prepared using the Advantage™ RT-for-PCR Kit (Clontech). Expression levels of PfPDE1 transcripts were examined by PCR amplification using the 5′-primer 5′-AGCATGCTTTTCATGCTAGACATGAACCAC-3′ and the 3′-primer 5′-TTATTCAAATTTGATGAGCTCAAGTTTGCTTAG-3′, which generates a DNA fragment of 795 bp (nt 1870–2655). An 840 bp DNA fragment of the P. falciparum cysteine protease falcipain-3 ([26]; GenBank™ accession number AF258791) was amplified by PCR using the 5′-primer 5′-AATAGTTTATATAAAAGGGGTATG-3′ and the 3′-primer 5′-TAATGGTACATAAGCTTCTGTTCC-3′ (nt 686–1528 of the cDNA) as a control. PCR amplification was carried out through 38 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min. PCR products were separated using 1.5% agarose gel electrophoresis, and the DNA fragments were stained with ethidium bromide.
Expression of PfPDE1 protein in Escherichia coli
To generate an expression plasmid for a catalytic domain of PfPDE1, the EcoT22I–SalI DNA fragment (approx. 1 kb) of pGEM-PfPDE1c was subcloned into the PstI and XhoI sites of the mammalian expression vector pcDNA4/HisMax A (Invitrogen) and the resultant plasmid named pHis-PfPDE1Δ. Transfection of this plasmid into COS-7 cells for recombinant PfPDE1 production was done according to the method described previously [12]. To express the PfPDE1 enzyme in E. coli, the BamHI–PstI DNA fragment (approx. 1 kb) of pHis-PfPDE1Δ was subcloned into the BamHI and PstI sites of the bacterial expression vector pQE-32 (Qiagen) and the resultant plasmid named pQE-PfPDE1Δ. Site-directed mutagenesis of the pQE-PfPDE1Δ plasmid was used to generate PfPDE1 mutants with alanine substitutions at Asp762 and Gly788; the QuikChange Site-Directed Mutagenesis Kit (Stratagene) was used following the manufacturer's protocol. To introduce the desired mutations, the following primers were used: 5′-ATTTTAAAGGCATCAGCTATTGGACACTCAACA-3′ with 5′-TGTTGAGTGTCCAATAGCTGATGCCTTTAAAAT-3′ for PfPDE1D762A, and 5′-GAATTCTATTTACAAGCTTTACTAGAAAAATCG-3′ with 5′-CGATTTTTCTAGTAAAGCTTGTAAATAGAATTC-3′ for PfPDE1G788A. The mutations were confirmed by DNA sequencing analysis.
The expression plasmid pQE-PfPDE1Δ, pQE-PfPDE1-ΔD762A, pQE-PfPDE1ΔG788A or the control vector pQE-32 was introduced into E. coli JM109 cells. The recombinant E. coli cells were grown overnight at 37 °C in LB (Luria–Bertani) medium containing ampicillin (100 μg/ml). The culture was diluted 1:20 with fresh LB medium containing 2% glucose and ampicillin (100 μg/ml), incubated at 37 °C in a shaking incubator for 2 h and then further incubated at 27 °C for 4 h, after addition of IPTG (isopropyl β-D-thiogalactoside) at a final concentration of 1 mM. The cells were then washed once with ice-cold PBS and resuspended in ice-cold lysis buffer (40 mM Tris/HCl, pH 7.5, 15 mM benzamidine, 5 μg/ml pepstatin A and 5 μg/ml leupeptin). After freeze–thaw treatment, suspended cells were disrupted by a sonicator (TOMY Seiko, Japan) for 15 s (5 times with 1 min intervals), and the lysates were centrifuged at 16000 g for 15 min at 4 °C. The supernatant was added to a plastic tube containing nickel nitrilotriacetate resin (Qiagen), equilibrated with the lysis buffer, and incubated with rotation at 4 °C for 3 h. The nitrilotriacetate resin was poured into a plastic column (0.8 cm×5 cm) and allowed to drain. The packed resin was washed with wash buffer (40 mM Tris/HCl, pH 7.5, 15 mM benzamidine, 200 mM NaCl, 5 mM imidazole, 5 μg/ml pepstatin A, and 5 μg/ml leupeptin), and the proteins were then eluted using elution buffer (40 mM Tris/HCl, pH 7.5, 15 mM benzamidine, 200 mM NaCl, 200 mM imidazole, 5 μg/ml pepstatin A, and 5 μg/ml leupeptin). The PfPDE1 fractions were dialysed against the lysis buffer and stored at −80 °C until use.
SDS/PAGE, immunoblotting, and MS analysis
Proteins were subjected to SDS/PAGE (12.5% gels) and stained using a Silver Staining Kit, Protein (Amersham Biosciences), omitting a step of fixation with glutardialdehyde. Immunoblot analysis was performed using PVDF membranes (Millipore) and anti-pentahistidine monoclonal antibody (Qiagen) as previously described [12]. Bound primary antibodies were detected using horseradish-peroxidase-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, U.S.A.) and visualized with ECL® Western Blotting Detection System (Amersham Biosciences). A gel slice containing a 41 kDa protein was treated with 10 mM DTT (dithiothreitol) at 56 °C for 45 min, and alkylation was performed in the dark with 50 mM iodoacetic acid at 25 °C for 30 min. The dried sample was immersed in 40 μl of 5 μg/ml trypsin solution in 25 mM NH4HCO3, and kept at 37 °C for 15 h. Tryptic peptides were extracted with 45 μl of 33% NH4HCO3/66% acetonitrile, 45 μl of 5% formic acid/66% acetonitrile and 75 μl of 5% formic acid/80% acetonitrile. The digests were subjected to automated LC (liquid chromatography)-MS/MS analysis HPLC using the Magic 2002 (Michrom BioResources, Auburn, CA, U.S.A.) connected online to a LCQ Deca ion-trap tandem mass spectrometer (Thermoquest, San Jose, CA, U.S.A.). Each chromatogram was subsequently analysed with the Mascot search algorithm (http://www.matrixscience.com).
PDE and protein assays
The PDE assay was performed using the radiolabelled nucleotide method as described previously [12,27]. In brief, the assay buffer contained 50 mM Tris/HCl, pH 8.0, 5mM MgCl2 or 1 mM MnCl2, 4 mM 2-mercaptoethanol, 0.33 mg/ml BSA, 13 nM [3H]cGMP or 5 nM [3H]cAMP, and unlabelled cGMP or cAMP. Reactions were started by adding enzyme solution to 500 μl of assay buffer and the tubes were incubated at 37 °C for 30 min. After boiling for 2 min, the mixtures were added to 100 μl of 1 mg/ml rattlesnake (Crotalus atrox) venom and incubated at 37 °C for 30 min. Reactions were stopped by the addition of 500 μl of methanol and the resultant solutions were applied to Dowex (1×8 200–400; Dow Chemical Company, Midland, MI, U.S.A.) columns. Aqueous scintillation mixture was added to each eluate and the radioactivity was measured using a scintillation counter. The Km and Vmax values were calculated from Lineweaver–Burk plots [28]. Relative Vmax values were determined according to the method of McPhee et al. [29]. Relative concentrations of PfPDE1 proteins produced in E. coli were calculated by immunoblotting as described above. The membranes were incubated with ECL® reagents at 25 °C for 1 min and then exposed to X-ray film for 2–10 s, under conditions in which each X-ray film exposure did not reach saturation. The resultant films were scanned by ARCUS II (Agfa-Gevaert), and quantified using the Quantity One program (PDI, Inc., Huntington Station, NY, U.S.A.). The absorbances were plotted versus the amount of hexahistidine-tagged protein to measure the relative concentrations of PfPDE1 proteins. The protein concentration was determined using DC Protein Assay Kit (Bio-Rad), using the BSA as a standard.
PDE assay of P. falciparum lysates
Erythrocytes infected with P. falciparum Honduras-1 were washed with AIM buffer (10 mM Pipes, pH 6.7, 120 mM KCl, 20 mM NaCl, 1 mM MgCl2, and 5 mM glucose) and treated with 0.075% (w/v) saponin in AIM buffer to obtain the parasite. The cells washed with AIM buffer were suspended in ice-cold lysis buffer and disrupted by freeze–thaw treatment and sonication as described above. To remove cell debris and haem polymer, the lysates were centrifuged at 10000 g for 15 min at 4 °C. Then the supernatants were centrifuged at 100000 g for 60 min at 4 °C and fractionated into pellets and soluble (cytosolic) fractions. The pellets were dissolved with ice-cold lysis buffer containing 0.5% Triton X-100, centrifuged at 10000 g for 15 min at 4 °C and the resultant supernatants were used as the membrane fractions. Prepared cytosolic and membrane fractions were used in a PDE assay.
In vitro antimalarial activity assay
The following procedure is based on the antimalarial activity assay previously described [30]. Test compounds were dissolved in appropriate solvent and a serial dilution was prepared. In the 24-well plate, each well contained 1 ml of asynchronous P. falciparum Honduras-1 culture with a final Ht concentration of 3% and 0.3% parasitaemia, which contained 10 μl of test compound solution. Prepared culture was then incubated for 72 h using the gas conditions described above. To evaluate the antimalarial activity of the test compounds, more than 1000 erythrocytes, stained with Giemsa (Merck), were examined by microscopy. All of the test compounds were assayed in duplicate and, for zaprinast, the experiment was repeated three times. The ED50 value, which is a dose giving 50% reduction in the increase of infected erythrocytes, was determined by comparison with drug-free controls cultured under the same conditions.
RESULTS
Cloning of chromosomal DNA and cDNA for a novel P. falciparum PDE
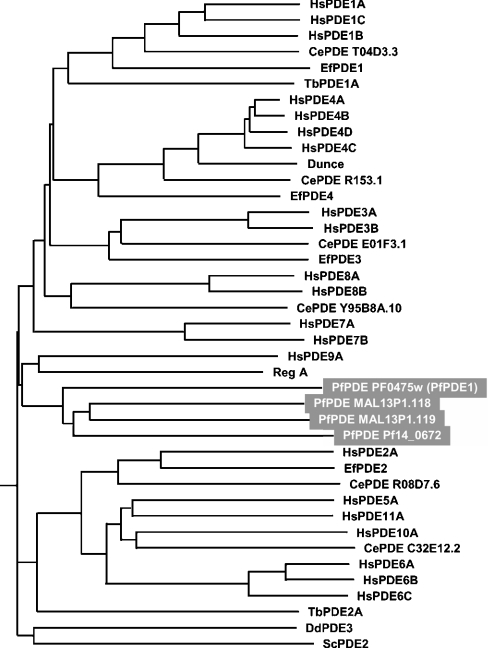
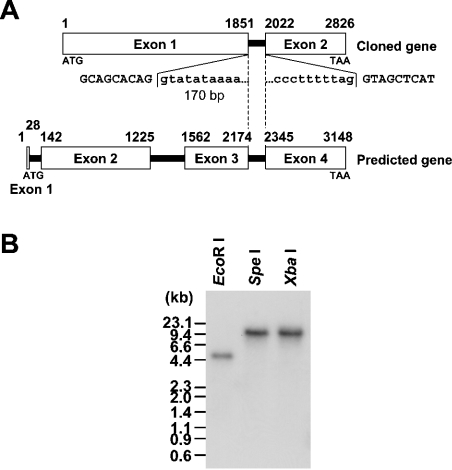
A search of draft genome sequence databases using the amino acid sequences of 11 mammalian PDEs (PDE 1–11) resulted in four putative PfPDE (P. falciparum PDE) genes. During the course of this study, whole genome sequencing of this organism was completed [1] and the protein-coding regions have been predicted by computer analysis (http://www.plasmodb.org/). On that web site, the above four putative PfPDE genes have been registered as PFL0475w, MAL13P1.118, MAL13P1.119 and PF14_0672. All these gene products contained a sequence consistent with the class I PDE signature sequence HDX2HX4N [31]. Based on the alignment of PDE catalytic domain sequences, a phylogenetic tree was inferred by the NJ method (Figure 1). The four putative PfPDEs did not belong to any PDE family previously described, but showed a low degree of evolutionary relatedness with PDE9A and the Dictyostelium PDE, RegA. Thus the PfPDEs constitute a new family of PDE. We focused on one of these PfPDEs, PFL0475w (here designated PfPDE1). The PfPDE1 gene was found on chromosome 12 in the genome database. Based on this sequence, we designed PCR primers and performed PCR amplification using P. falciparum chromosomal DNA as a template to obtain chromosomal DNA for PfPDE1. PCR products of the appropriate size were detected, subcloned into the TA-cloning vector pGEM-T Easy and sequenced. The nucleotide sequences of the isolated clones carrying a chromosomal DNA for PfPDE1 were in full agreement with the sequences found in the genome database (Figure 2A). Southern blot analysis of P. falciparum chromosomal DNA, digested with restriction endonucleases, EcoRI, SpeI and XbaI, indicated that PfPDE1 is a single-copy gene (Figure 2B).
Figure 1. Phylogenetic tree of the PDE families inferred from their catalytic domain sequences.
The phylogenetic tree was generated using the NJ algorithm of PHYLIP on the basis of a multiple alignment of the catalytic domain sequences of PDEs analysed with CLUSTALW. The following PDEs were included in the analysis: human PDE1A (HsPDE1A) (GenBank accession number, U40370), HsPDE1B (U56976), HsPDE1C (U40371), HsPDE2A (U67733), HsPDE3A (U36798), HsPDE3B (U38178), HsPDE4A (U68532), HsPDE4B (U85048), HsPDE4C (Z46632), HsPDE4D (L20969), HsPDE5A (D89094), HsPDE6A (M26061), HsPDE6B (X66142), HsPDE6C (X94354), HsPDE7A (U67932), HsPDE7B (AB038040), HsPDE8A (AF056490), HsPDE8B (AB085824), HsPDE9A (AF048837), HsPDE10A (AB020593), HsPDE11A (AB036704), Ephydatia fluviatilis PDE1 (EfPDE1) (AB017021), EfPDE2 (AB017022), EfPDE3 (AB017023), EfPDE4 (AB017024), Caenorhabditis elegans PDE (CePDE) T04D3.3 (Z81114), CePDE R153.1 (U28729), CePDE E01F3.1 (Z93376), CePDE Y95B8A.10 (AC024877), CePDE R08D7.6 (Z12017), CePDE C32E12.2 (U80032), Trypanosoma brucei PDE1A (TbPDE1A) (AF253418), TbPDE2A (AF263280), Saccharomyces cerevisiae (yeast) PDE2 (ScPDE2) (M14563), Drosophila melanogaster Dunce (AH006406), Dictyostelium discoideum RegA (AJ005398), D. discoideum PDE3 (DdPDE3) (AY162269).
Figure 2. Structure of the PfPDE1 gene.
(A) The PfPDE1 gene structure is illustrated. Open boxes with numbers represent exons. The computationally predicted organization is indicated above. Exon–intron organization, determined by comparison of the cDNA and genomic sequences, is shown below. Exon sequences are indicated by upper-case letters and intron sequences by lower-case letters. Exon–intron boundaries in common to both organizations are indicated by a broken line. A chromosomal region, which is computationally predicted as a second intron but actually is a coding region, is shown between thin lines. The initiation ATG codon of the cloned cDNA was indicated by a thick line. (B) Southern-blot analysis. P. falciparum chromosomal DNA, digested with the indicated (above the gel) restriction endonucleases, was fractionated and transferred on to a nylon membrane. The blot was hybridized with a 32P-labelled PfPDE1 cDNA fragment.
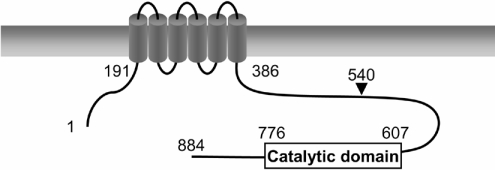
PfPDE1 cDNA was also obtained in the same way by PCR amplification using P. falciparum cDNA as a template. An open reading frame of 2655 bp was identified and predicted to encode an 884-amino-acid protein with a predicted molecular mass of 107 kDa. The cDNA sequence was A/T-rich [75.6% (2112 bp/2655 bp)] and showed a characteristic codon usage similar to those of the P. falciparum genes reported to date. Two motifs of a bivalent-cation-binding domain (YHNX2HG/AX23E and HDX2HX24–26E) conserved in class I PDEs [32] are also found in PfPDE1 (Y609HtX2HAX23d639 and H650DX2HX25E680, conserved and irregular amino acids are shown in upper- and lower-case letters respectively). [32]. The catalytic domain sequence of PfPDE1 (amino acid residues 607–776) was compared with those of PDEs from human, yeast, Dictyostelium and trypanosome. The catalytic domain sequence of PfPDE1 exhibited the highest identity (36%) to PDE9A among these PDE sequences. Comparison of the catalytic domain sequence of PfPDE1 with those of the three other predicted PfPDEs revealed the highest identity (40%) and similarity (60%) to MAL13P1.118. The amino acid sequence of PfPDE1 outside the catalytic domain was searched using SMART and BLAST. The SMART program revealed that N-terminal region of PfPDE1 has six putative transmembrane regions (Figure 3), suggesting that PfPDE1 is a membrane protein. The BLAST search revealed no significant similarity to other reported sequences.
Figure 3. Schematic structure of PfPDE1.
The cylinders represent hydrophobic segments that are thought to form transmembrane α-helices. An arrowhead indicates the start site of PfPDE1Δ.
The PfPDE1 cDNA sequence was compared with a computationally predicted coding sequence for PFL0475w and the PfPDE1 chromosomal DNA sequence. As shown in Figure 2(A), the PfPDE1 gene was revealed to consist of two exons and a 170 bp intron, although the presence of three introns (nt 29–141, 1226–1561, and 2175–2344 in the predicted organization) has been predicted in the coding region of the genome sequence (accession no. NC004316). The nucleotide sequence, observed at the 5′ donor and 3′ acceptor splice sites, demonstrated that the sites are consistent with the canonical GT-AG rule (5′ donor; GTATATAAAAAA… and 3′ acceptor; …ATCCCTTTTTAG) [33]. The second intron in the predicted organization (nt 1226–1561; see accession no. AE014845) was revealed to be a coding region of PfPDE1, composed of 112 amino acid residues (Figure 2A). Finally, PfPDE1 contains six potential transmembrane regions (PFL0475w has been predicted as a membrane protein with three potential transmembrane regions).
Stage-specificity of PfPDE1 expression in P. falciparum
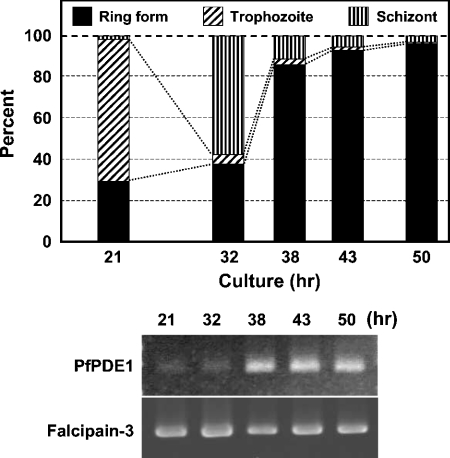
Plasmodium has a complex life cycle that differs at morphological and biochemical levels. To investigate changes in PfPDE1 expression during blood stage development, RT (reverse transcriptase)-PCR analysis was performed using total RNA isolated from synchronously cultured parasites as shown in Figure 4. Cell populations were determined by counting the numbers of the three cell forms. To eliminate PCR products amplified from contaminating chromosomal DNA, sense and antisense PCR primers were designed from coding sequences of exons 1 and 2 respectively. PCR products amplified from chromosomal DNA were not detected. The levels of PfPDE1 transcripts were in accordance with the proportion of ring form. Amounts of mRNA loaded in each lane were confirmed by RT-PCR analysis of falcipain-3, which is expressed in all stages of asexual blood stages [26]. Thus stage-specific transcriptional regulation of PfPDE1 was observed.
Figure 4. Stage-specific expression of PfPDE1 transcripts.
In order to obtain a synchronous culture, ring form-rich parasites, prepared as described in the Experimental section, were cultured. After 21, 32, 38, 43 and 50 h, each sample was collected and the stage-specific cDNAs were prepared. The percentage of ring form, trophozoite, and schizont was calculated by counting the numbers of each cell form (upper panel). Expression levels of PfPDE1 transcripts (nt 1870–2655) and falcipain-3 (GenBank® accession number AF258791, nt 686–1528) were examined by RT-PCR. After separation on a 1.5% agarose gel, PCR products were detected with ethidium bromide staining (lower panel).
Production of recombinant PfPDE1 in E. coli
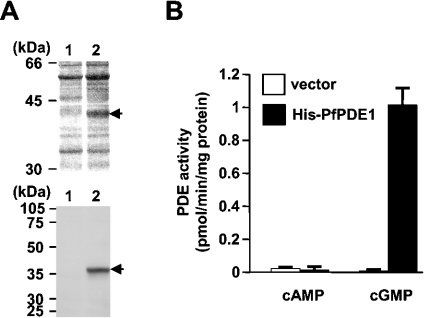
Production of the recombinant PfPDE1 protein as a hexahistidine-tagged N-terminally truncated form (PfPDE1Δ) was first attempted with COS cells, which we have previously employed successfully as described in [12]. However, no significant plasmid-directed PDE activity was detected in transfected COS cells, probably owing to an A/T-rich coding sequence of PfPDE1. Therefore an E. coli expression plasmid encoding a hexahistidine-tagged PfPDE1Δ (pQE-PfPDE1Δ) was generated and introduced into E. coli. After induction with IPTG, the cell extracts prepared were subjected to nickel affinity column purification, as described in the Experimental section. SDS/PAGE analysis visualized by silver staining and immunoblot analysis using anti-pentahistidine antibody demonstrated the production of a 41 kDa protein in the lysates of the E. coli cells carrying pQE-PfPDE1Δ (Figure 5A). The value was in reasonable agreement with the molecular mass predicted for the hexahistidine-tagged PfPDE1Δ. The masses of tryptic peptides of the 41 kDa protein, determined by LC-MS/MS, corresponded to those of predicted tryptic fragments derived from the PfPDE1Δ sequence, such as ‘ANTFISIGYK’, ‘LLYPLGVLEANFDKEK’, ‘AILSTDMK’, ‘LELIKFE’, etc. (results not shown). The eluates were employed in the PDE assay using either 5 nM cAMP or 13 nM cGMP as a substrate. Partially purified proteins from PfPDE1Δ-transformed cells exhibited approx.135-fold higher levels of cGMP PDE activity than those from cells transformed with the control vector (Figure 5B). No significant cAMP hydrolytic activity of the PfPDE1Δ enzyme was observed under these conditions. cGMP hydrolysis was neither activated nor inhibited by cAMP at concentrations of up to 100 μM (results not shown). These results indicated that PfPDE1 is a cGMP-specific PDE.
Figure 5. Production of the recombinant PfPDE1 protein.
(A) Expression of the catalytic domain of PfPDE1 was examined by SDS/PAGE and immunoblotting. The control vector pQE-32 (lane 1) and pQE-PfPDE1Δ (lane 2) were used to transform the E. coli JM109 cells. The hexahistidine-tagged PfPDE1Δ was partially purified using a nickel affinity column. Partially purified PfPDE1 protein was separated by SDS/PAGE (12.5% gels), and visualized with silver staining (upper panel) and with immunoblotting using anti-pentahistidine antibody (lower panel). (B) Cyclic nucleotide hydrolytic activities of partially purified proteins from the cells carrying a control vector (open bars) and pQE-PfPDE1Δ (filled bars) were measured using 5 nM cAMP or 13 nM cGMP as a substrate. Bivalent cation used was 5 mM MgCl2.
Characterization of PfPDE1 activity
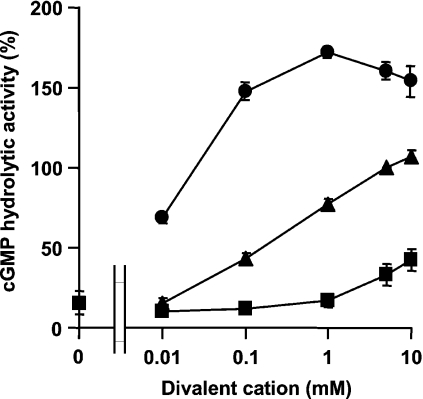
PDEs require bivalent cations for their activities [32,34]. For example, cGMP hydrolytic activity of human PDE9A is supported by Ca2+, Mg2+ and Mn2+, and this enzyme exhibits the highest activity with Mn2+ [34]. The catalytic domain sequence of PfPDE1 shows the highest similarity with that of PDE9A among mammalian PDEs, and both PfPDE1 and PDE9A are cGMP-specific PDEs, suggesting that PfPDE1 activity also may be supported by Mn2+. The effect of several bivalent cations (Ca2+, Mg2+ and Mn2+) on PfPDE1 enzyme activity was examined, and as expected, Mn2+ was revealed to be a more potent activator of PfPDE1 than Mg2+ and Ca2+ at concentrations of 0.01–10 mM. Maximum activity was observed at 1 mM MnCl2 (Figure 6). Ca2+ was less potent at concentrations of 0.01–1 mM.
Figure 6. Effect of bivalent cation on PfPDE1.
PDE activity of the catalytic domain of PfPDE1 expressed in E. coli was measured with 13 nM cGMP in the presence of different concentrations (0–10 mM) of MgCl2 (▲), MnCl2 (●), and CaCl2 (■). The results are presented relative to the activity with 5 mM MgCl2.
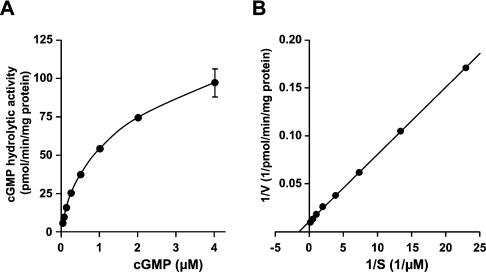
The Km and Vmax values of PfPDE1 in the presence of Mn2+ were calculated from Lineweaver–Burk plots of activities using cGMP (0.03125–4 μM) as a substrate. As shown in Figure 7, Lineweaver–Burk plots showed apparent linearity over the substrate concentration range examined. The Km value of PfPDE1 for cGMP was 0.65±0.033 μM, indicating that PfPDE1 is categorized into high-affinity cGMP PDEs. The Vmax value was 91±4.1 pmol/min per mg of protein with the partially purified recombinant protein. Consistent with the HDX2HX4N motif in the PfPDE1 catalytic domain, high affinity for cGMP also indicated that PfPDE1 is a class I, not a class II, PDE.
Figure 7. Kinetic analysis of partially purified PfPDE1.
(A) Michaelis–Menten kinetics of PfPDE1. Partially purified PfPDE1 protein was prepared as described in the Experimental section. PfPDE1 was assayed at various cGMP concentrations (0.03125–4 μM). (B) Lineweaver–Burk plots of the same sets of data are shown. The data are expressed as the means±S.E.M. for three independent experiments. Km and Vmax values are expressed as the means±S.E.M. for triplicate assays. The bivalent cation used was 1 mM MnCl2.
Inhibitory profile of PfPDE1
The effects of various PDE inhibitors on PfPDE1 were examined (Table 1). IC50 values for the non-specific PDE inhibitors IBMX, papaverine, theophylline and pentoxifylline were more than 100 μM. Furthermore, vinpocetine, EHNA, milrinone and rolipram, which are inhibitors for mammalian PDE1, PDE2, PDE3 and PDE4 respectively, were also inactive up to 100 μM. Compounds that inhibit PDE5 showed inhibitory effects on PfPDE1. Dipyridamole, E4021 and sildenafil demonstrated a moderate inhibitory effect (IC50=22±0.58, 46±1.8, and 56±11 μM, respectively). Among the PDE inhibitors tested, zaprinast was the most effective antagonist for PfPDE1 with an IC50 value of 3.8±0.23 μM. This value is approximately 10 times higher than that obtained for human PDE5 (IC50=0.5 μM; [35]). A potent antimalarial, chloroquine, showed 35% inhibition at 100 μM.
Table 1. Inhibitory effect of the various PDE inhibitors and chloroquine on PfPDE1.
Partially purified PfPDE1 proteins produced in E. coli were used for the assay. The concentrations of cGMP used were 0.6 μM. IC50 values were calculated by linear regression. Data are expressed as the means±S.E.M. for three independent determinations. All assays were performed in duplicate. The bivalent cation used was 1 mM MnCl2.
| Inhibitor | IC50 for PfPDE1 (μM) |
|---|---|
| Chloroquine | >100 |
| IBMX | >100 |
| Papaverine | >100 |
| Theophylline | >100 |
| Pentoxifylline | >100 |
| Vinpocetine | >100 |
| EHNA | >100 |
| Milrinone | >100 |
| Rolipram | >100 |
| Sildenafil | 56±11 |
| E4021 | 46±1.8 |
| Dipyridamole | 22±0.58 |
| Zaprinast | 3.8±0.23 |
Previous reports revealed that Asp754 and Gly780 of bovine PDE5A are critical for interaction with zaprinast [36,37]. The Asp residue is conserved among PDEs and the involvement of this residue in binding to the substrate cGMP has been reported [38]. Since PfPDE1 also contained Asp762 and Gly788 in the conserved positions, we examined a role of these residues for cGMP hydrolytic activity and zaprinast-sensitivity of PfPDE1. Two PfPDE1 mutants, PfPDE1D762A and PfPDE1G788A, with alanine substitutions at Asp762 and Gly788 respectively (see the Experimental section) were used. PfPDE1D762A showed no cGMP hydrolytic activity (Table 2), although the protein was produced at almost the same level as PfPDE1Δ in immunoblot analysis (results not shown), indicating that Asp762 is indispensable for the PDE activity. On the other hand, another PfPDE1 mutant, PfPDE1G788A, retained the cGMP-PDE activity with a Km value of 0.77±0.054 μM, which was about the same as that of the wild-type (0.65±0.033 μM). The IC50 value of the mutant for zaprinast (PfPDE1G788A) was 5.6±0.67 μM, and introduction of this amino acid substitution did not alter the zaprinast-sensitivity drastically.
Table 2. Comparison of IC50 for zaprinast of PfPDE1 mutants.
Wild-type PfPDE1 enzyme and its mutants, PfPDE1D762A and PfPDE1G788A, were produced in E. coli and partially purified by using a nickel affinity column. The Km values of PfPDE1 were calculated from Lineweaver–Burk plots of activities using cGMP. IC50 values for zaprinast were measured with 0.6 μM cGMP by linear regression. Data are expressed as the means±S.E.M. for three independent determinations All assays were performed in duplicate. ND, not detected; NT, not tested.
| Protein | Km (μM) | IC50 for zaprinast (μM) |
|---|---|---|
| PfPDE1 (wild-type) | 0.65±0.033 | 3.8±0.23 |
| PfPDE1D762A | ND | NT |
| PfPDE1G788A | 0.77±0.054 | 5.6±0.67 |
cGMP hydrolytic activity in P. falciparum
Endogenous cGMP PDE activity in P. falciparum was examined. The cytosolic and membrane fractions were prepared from mixed asexual blood-stage malaria parasites and cGMP PDE activities and their subcellular localization were tested. Consistent with the predicted protein feature of PfPDE1 as a membrane protein, most cGMP hydrolytic activity was detected in membrane fractions prepared from infected erythrocytes (Figure 8A). Uninfected erythrocytes showed no significant cGMP hydrolytic activities in either cytosolic or membrane fractions. Interestingly, zaprinast inhibited P. falciparum cGMP PDE activity of membrane fractions with an IC50 value of 4.1±0.32 μM, which was in good agreement with the value obtained with recombinant PfPDE1 (Figure 8B). Furthermore, E4021 inhibited 85% of cGMP hydrolytic activity at 100 μM. On the other hand, IBMX and theophylline were also weak inhibitors at a concentration of 100 μM. At this concentration, chloroquine did not inhibit the cGMP-hydrolytic activity at all.
Figure 8. cGMP hydrolytic activity in P. falciparum.
(A) Cytosolic and membrane fractions of P. falciparum were prepared as described in the Experimental section. PDE activity was measured using 13 nM cGMP as a substrate. The data are expressed as the means±S.E.M. for three independent experiments. (B) Membrane fractions of P. falciparum were used for the PDE assay using 13 nM cGMP. Data are expressed as the means±S.E.M. for three independent experiments. All assays were performed in duplicate. The bivalent cation used was 1 mM MnCl2.
In vitro antimalarial activity of zaprinast
Finally, we examined the effect of PDE inhibitors in the cell proliferation of asexual blood parasites using an in vitro antimalarial activity assay. A solvent for PDE inhibitors, DMSO, did not affect the increase in the P. falciparum cell number (results not shown). Intriguingly, treatment with zaprinast, a potent inhibitor of both recombinant PfPDE1 enzyme and endogenous cGMP PDE activity in P. falciparum, resulted in growth inhibition of the parasites with ED50=35±4.2 μM (Table 3). In contrast, neither IBMX nor theophylline inhibited parasite growth at the concentration of 100 μM. These data reaffirm the importance of the involvement of zaprinast-sensitive PDE activity, including PfPDE1, in the asexual stage development.
Table 3. In vitro antimalarial activity of zaprinast.
Asynchronous P. falciparum Honduras-1 were cultured with final Ht 3% and 0.3% parasitaemia in the absence or presence of PDE inhibitors. After 72 h, more than 1000 erythrocytes stained with Giemsa were examined under microscopy. The ED50 value, which is a dose giving 50% reduction of the increase of infected erythrocytes, was determined by comparison to drug-free controls cultured under the same conditions. Data are expressed as the means±S.E.M. for three independent determinations.
| Inhibitors | ED50 (μM) |
|---|---|
| IBMX | >100 |
| Theophylline | >100 |
| Zaprinast | 35±4.2 |
DISCUSSION
Currently, PDEs comprise a superfamily of enzymes that are divided into three major classes (class I, II and III) on the basis of sequence similarity. Class I PDEs contain a signature sequence, HDX2HX4N, show high affinity for cAMP and cGMP and have been identified in organisms ranging from lower eukaryotes to mammals. P. falciparum was predicted to contain four class I PDE genes in its genome, based on sequence analysis. One of the P. falciparum PDEs, PfPDE1, showed high affinity for cGMP and was sensitive to some of the generally used PDE inhibitors, typical enzymatic characteristics of class I PDEs. Moreover, the PDE activity was lost by mutagenesis of the conserved Asp762, which is predicted to be involved in the formation of a metal-binding pocket essential for class I PDEs [39]. Thus intracellular cyclic nucleotide levels in this lower organism would be multiply controlled by the four class I PDEs including PfPDE1.
The presence of six putative transmembrane domains in the N-terminal to central region of PfPDE1 suggested that PfPDE1 is a membrane protein. Interestingly, we found that the inhibitor sensitivity of cGMP hydrolytic activity in membrane fractions of P. falciparum at mixed asexual blood stages is similar to that of the recombinant PfPDE1 enzyme, suggesting the presence of PfPDE1 in these fractions. However, as well as PfPDE1, the other three putative PfPDEs are also predicted to contain transmembrane regions in their N-termini and it has been reported that theophylline-sensitive cGMP PDE activity, not a characteristic of PfPDE1, may exist in gametocytes of P. falciparum [6]. Thus, although inhibitor sensitivity of endogenous PDE activity is analogous to that of PfPDE1, it is unclear whether PfPDE1 is a major cGMP-PDE in asexual blood stages of P. falciparum or not. Therefore analysis of the three other putative PfPDEs is needed to clarify the regulation of cyclic nucleotide signalling through hydrolysis by the PDEs in P. falciparum.
Previous reports have discussed the cyclic nucleotide signalling in P. falciparum. cAMP is involved in the asexual stage of development and erythrocyte invasion in P. falciparum and Plasmodium berghei [40,41]. In contrast, cGMP has been shown to play an important role in gametogenesis of both parasites [4,5]. Guanylate cyclase (PfGC) activity has been demonstrated in gametocytes. A gametocyte-activating factor, xanthurenic acid, is known to increase PfGC activity [6], suggesting that exflagellation, a requirement for fertilization, may be mediated by cGMP signalling. However, Deng and Baker [8] have reported predominant expression of P. falciparum cGMP-dependent protein kinase (PfPKG) in the ring stage. In mammals, cGMP has three major receptor proteins, namely cGMP-dependent protein kinase, cyclic nucleotide-gated ion channel and cGMP-regulated PDE. Since PfPDE1 and the other three putative PfPDEs do not have a cGMP-binding domain, and a cyclic nucleotide-gated ion channel has not yet been identified in Plasmodium, PfPKG is the only effector of cGMP. Stage-specific expression of PfPDE1 transcripts was consistent with PfPKG, but not PfGC, and the lysates prepared from the parasite in asexual blood stages actually contained cGMP hydrolytic activity. The presence of cGMP-PDE and PKG activities suggests a role for cGMP signalling in the development of the parasite at the asexual blood stages.
The in vitro antimalarial activity assay demonstrated that treatment of P. falciparum with zaprinast results in significant growth inhibition of the parasites in the asexual blood stages. Interestingly, a cell-permeable cGMP analogue, 8-bromo-cGMP, inhibited growth of the parasites in the asexual blood stages with ED50 values less than 10 μM (results not shown), indicating the significance of cGMP signalling in the survival of this organism. Zaprinast is a potent mammalian PDE5 inhibitor, and therefore it is important to know whether synthesis of a specific inhibitor for PfPDE1 is promising or not. Mutagenesis of Gly788 in PfPDE1, which is implicated in the zaprinast binding of bovine PDE5 [36,37], did not alter the inhibitory effects of this compound on PfPDE1. This indicates that the amino acid residues concerned with the binding of zaprinast differ between mammalian PDE5 and PfPDE1; that is, the discovery of a PfPDE1 specific inhibitor is achievable.
In addition to zaprinast (IC50=3.8 μM), PfPDE1 was also inhibited moderately by dipyridamole (IC50=22 μM). The antimalarial activity of dipyridamole has been reported previously [42,43]. A 50% inhibition of P. falciparum has been observed at 30 nM dipyridamole after 24 h treatment [43]. However, the reported antimalarial activity of dipyridamole [42] is strong compared with that of zaprinast observed here. Dipyridamole is known to have various effects such as nucleoside transport inhibition [44], anti-tumour-drug-effluxing inhibition [45], blockage of chloride transport [46], band-3-mediated anion-exchange inhibition [47] and antioxidant properties [48]. It has been assumed that the antimalarial effects of dipyridamole are associated with the inhibition of some transport system [42]. On the other hand, dipyridamole is also established as a potent PDE inhibitor [49]; nevertheless, the involvement of its inhibitory effects on PDEs in the organism was not discussed in [42]. Dipyridamole-sensitive PDE may be included in the three other Plasmodium PDEs and dipyridamole might exhibit more potent antimalarial effects through inhibition of the enzyme than zaprinast.
In summary, we have described the first P. falciparum PDE, PfPDE1, and the effect of a PfPDE1 inhibitor, zaprinast, on antimalarial activity. PfPDEs in the asexual blood stages are not a target molecule of chloroquine, and therefore, considering the need for the development of a novel treatment of chloroquine-resistant malaria, PfPDEs must be a unique target for chemotherapy. Further investigation of the PfPDEs of P. falciparum will elucidate the involvement of cyclic nucleotide signalling in the physiological responses of this parasite and will provide us with a new treatment of the most lethal malaria.
Acknowledgments
We are very grateful to Mr T. Sasaki (Discovery Research Laboratories, Tanabe Seiyaku Co, Ltd, Toda, Saitama, Japan) for helpful discussion. We also wish to express thanks to Dr A. Tanaka (Riken Genomic Sciences Center, Wako, Saitama, Japan) for her great interest in this work.
References
- 1.Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature (London) 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Bras J., Durand R. The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum. Fundam. Clin. Pharmacol. 2003;17:147–153. doi: 10.1046/j.1472-8206.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller L. H., Baruch D. I., Marsh K., Doumbo O. K. The pathogenic basis of malaria. Nature (London) 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto F., Alejo-Blanco R., Fleck S. L., Kawamoto Y., Sinden R. E. Possible roles of Ca2+ and cGMP as mediators of the exflagellation of Plasmodium berghei and Plasmodium falciparum. Mol. Biochem. Parasitol. 1990;42:101–108. doi: 10.1016/0166-6851(90)90117-5. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto F., Fujioka H., Murakami R., Syafruddin Hagiwara M., Ishikawa T., Hidaka H. The roles of Ca2+/calmodulin- and cGMP-dependent pathways in gametogenesis of a rodent malaria parasite, Plasmodium berghei. Eur. J. Cell. Biol. 1993;60:101–107. [PubMed] [Google Scholar]
- 6.Muhia D. K., Swales C. A., Deng W., Kelly J. M., Baker D. A. The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 2001;42:553–560. doi: 10.1046/j.1365-2958.2001.02665.x. [DOI] [PubMed] [Google Scholar]
- 7.Carucci D. J., Witney A. A., Muhia D. K., Warhurst D. C., Schaap P., Meima M., Li J. L., Taylor M. C., Kelly J. M., Baker D. A. Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum. J. Biol. Chem. 2000;275:22147–22156. doi: 10.1074/jbc.M001021200. [DOI] [PubMed] [Google Scholar]
- 8.Deng W., Baker D. A. A novel cyclic GMP-dependent protein kinase is expressed in the ring stage of the Plasmodium falciparum life cycle. Mol. Microbiol. 2002;44:1141–1151. doi: 10.1046/j.1365-2958.2002.02948.x. [DOI] [PubMed] [Google Scholar]
- 9.Soderling S. H., Beavo J. A. Regulation of cAMP and cGMP signalling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 10.Francis S. H., Turko I. V., Corbin J. D. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 11.Hetman J. M., Robas N., Baxendale R., Fidock M., Phillips S. C., Soderling S. H., Beavo J. A. Cloning and characterization of two splice variants of human phosphodiesterase 11A. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12891–12895. doi: 10.1073/pnas.200355397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuasa K., Kotera J., Fujishige K., Michibata H., Sasaki T., Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J. Biol. Chem. 2000;275:31469–31479. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]
- 13.Shabsigh R. Therapy of ED: PDE-5 Inhibitors. Endocrine. 2004;23:135–141. doi: 10.1385/ENDO:23:2-3:135. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Shakur Y., Yoshitake M., Kambayashi Ji J. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc. Drug Rev. 2001;9:369–386. doi: 10.1111/j.1527-3466.2001.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 15.Spina D. The potential of PDE4 inhibitors in respiratory disease. Curr. Drug Targets Inflamm. Allergy. 2004;3:231–236. doi: 10.2174/1568010043343822. [DOI] [PubMed] [Google Scholar]
- 16.Seebeck T., Gong K., Kunz S., Schaub R., Shalaby T., Zoraghi R. cAMP signalling in Trypanosoma brucei. Int. J. Parasitol. 2001;31:491–498. doi: 10.1016/s0020-7519(01)00164-3. [DOI] [PubMed] [Google Scholar]
- 17.Zoraghi R., Kunz S., Gong K., Seebeck T. Characterization of TbPDE2A, a novel cyclic nucleotide-specific phosphodiesterase from the protozoan parasite Trypanosoma brucei. J. Biol. Chem. 2001;276:11559–11566. doi: 10.1074/jbc.M005419200. [DOI] [PubMed] [Google Scholar]
- 18.Rascón A., Soderling S. H., Schaefer J. B., Beavo J. A. Cloning and characterization of a cAMP-specific phosphodiesterase (TbPDE2B) from Trypanosoma brucei. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4714–4719. doi: 10.1073/pnas.002031599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoraghi R., Seebeck T. The cAMP-specific phosphodiesterase TbPDE2C is an essential enzyme in bloodstream form Trypanosoma brucei. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4343–4348. doi: 10.1073/pnas.062716599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science (Washington D.C.) 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 21.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 22.Schultz J., Milpetz F., Bork P., Ponting C. P. SMART, a simple modular architecture research tool: identification of signalling domains. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 25.Rivadeneira E. M., Wasserman M., Espinal C. T. Separation and concentration of schizonts of Plasmodium falciparum by Percoll gradients. J. Protozool. 1983;30:367–370. doi: 10.1111/j.1550-7408.1983.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 26.Sijwali P. S., Shenai B. R., Gut J., Singh A., Rosenthal P. J. Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3. Biochem. J. 2001;360:481–489. doi: 10.1042/0264-6021:3600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv. Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- 28.Lineweaver H., Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934;56:658–666. [Google Scholar]
- 29.McPhee I., Pooley L., Lobban M., Bolger G., Houslay M. D. Identification, characterization and regional distribution in brain of RPDE-6 (RNPDE4A5), a novel splice variant of the PDE4A cyclic AMP phosphodiesterase family. Biochem. J. 1995;310:965–974. doi: 10.1042/bj3100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H.-S., Shibata Y., Wataya Y., Tsuchiya K., Masuyama A., Nojima M. Synthesis and antimalarial activity of cyclic peroxides, 1,2,4,5,7-pentoxocanes and 1,2,4,5-tetroxanes. J. Med. Chem. 1999;42:2604–2609. doi: 10.1021/jm990014j. [DOI] [PubMed] [Google Scholar]
- 31.Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol. Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- 32.Francis S. H., Colbran J. L., McAllister-Lucas L. M., Corbin J. D. Zinc interactions and conserved motifs of the cGMP-binding cGMP-specific phosphodiesterase suggest that it is a zinc hydrolase. J. Biol. Chem. 1994;269:22477–22480. [PubMed] [Google Scholar]
- 33.Senapathy P., Shapiro M. B., Harris N. L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- 34.Fisher D. A., Smith J. F., Pillar J. S., St Denis S. H., Cheng J. B. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J. Biol. Chem. 1998;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- 35.Loughney K., Hill T. R., Florio V. A., Uher L., Rosman G. J., Wolda S. L., Jones B. A., Howard M. L., McAllister-Lucas L. M., Sonnenburg W. K., et al. Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3′,5′-cyclic nucleotide phosphodiesterase. Gene. 1998;216:139–147. doi: 10.1016/s0378-1119(98)00303-5. [DOI] [PubMed] [Google Scholar]
- 36.Turko I. V., Francis S. H., Corbin J. D. Potential roles of conserved amino acids in the catalytic domain of the cGMP-binding cGMP-specific phosphodiesterase. J. Biol. Chem. 1998;273:6460–6466. doi: 10.1074/jbc.273.11.6460. [DOI] [PubMed] [Google Scholar]
- 37.Turko I. V., Ballard S. A., Francis S. H., Corbin J. D. Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compounds. Mol. Pharmacol. 1999;56:124–130. doi: 10.1124/mol.56.1.124. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W., Ke H., Tretiakova A. P., Jameson B., Colman R. W. Identification of overlapping but distinct cAMP and cGMP interaction sites with cyclic nucleotide phosphodiesterase 3A by site-directed mutagenesis and molecular modeling based on crystalline PDE4B. Protein Sci. 2001;10:1481–1489. doi: 10.1110/ps.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Card G. L., England B. P., Suzuki Y., Fong D., Powell B., Lee B., Luu C., Tabrizizad M., Gillette S., Ibrahim, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233–2247. doi: 10.1016/j.str.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Rangachari K., Dluzewski A., Wilson R. J., Gratzer W. B. Control of malarial invasion by phosphorylation of the host cell membrane cytoskeleton. Nature (London) 1986;324:364–365. doi: 10.1038/324364a0. [DOI] [PubMed] [Google Scholar]
- 41.McColm A. A., Hommel M., Trigg P. I. Inhibition of malaria parasite invasion into erythrocytes pretreated with membrane-active drugs. Mol. Biochem. Parasitol. 1980;1:119–127. doi: 10.1016/0166-6851(80)90006-7. [DOI] [PubMed] [Google Scholar]
- 42.Akaki M., Nakano Y., Ito Y., Nagayasu E., Aikawa M. Effects of dipyridamole on Plasmodium falciparum-infected erythrocytes. Parasitol. Res. 2002;88:1044–1050. doi: 10.1007/s00436-002-0690-8. [DOI] [PubMed] [Google Scholar]
- 43.Gero A. M., Scott H. V., O'sullivan W. J., Christopherson R. I. Antimalarial action of nitrobenzylthioinosine in combination with purine nucleoside antimetabolites. Mol. Biochem. Parasitol. 1989;34:87–97. doi: 10.1016/0166-6851(89)90023-6. [DOI] [PubMed] [Google Scholar]
- 44.Scholtissek C. Studies on the uptake of nucleic acid precursors into cells in tissue culture. Biochim. Biophys. Acta. 1968;158:435–447. doi: 10.1016/0304-4165(68)90297-3. [DOI] [PubMed] [Google Scholar]
- 45.Chen H. X., Bamberger U., Heckel A., Guo X., Cheng Y. C. BIBW 22, a dipyridamole analogue, acts as a bifunctional modulator on tumor cells by influencing both P-glycoprotein and nucleoside transport. Cancer Res. 1993;53:1974–1977. [PubMed] [Google Scholar]
- 46.Garcia A. M., Lodish H. F. Lysine 539 of human band 3 is not essential for ion transport or inhibition by stilbene disulfonates. J. Biol. Chem. 1989;264:19607–19613. [PubMed] [Google Scholar]
- 47.Falke J. J., Chan S. I. Molecular mechanisms of band 3 inhibitors. 2. Channel blockers. Biochemistry. 1986;25:7895–7898. doi: 10.1021/bi00372a016. [DOI] [PubMed] [Google Scholar]
- 48.Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 49.Thompson W. J. Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and function. Pharmacol. Ther. 1991;51:13–33. doi: 10.1016/0163-7258(91)90039-o. [DOI] [PubMed] [Google Scholar]