Abstract
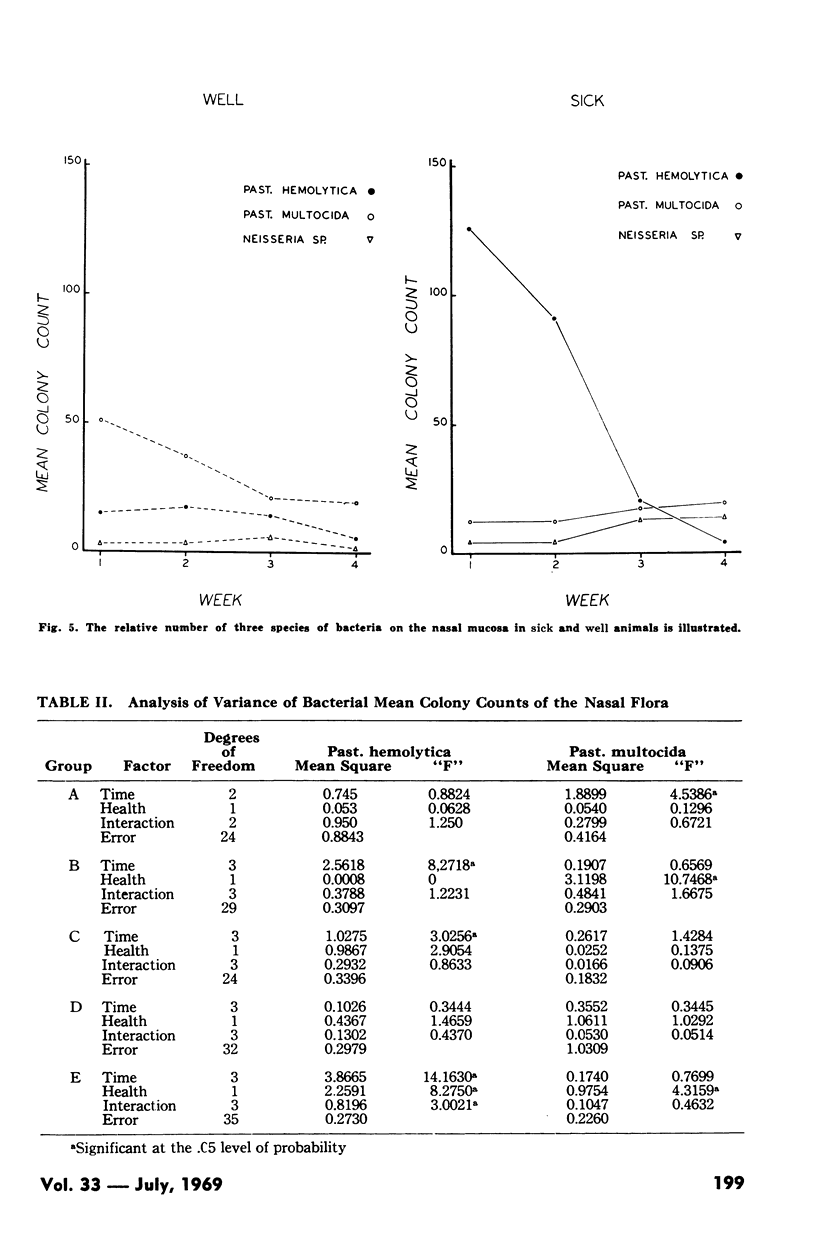
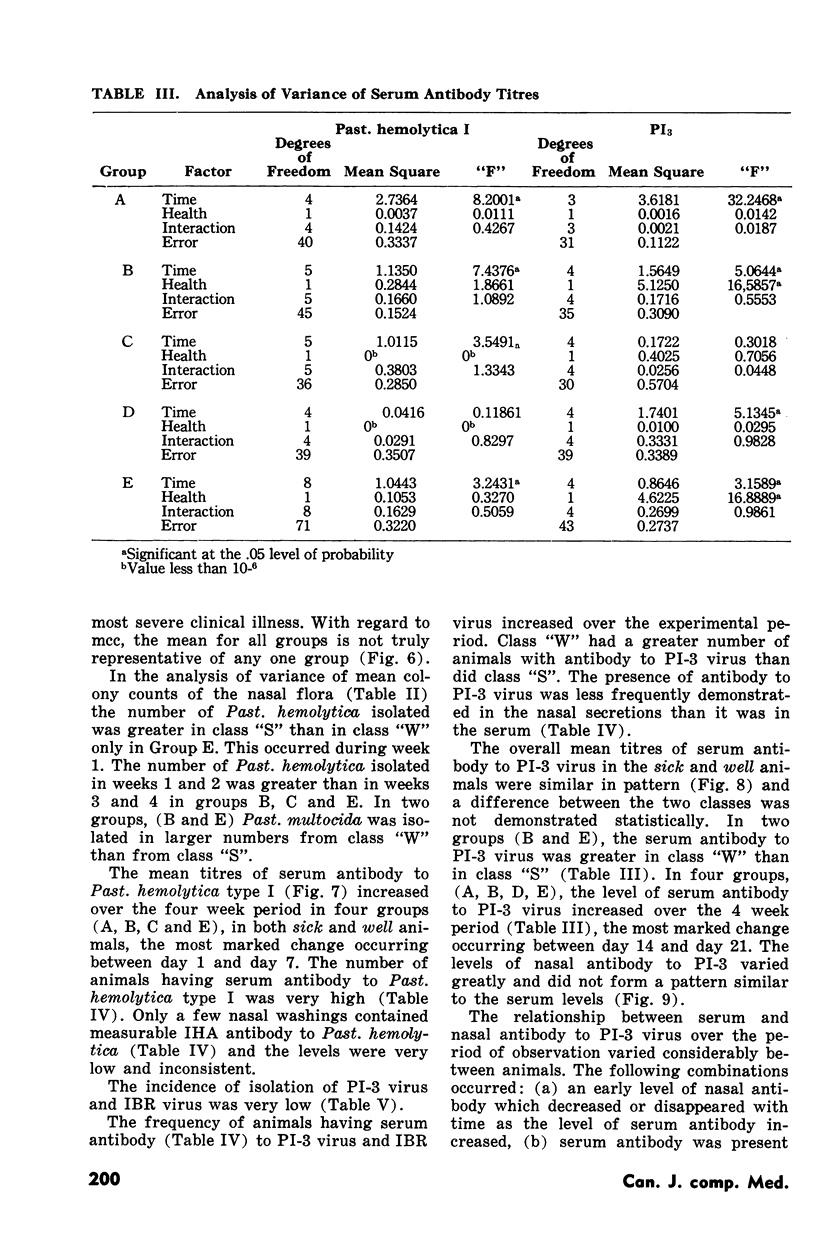
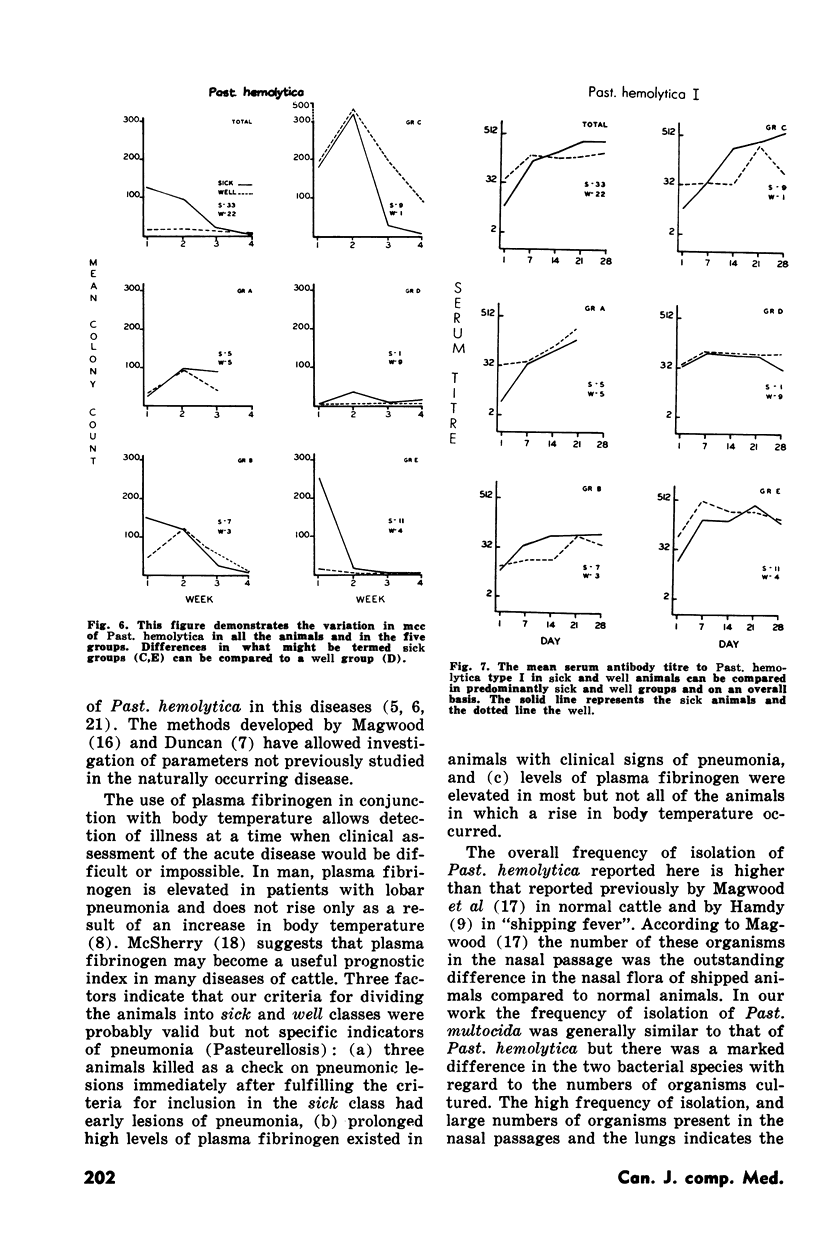
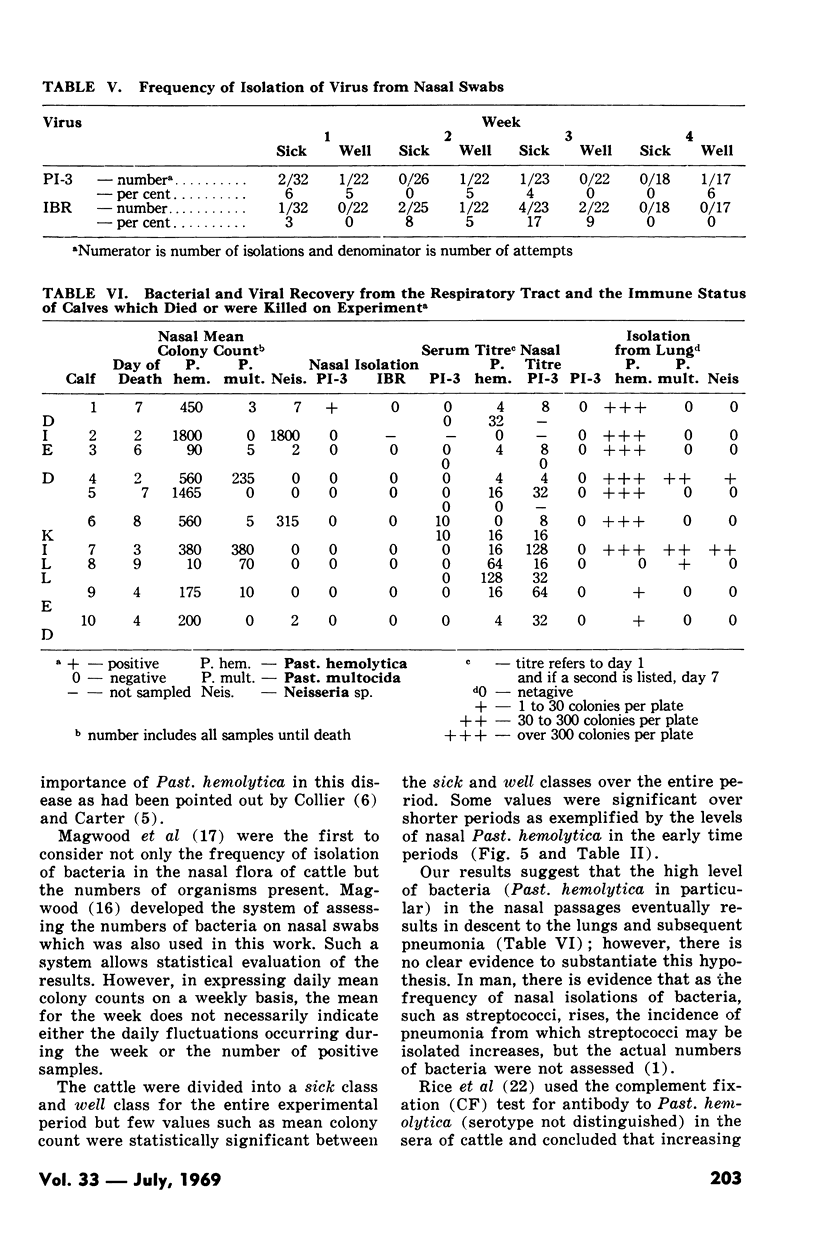
Pasteurellosis was investigated under natural conditions by comparing bacterial and viral nasal flora and levels of bacterial and viral antibody in sera and nasal secretions between animals sick with the disease and those that remained well. The animals were classified sick or well on the basis of the levels of body temperature and plasma fibrinogen. The most significant feature of the bacterial flora was the higher frequency of isolation and the numbers of Past. hemolytica in the nasal flora in the first two weeks after shipment. As indicated by the number of animals with serum antibody to PI-3 virus, infection with this virus was active in both sick and well animals, and serologically, the incidence of infection was higher in animals that remained well. Nasal antibody to PI-3 virus was slightly lower in incidence than serum antibody. Examination of untreated fatal cases and animals killed during the experiment suggests that in some animals there may be a relationship between the high numbers of a bacterial species in the nasal passage and infection by that organism in the lung.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIBERSTEIN E. L., GILLS M., KNIGHT H. Serological types of Pasteurella hemolytica. Cornell Vet. 1960 Jul;50:283–300. [PubMed] [Google Scholar]
- Basiliere J. L., Bistrong H. W., Spence W. F. Streptococcal pneumonia. Recent outbreaks in military recruit populations. Am J Med. 1968 Apr;44(4):580–589. doi: 10.1016/0002-9343(68)90058-2. [DOI] [PubMed] [Google Scholar]
- Hamdy A. H., Trapp A. L. Investigation of nasal microflora of feedlot calves before and after weaning. Am J Vet Res. 1967 Jul;28(125):1019–1025. [PubMed] [Google Scholar]
- MADIN S. H., ANDRIESE P. C., DARBY N. B. The in vitro cultivation of tissues of domestic and laboratory animals. Am J Vet Res. 1957 Oct;18(69):932–941. [PubMed] [Google Scholar]
- Mufson M. A., Chang V., Gill V., Wood S. C., Romansky M. J., Chanock R. M. The role of viruses, mycoplasmas and bacteria in acute pneumonia in civilian adults. Am J Epidemiol. 1967 Nov;86(3):526–544. doi: 10.1093/oxfordjournals.aje.a120763. [DOI] [PubMed] [Google Scholar]
- Omar A. R., Jennings A. R., Betts A. O. The experimental disease produced in calves by the J-121 strain of parainfluenza virus type 3. Res Vet Sci. 1966 Oct;7(4):379–388. [PubMed] [Google Scholar]
- PALOTAY J. L., NEWHALL J. H. Pneumonia in newly weaned calves; report of a field study. J Am Vet Med Assoc. 1958 Oct 1;133(7):353–357. [PubMed] [Google Scholar]
- Rice C. E., Beauregard M., Maybee T. K. Survey of Shipping Fever in Canada: Serological Studies. Can J Comp Med Vet Sci. 1955 Nov;19(11):329–349. [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- STUDDERT M. J., BARKER C. A., SAVAN M. INFECTIOUS PUSTULAR VULVOVAGINITIS VIRUS INFECTION OF BULLS. Am J Vet Res. 1964 Mar;25:303–314. [PubMed] [Google Scholar]
- Sweat R. L. Epizootiologic studies of bovine myxovirus parainfluenza 3. J Am Vet Med Assoc. 1967 Jan 15;150(2):178–183. [PubMed] [Google Scholar]


