Abstract
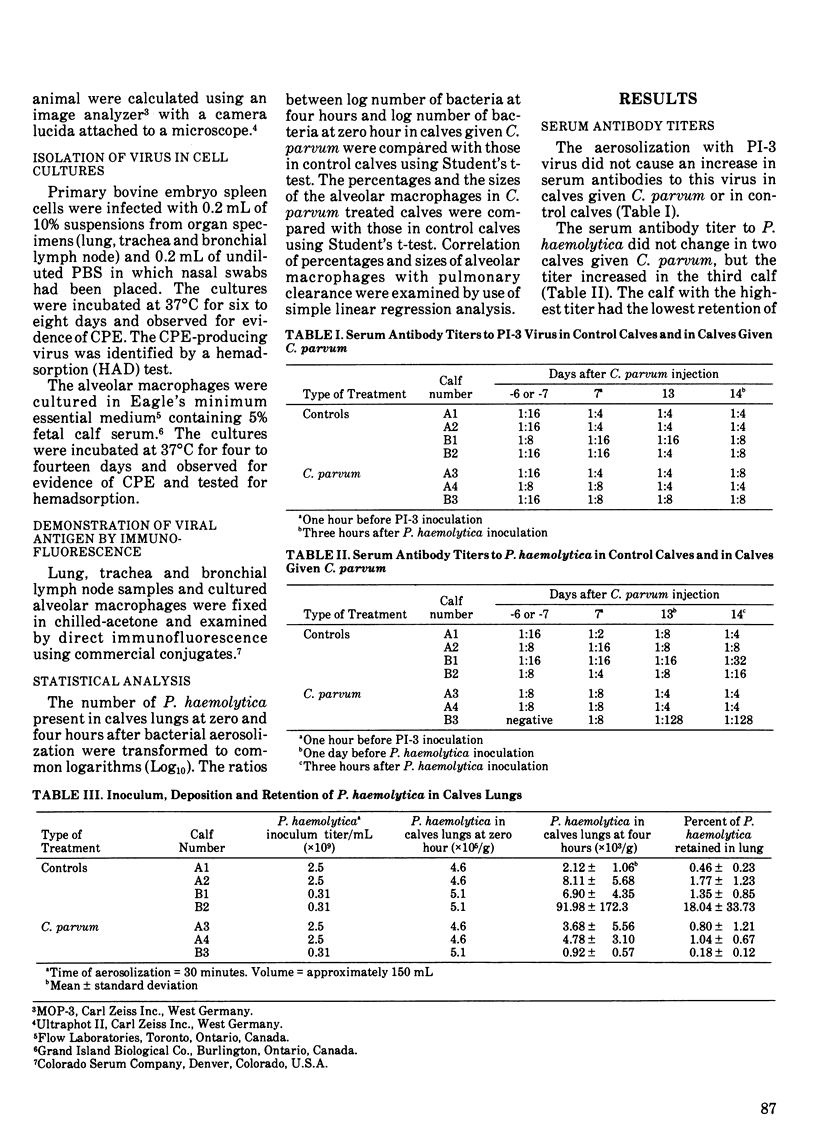
Four control calves were aerosolized with parainfluenza-3 and one week later with Pasteurella haemolytica. Three calves were given Corynebacterium parvum at a dose of 15 mg/m2 body surface area, infected with parainfluenza-3 virus one week later, and aerosolized with P. haemolytica two weeks after C. parvum injection. All calves were killed four hours after P. haemolytica exposure and the bacterial retention in the lung was determined. Parainfluenza-3 viral infection did not exert any suppressive effect on pulmonary clearance of P. haemolytica in six out of seven calves used. However, the bacterial colony counts in the lungs of control calves were higher (P less than 0.05) than those in calves given C. parvum. Hence, C. parvum appeared to enhance bacterial clearance. Despite the marked influx of neutrophils into the lungs after the bacterial inoculation, the neutrophil:macrophage ratio in lavage samples was less in calves given C. parvum than in the control calves. The alveolar macrophages in C. parvum treated calves were generally larger but did not differ significantly (P less than 0.05) from those in the controls. There was no significant (P less than 0.05) correlation between the percentages of alveolar macrophages and the bacterial clearance. In calves given C. parvum, bacterial clearance was enhanced in those calves which had larger macrophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Allan E. M., Pirie H. M., Selman I. E., Snodgrass D. R. Some characteristics of a natural infection by parainfluenza-3 virus in a group of calves. Res Vet Sci. 1978 May;24(3):339–346. [PubMed] [Google Scholar]
- Baldwin D. E., Marshall R. G., Wessman G. E. Experimental infection of calves with myxovirus parainfluenza-3 and Pasteurella haemolytica. Am J Vet Res. 1967 Nov;28(127):1773–1782. [PubMed] [Google Scholar]
- Bursuker I., Goldman R. Quantitative and functional differences induced in bone marrow-derived mononuclear phagocytes by inflammatory stimuli. J Reticuloendothel Soc. 1979 May;25(5):533–544. [PubMed] [Google Scholar]
- CARTER G. R. A serological study of Pasteurella haemolytica. Can J Microbiol. 1956 Aug;2(5):483–488. doi: 10.1139/m56-058. [DOI] [PubMed] [Google Scholar]
- Cantrell J. L., Wheat R. W. Antitumor activity and lymphoreticular stimulation properties of fractions isolated from Corynebacterium parvum. Cancer Res. 1979 Sep;39(9):3554–3563. [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilka F., Thomson R. G., Savan M. The effect of edema, hydrocortisone acetate, concurrent viral infection and immunization on the clearance of Pasteurella hemolytica from the bovine lung. Can J Comp Med. 1974 Jul;38(3):251–259. [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976 Jun;57(6):1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. The effect of Sendai virus infection on bactericidal and transport mechanisms of the murine lung. J Clin Invest. 1972 Aug;51(8):1989–1998. doi: 10.1172/JCI107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Hirt H. M., Becker H., Munk K. Production of an antiviral factor by murine spleen cells after treatment with Corynebacterium parvum. Cell Immunol. 1977 Jun 1;31(1):172–176. doi: 10.1016/0008-8749(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Klein J. O., Green G. M., Tilles J. G., Kass E. H., Finland M. Effect of intranasal reovirus infection on antibacterial activity of mouse lung. J Infect Dis. 1969 Jan;119(1):43–50. doi: 10.1093/infdis/119.1.43. [DOI] [PubMed] [Google Scholar]
- Lopez A., Thomson R. G., Savan M. The pulmonary clearance of Pasteurella hemolytica in calves infected with bovine parainfluenza-3 virus. Can J Comp Med. 1976 Oct;40(4):385–391. [PMC free article] [PubMed] [Google Scholar]
- Miller G. A., Campbell M. W., Hudson J. L. Separation of rat peritoneal macrophages into functionally distinct subclasses by centrifugal elutriation. J Reticuloendothel Soc. 1980 Feb;27(2):167–174. [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Neumann C., Macher E., Sorg C. Interferon production by Corynebacterium parvum and BCG-activated murine spleen macrophages. Immunobiology. 1980 Apr;157(1):12–23. doi: 10.1016/S0171-2985(80)80057-X. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S. Increased nonspecific resstance to malaria produced by administration of killed Corynebacterium parvum. Exp Parasitol. 1967 Oct;21(2):224–231. doi: 10.1016/0014-4894(67)90084-7. [DOI] [PubMed] [Google Scholar]
- Scharfman A., Houdret N., Roussel P., Biserte G., Aerts C., Voisin C. Action de Corynebacterium parvum sur les glycosidases des macrophages alvéolaires de cobaye. Influence de la voie d'introduction. Bull Eur Physiopathol Respir. 1978 Sep-Oct;14(5):485–492. [PubMed] [Google Scholar]
- Swartzberg J. E., Krahenbuhl J. L., Remington J. S. Dichotomy between macrophage activation and degree of protection against Listeria monocytogenes and Toxoplasma gondii in mice stimulated with Corynebacterium parvum. Infect Immun. 1975 Nov;12(5):1037–1043. doi: 10.1128/iai.12.5.1037-1043.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


