Abstract
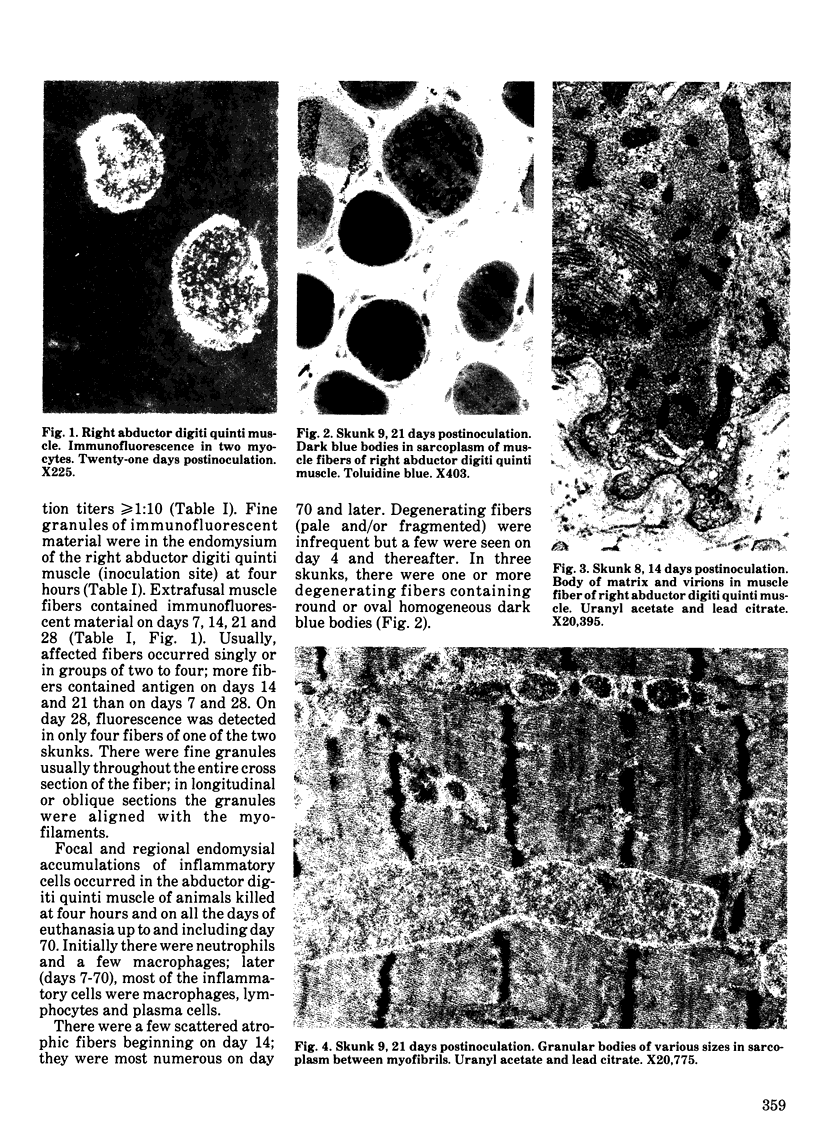
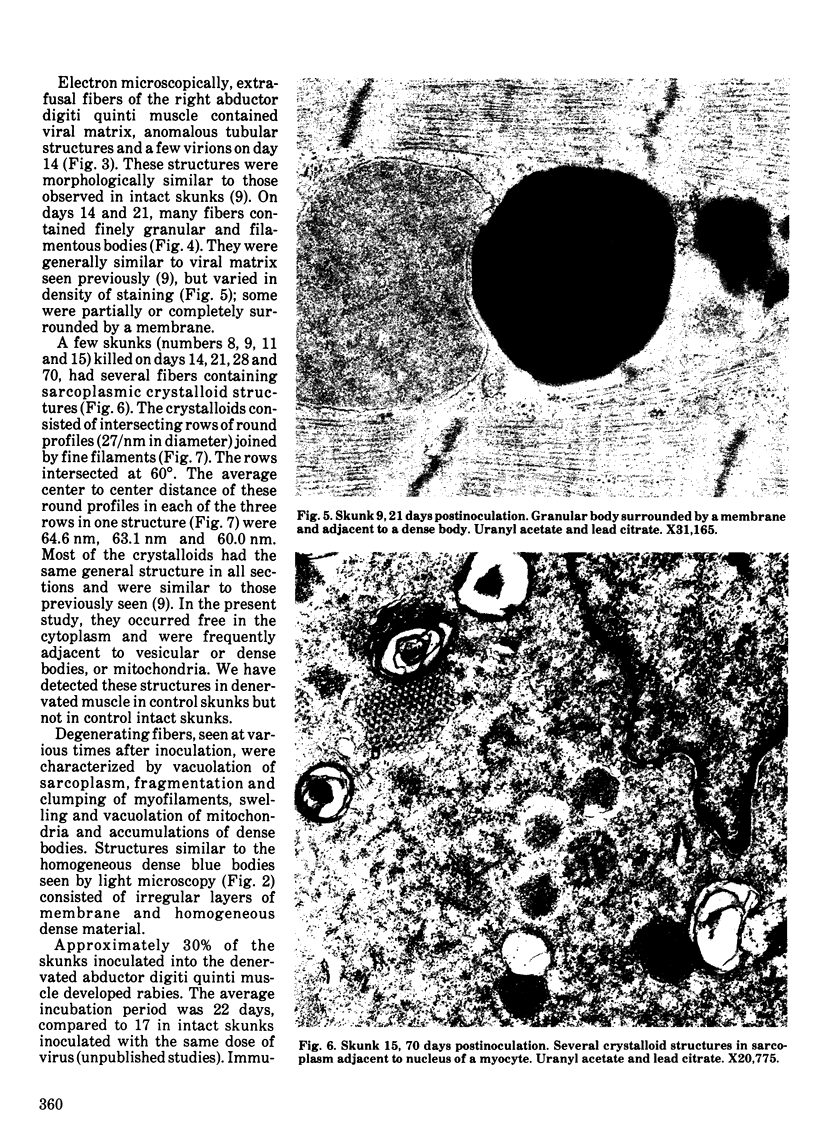
Striped skunks (Mephitis mephitis) were inoculated into the denervated abductor digiti quinti muscle with street rabies virus. They were killed at various times after inoculation and several tissues were examined by immunofluorescence and light microscopy. Muscle at the inoculation site was examined electron microscopically. Rabies antigen was detected in muscle fibers first on day 7 and persisted until day 28. Light and electron microscopic lesions at the inoculation site included atrophic and degenerating muscle fibers and a few focal and regional endomysial accumulations of macrophages, lymphocytes and plasma cells. Scattered myocytes contained bodies of matrix, virions and anomalous tubular structures on electron microscopic examination. The results indicate that replication of rabies virus may occur in infected muscle fibers at the inoculation site until 28 days after exposure. This could contribute to variations in the incubation period for the first two to three months after exposure. However, the results do not support the contention that virus is contained in striated muscle cells throughout the long incubation periods.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS K. F., FARINACCI C. J. Rabies in nonsanguivorous bats of Texas. J Infect Dis. 1955 Sep-Oct;97(2):211–218. doi: 10.1093/infdis/97.2.211. [DOI] [PubMed] [Google Scholar]
- Baer G. M., Cleary W. F. A model in mice for the pathogenesis and treatment of rabies. J Infect Dis. 1972 May;125(5):520–527. doi: 10.1093/infdis/125.5.520. [DOI] [PubMed] [Google Scholar]
- Baer G. M., Shantha T. R., Bourne G. H. The pathogenesis of street rabies virus in rats. Bull World Health Organ. 1968;38(1):119–125. [PMC free article] [PubMed] [Google Scholar]
- Baer G. M., Shanthaveerappa T. R., Bourne G. H. Studies on the pathogenesis of fixed rabies virus in rats. Bull World Health Organ. 1965;33(6):783–794. [PMC free article] [PubMed] [Google Scholar]
- Bulenga G., Heaney T. Post-exposure local treatment of mice infected with rabies with two axonal flow inhibitors, colchicine and vinblastine. J Gen Virol. 1978 May;39(2):381–385. doi: 10.1099/0022-1317-39-2-381. [DOI] [PubMed] [Google Scholar]
- Caulfield J. B., Rebeiz J., Adams R. D. Viral involvement of human muscle. J Pathol Bacteriol. 1968 Jul;96(1):232–234. doi: 10.1002/path.1700960128. [DOI] [PubMed] [Google Scholar]
- Charlton K. M., Casey G. A. Experimental rabies in skunks: immunofluorescence light and electron microscopic studies. Lab Invest. 1979 Jul;41(1):36–44. [PubMed] [Google Scholar]
- Charlton K. M., Casey G. A. Experimental rabies in skunks: oral, nasal, tracheal and intestinal exposure. Can J Comp Med. 1979 Apr;43(2):168–172. [PMC free article] [PubMed] [Google Scholar]
- Chou S. M., Gutmann L. Picornavirus-like crystals in subacute polymyositis. Neurology. 1970 Mar;20(3):205–213. doi: 10.1212/wnl.20.3.205. [DOI] [PubMed] [Google Scholar]
- Chou S. M. Myxovirus-like structures and accompanying nuclear changes in chronic polymyositis. Arch Pathol. 1968 Dec;86(6):649–658. [PubMed] [Google Scholar]
- DEAN D. J., EVANS W. M., MCCLURE R. C. PATHOGENESIS OF RABIES. Bull World Health Organ. 1963;29:803–811. [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N. Electron microscopical demonstration of nucleic acids in virus-like particles in the skeletal muscle of a traffic accident victim. Acta Neuropathol. 1979 Jun 15;47(1):55–59. doi: 10.1007/BF00698273. [DOI] [PubMed] [Google Scholar]
- Gough P. M., Niemeyer C. A rabies epidemic in recently captured skunks. J Wildl Dis. 1975 Apr;11(2):170–176. doi: 10.7589/0090-3558-11.2.170. [DOI] [PubMed] [Google Scholar]
- Gyorkey F., Cabral G. A., Gyorkey P. K., Uribe-Botero G., Dreesman G. R., Melnick J. L. Coxsackievirus aggregates in muscle cells of a polymyositis patient. Intervirology. 1978;10(2):69–77. doi: 10.1159/000148970. [DOI] [PubMed] [Google Scholar]
- Jézéquel A. M., Steiner J. W. Some ultrastructural and histochemical aspects of Coxsackie virus-cell interactions. Lab Invest. 1966 Jun;15(6):1055–1083. [PubMed] [Google Scholar]
- Kirk J. Pseudoviral hollow-cored vesicles in multiple sclerosis brain. Acta Neuropathol. 1979 Oct;48(1):63–66. doi: 10.1007/BF00691793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martocci R. J., Jones M. Z. Crystalline muscle inclusions in atypical postinfectious polyradiculoneuropathy. Ann Clin Lab Sci. 1977 Mar-Apr;7(2):130–140. [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P., Harrison A. K., Winn W. C., Jr Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab Invest. 1973 Mar;28(3):361–376. [PubMed] [Google Scholar]
- Otte G., de Coster W., Thiery E., de Reuck J., vander Eecken H. Ultrastructural study of a muscle biopsy from a patient with subacute myelo-optic neuropathy. J Neurol. 1977 May 13;215(2):91–102. [PubMed] [Google Scholar]
- Sato T., Chou S. M. Effect of denervation on coxsackie A virus myositis in mice: an electronmicroscopic study. Neurology. 1978 Dec;28(12):1232–1240. doi: 10.1212/wnl.28.12.1232. [DOI] [PubMed] [Google Scholar]
- Shafiq S. A., Milhorat A. T., Gorycki M. A. Crystals in muscle fibers in patients with diabetic amyotrophy and neuropathy. Neurology. 1968 Aug;18(8):785–790. doi: 10.1212/wnl.18.8.785. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Bhawan J., Keller R. B., DeGirolami U. Charcot-Marie-Tooth disease associated with hypertrophic neuropathy: a neuropathologic study of two cases. J Neuropathol Exp Neurol. 1980 Jul;39(4):420–440. doi: 10.1097/00005072-198007000-00003. [DOI] [PubMed] [Google Scholar]









