Abstract
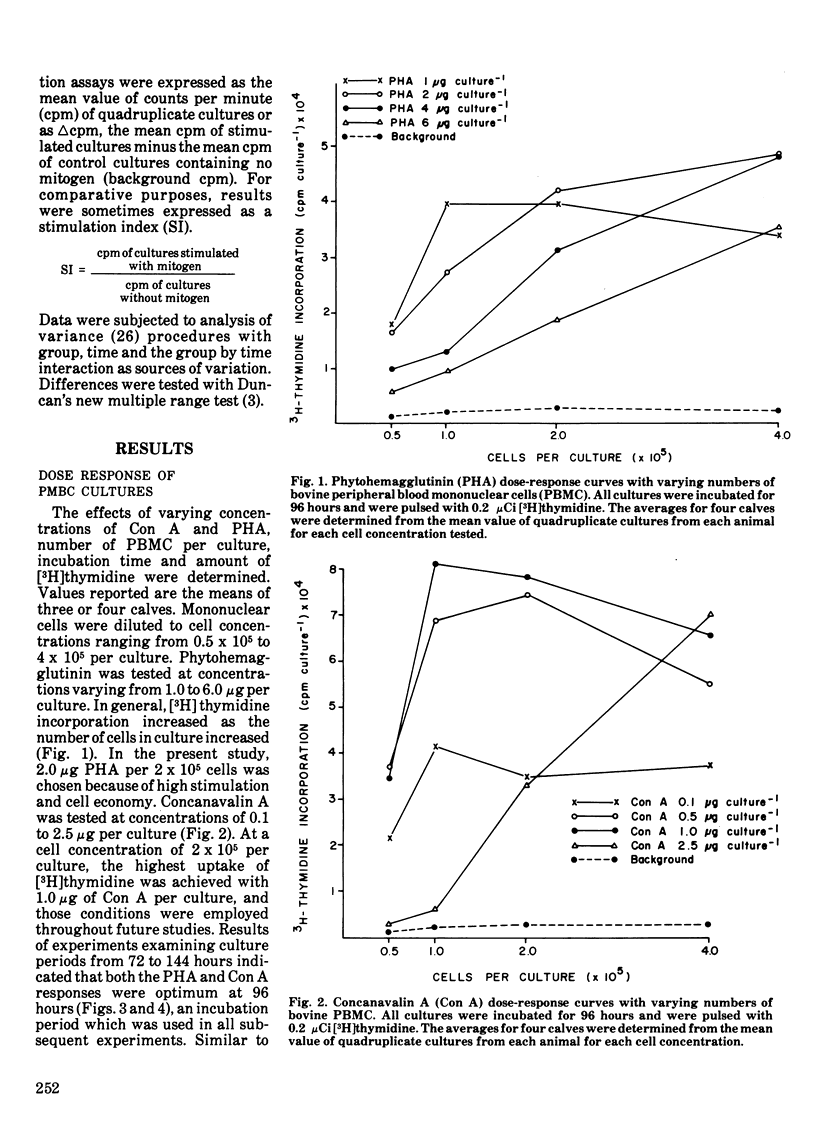
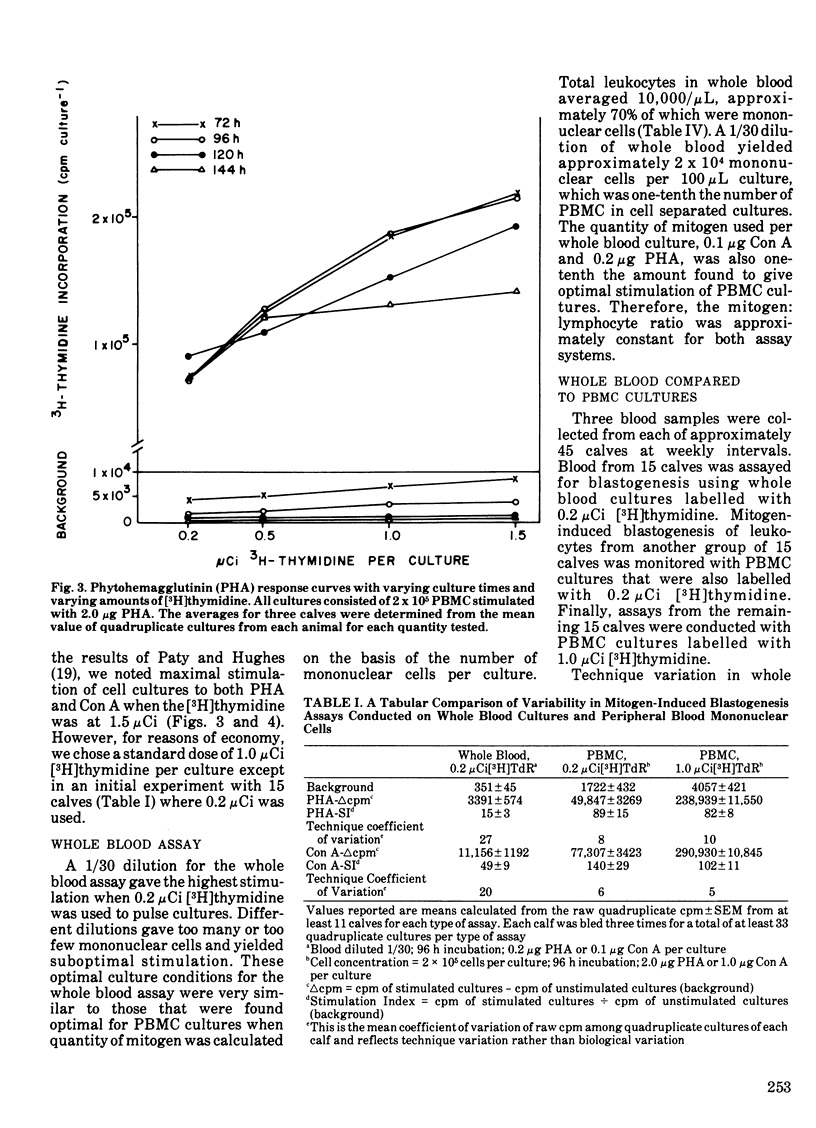
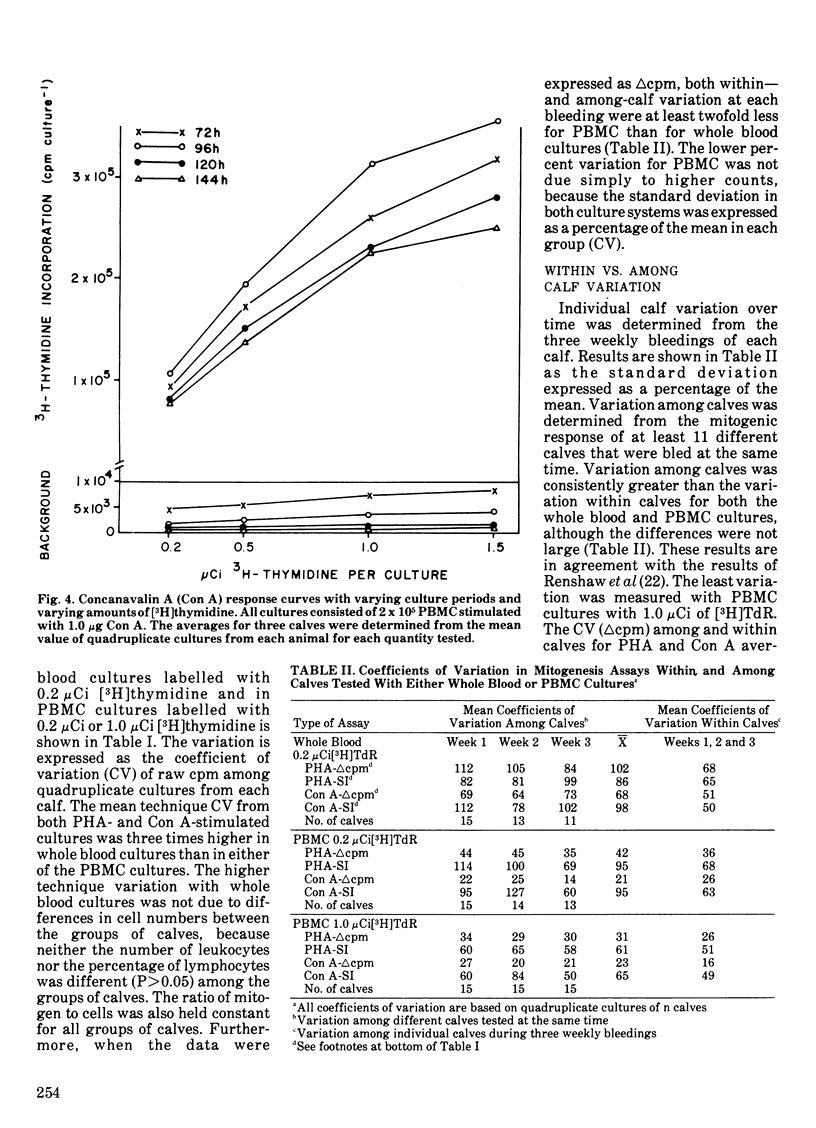
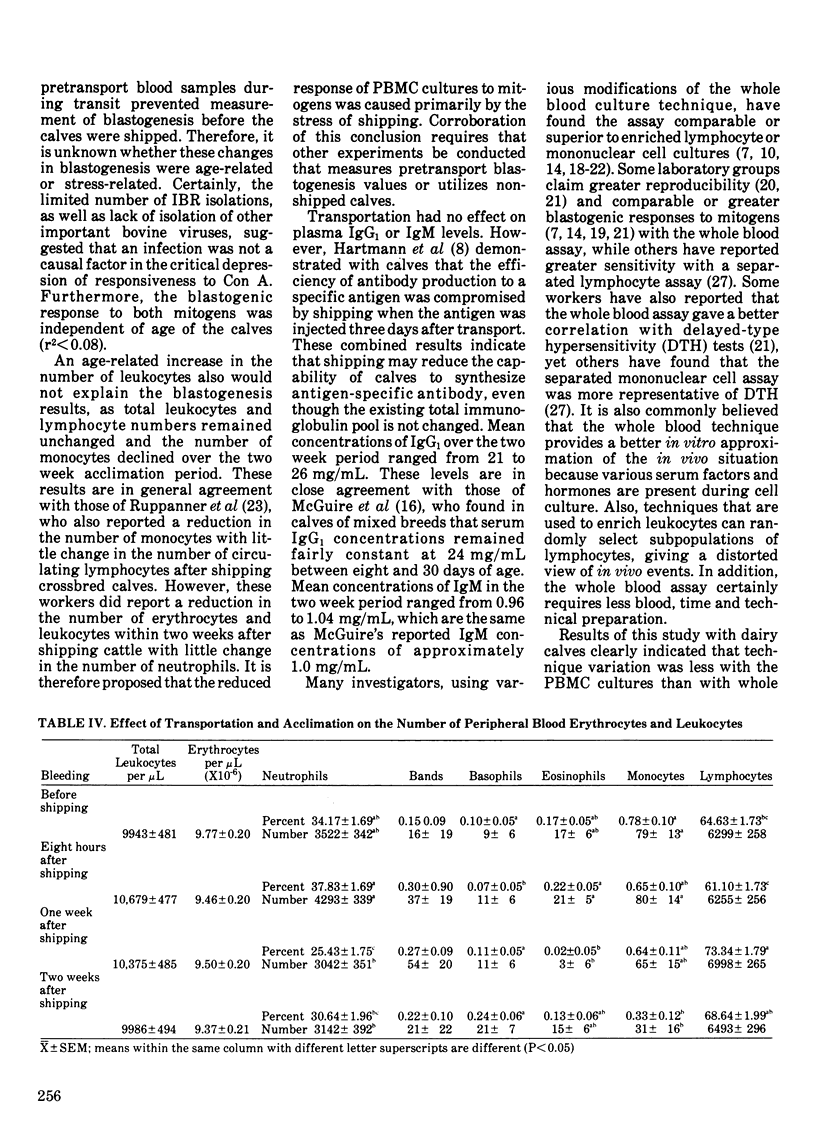
The blastogenic response of peripheral blood mononuclear cells to mitogenic stimulation by concanavalin A was lower (P less than 0.01) after transporting 60 dairy calves 480 km than it was either one or two weeks later. The response was similar for phytohemagglutinin. There was a decrease (P less than 0.05) in the number of peripheral blood monocytes and neutrophils two weeks after shipping. The transportation of calves did not affect plasma IgG1 or IgM level. The mitogenic stimulation of peripheral blood leukocytes by both phytohemagglutinin and concanavalin A in whole blood cultures was more variable than with the culture of peripheral blood mononuclear cells. Technique variation, which was defined as the coefficient of variation among quadruplicate cultures, was greater than 20% for while blood assays and less than 10% for cultures of peripheral blood mononuclear cells. The variation among different calves tested at the same time and the variation within single calves tested at different times were also lower in peripheral blood mononuclear cell cultures than in whole blood mononuclear cell cultures than in whole blood assays. It is suggested that the variation among replicate cultures be reported in blastogenesis studies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus K., Yang T. J. Lymphocyte response to phytohemagglutinin: temporal variation in normal dogs. J Immunol Methods. 1978;21(3-4):261–269. doi: 10.1016/0022-1759(78)90152-7. [DOI] [PubMed] [Google Scholar]
- Crookshank H. R., Elissalde M. H., White R. G., Clanton D. C., Smalley H. E. Effect of transportation and handling of calves upon blood serum composition. J Anim Sci. 1979 Mar;48(3):430–435. doi: 10.2527/jas1979.483430x. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Gisler R. H., Bussard A. E., Mazié J. C., Hess R. Hormonal regulation of the immune response. I. Induction of an immune response in vitro with lymphoid cells from mice exposed to acute systemic stress. Cell Immunol. 1971 Dec;2(6):634–645. doi: 10.1016/0008-8749(71)90011-6. [DOI] [PubMed] [Google Scholar]
- Han T., Pauly J. Simplified whole blood method for evaluating in vitro lymphocyte reactivity of laboratory animals. Clin Exp Immunol. 1972 May;11(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Hartmann H., Bruer W., Herzog A., Meyer H., Rhode H., Schulze F., Steinbach G. Allgemeines Adaptationssyndrom (Selye) beim Kalb 6. Mitteilung: Einfluss von Stresszuständen auf den Antikörperspiegel nach aktiver und passiver Immunisierung sowie auf die topographische Verteilung einiger Keimgruppen im Magen-Darm-Kanal. Arch Exp Veterinarmed. 1976;30(4):553–566. [PubMed] [Google Scholar]
- Irwin M. R., McConnell S., Coleman J. D., Wilcox G. E. Bovine respiratory disease complex: a comparison of potential predisposing and etiologic factors in Australia and the United States. J Am Vet Med Assoc. 1979 Nov 15;175(10):1095–1099. [PubMed] [Google Scholar]
- Junge U., Hoekstra J., Wolfe L., Deinhardt F. Microtechnique for quantitative evaluation of in vitro lymphocyte transformation. Clin Exp Immunol. 1970 Sep;7(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Kaneene J. M., Johnson D. W., Anderson R. K., Angus R. D., Pietz D. E., Muscoplat C. C. Specific lymphocyte stimulation in cattle naturally infected with strains of Brucella abortus and cattle vaccinated with Brucella abortus strain 19. Am J Vet Res. 1978 Apr;39(4):585–589. [PubMed] [Google Scholar]
- Koller L. D., Roan J. G., Kerkvliet N. I. Evaluation of data from mitogen studies in CBA mice: comparison of counts per minute, stimulation index and relative proliferation index. Am J Vet Res. 1979 Jun;40(6):863–865. [PubMed] [Google Scholar]
- Lee L. F. Chicken lymphocyte stimulation by mitogens: a microassay with whole-blood cultures. Avian Dis. 1978 Apr-Jun;22(2):296–307. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Pfeiffer N. E., Weikel J. M., Bartsch R. C. Failure of colostral immunoglobulin transfer in calves dying from infectious disease. J Am Vet Med Assoc. 1976 Oct 1;169(7):713–718. [PubMed] [Google Scholar]
- Muscoplat C. C., Alhaji I., Johnson D. W., Pomeroy K. A., Olson J. M., Larson V. L., Stevens J. B., Sorensen D. K. Characteristics of lymphocyte responses to phytomitogens: comparison of responses of lymphocytes from normal and lymphocytotic cows. Am J Vet Res. 1974 Aug;35(8):1053–1055. [PubMed] [Google Scholar]
- Muscoplat C. C., Chen A. W., Johnson D. W., Alhaji I. In vitro stimulation of bovine peripheral blood lymphocytes: standardization and kinetics of the response. Am J Vet Res. 1974 Dec;35(12):1557–1561. [PubMed] [Google Scholar]
- Paty D. W., Hughes D. Lymphocyte transformation using whole blood cultures: an analysis of responses. J Immunol Methods. 1972 Nov;2(1):99–114. doi: 10.1016/0022-1759(72)90022-1. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Sokal J. E. A simplified technique for in vitro studies of lymphocyte reactivity. Proc Soc Exp Biol Med. 1972 May;140(1):40–44. doi: 10.3181/00379727-140-36391. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Sokal J. E., Han T. Whole-blood culture technique for functional studies of lymphocyte reactivity to mitogens, antigens, and homologous lymphocytes. J Lab Clin Med. 1973 Sep;82(3):500–512. [PubMed] [Google Scholar]
- Renshaw H. W., Eckblad W. P., Everson D. O., Tassinari P. D., Amos D. Ontogeny of immunocompetence in cattle: evaluation of phytomitogen-induced in vitro bovine fetal lymphocyte blastogenesis, using a whole blood culture technique. Am J Vet Res. 1977 Aug;38(8):1141–1150. [PubMed] [Google Scholar]
- Ruppanner R., Norman B. B., Adams C. J., Addis D. G., Lofgreen G. P., Clark J. G., Dunbar J. R. Metabolic and cellular profile testing in calves uncer feedlot conditions: blood cellular components--reference values and changes over time in feedlot. Am J Vet Res. 1978 May;39(5):851–854. [PubMed] [Google Scholar]
- Soper F. F., Muscoplat C. C., Johnson D. W. In vitro stimulation of bovine peripheral blood lymphocytes: analysis of variation of lymphocyte blastogenic response in normal dairy cattle. Am J Vet Res. 1978 Jun;39(6):1039–1042. [PubMed] [Google Scholar]
- Staples G. E., Haugse C. N. Losses in young calves after transportation. Br Vet J. 1974 Jul-Aug;130(4):374–379. doi: 10.1016/s0007-1935(17)35841-4. [DOI] [PubMed] [Google Scholar]
- Woodard L. F., Eckblad W. P., Olson D. P., Bull R. C., Everson D. O. Effects of maternal protein-energy malnutrition on lymphoblastogenic responses of bovine neonates subjected to cold stress. Am J Vet Res. 1980 Apr;41(4):561–563. [PubMed] [Google Scholar]
- Woodard L. F., Renshaw H. W., Burger D. Cell-mediated immunity in neonatal calves: delayed-type hypersensitivity and lymphocyte blastogenesis following immunization with a mycobacterial immunopotentiating glycolipid and tuberculoproteins of Mycobacterium bovis. Am J Vet Res. 1978 Apr;39(4):579–584. [PubMed] [Google Scholar]


