Abstract
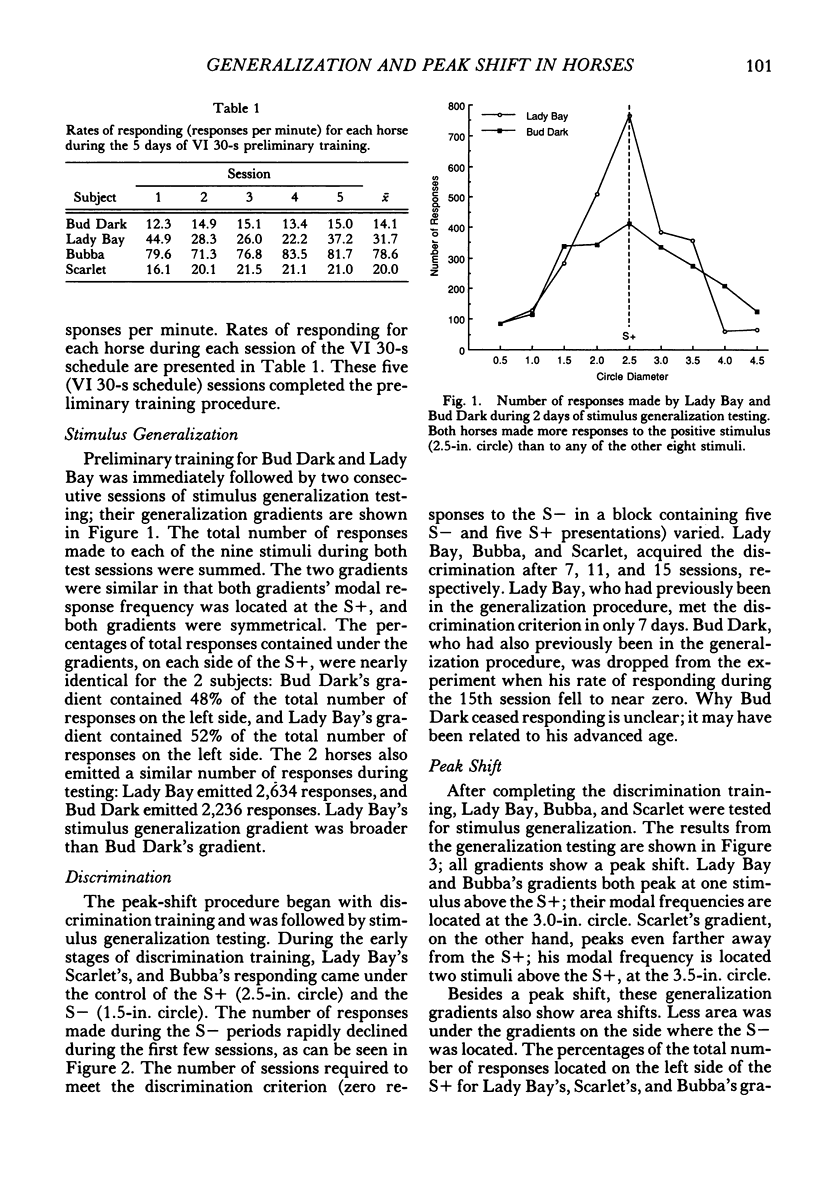
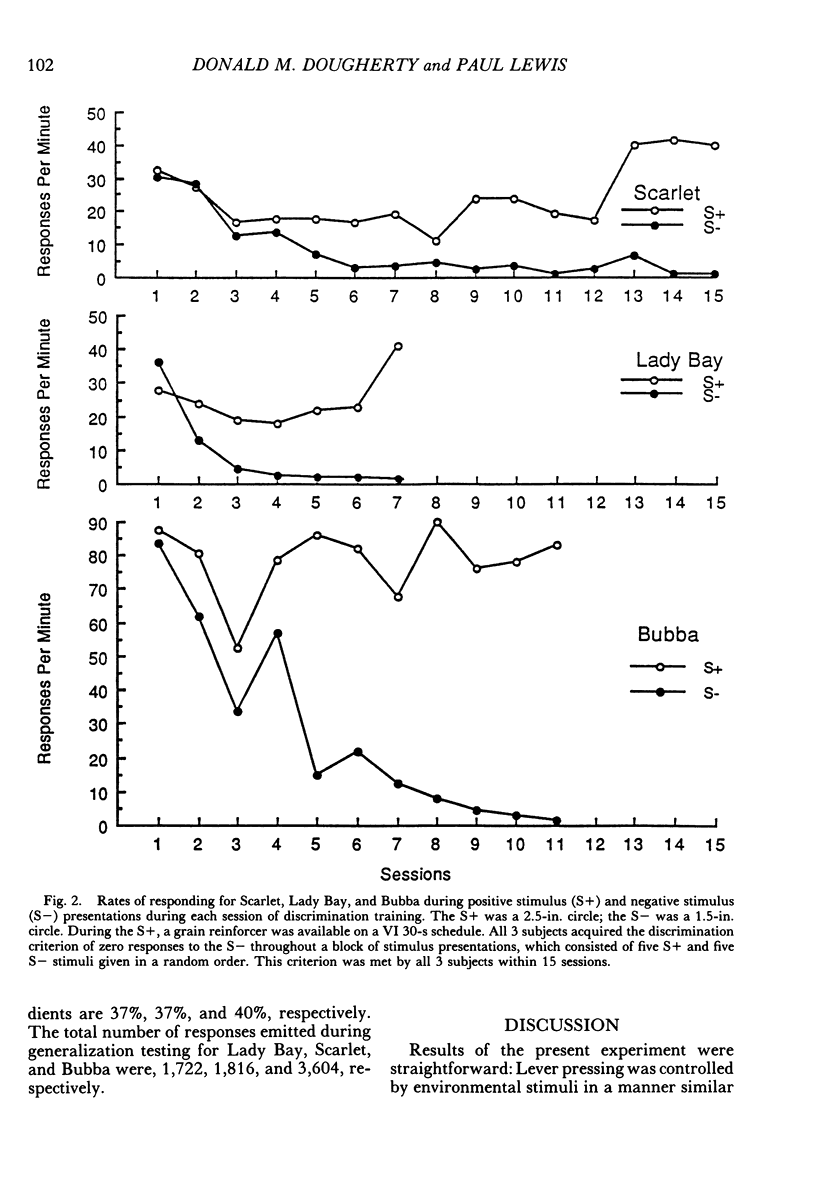
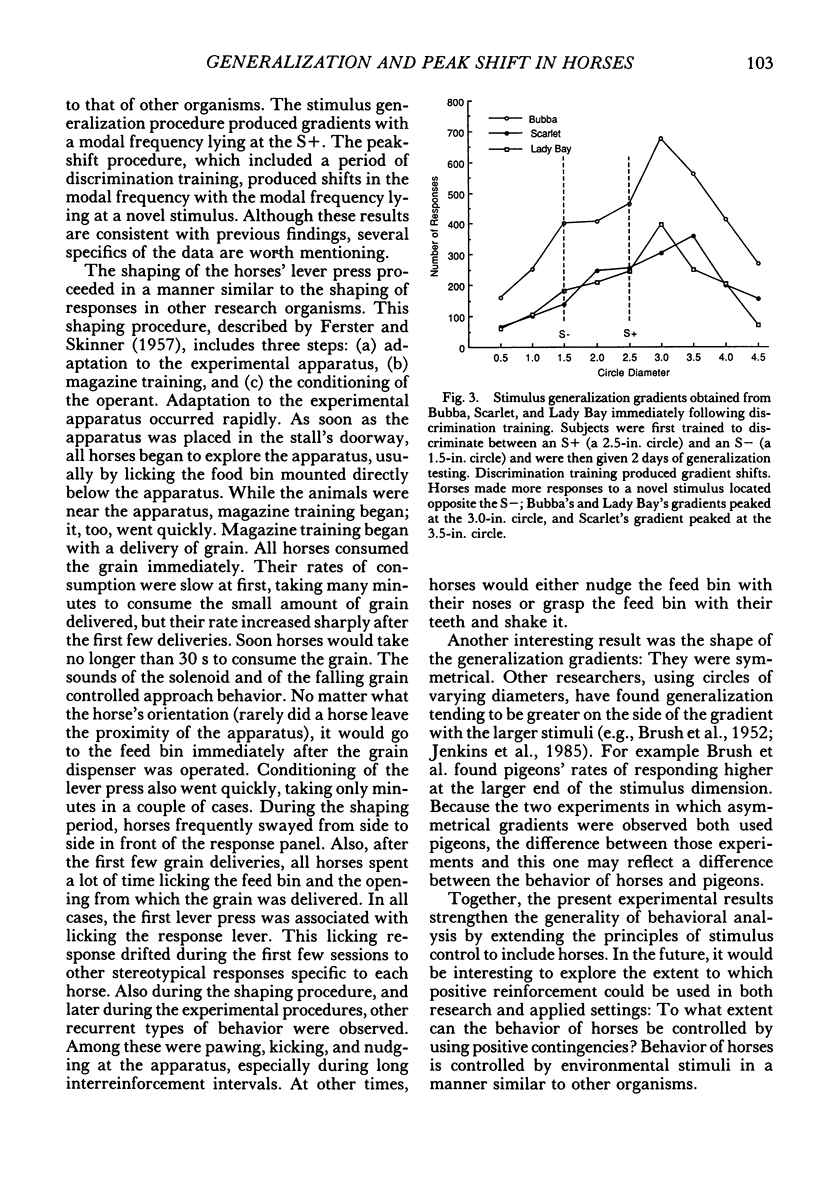
Using horses, we investigated three aspects of the stimulus control of lever-pressing behavior: stimulus generalization, discrimination learning, and peak shift. Nine solid black circles, ranging in size from 0.5 in. to 4.5 in. (1.3 cm to 11.4 cm) served as stimuli. Each horse was shaped, using successive approximations, to press a rat lever with its lip in the presence of a positive stimulus, the 2.5-in. (6.4-cm) circle. Shaping proceeded quickly and was comparable to that of other laboratory organisms. After responding was maintained on a variable-interval 30-s schedule, stimulus generalization gradients were collected from 2 horses prior to discrimination training. During discrimination training, grain followed lever presses in the presence of a positive stimulus (a 2.5-in circle) and never followed lever presses in the presence of a negative stimulus (a 1.5-in. [3.8-cm] circle). Three horses met a criterion of zero responses to the negative stimulus in fewer than 15 sessions. Horses given stimulus generalization testing prior to discrimination training produced symmetrical gradients; horses given discrimination training prior to generalization testing produced asymmetrical gradients. The peak of these gradients shifted away from the negative stimulus. These results are consistent with discrimination, stimulus generalization, and peak-shift phenomena observed in other organisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOUGH D. S. Generalization and preference on a stimulus-intensity continuum. J Exp Anal Behav. 1959 Oct;2:307–317. doi: 10.1901/jeab.1959.2-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUSH F. R., BUSH R. R., JENKINS W. O., JOHN W. F., WHITING J. W. M. Stimulus generalization after extinction and punishment: an experimental study of displacement. J Abnorm Psychol. 1952 Jul;47(3):633–640. doi: 10.1037/h0055188. [DOI] [PubMed] [Google Scholar]
- Bloomfield T. M. A peak shift on a line-tilt continuum. J Exp Anal Behav. 1967 Jul;10(4):361–366. doi: 10.1901/jeab.1967.10-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough D. S. Generalization gradient shape and summation in steady-state tests. J Exp Anal Behav. 1969 Jan;12(1):91–104. doi: 10.1901/jeab.1969.12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A. J., Engberg L., Thomas D. R. On the form of stimulus generalization curves for visual intensity. J Exp Anal Behav. 1971 Sep;16(2):177–180. doi: 10.1901/jeab.1971.16-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLESHLER M., HOFFMAN H. S. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962 Oct;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay R. R. Auditory frequency generalization in the goldfish (Carassius auratus). J Exp Anal Behav. 1970 Nov;14(3):353–360. doi: 10.1901/jeab.1970.14-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRICE G. R., SALTZ E. The generalization of an instrumental response to stimuli varying in the size dimension. J Exp Psychol. 1950 Dec;40(6):702–708. doi: 10.1037/h0054435. [DOI] [PubMed] [Google Scholar]
- GUTTMAN N., KALISH H. I. Discriminability and stimulus generalization. J Exp Psychol. 1956 Jan;51(1):79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- HANSON H. M. Effects of discrimination training on stimulus generalization. J Exp Psychol. 1959 Nov;58:321–334. doi: 10.1037/h0042606. [DOI] [PubMed] [Google Scholar]
- Heffner H. E., Heffner R. S. Sound localization in large mammals: localization of complex sounds by horses. Behav Neurosci. 1984 Jun;98(3):541–555. doi: 10.1037//0735-7044.98.3.541. [DOI] [PubMed] [Google Scholar]
- Heffner R. S., Heffner H. E. Localization of tones by horses: use of binaural cues and the role of the superior olivary complex. Behav Neurosci. 1986 Feb;100(1):93–103. doi: 10.1037//0735-7044.100.1.93. [DOI] [PubMed] [Google Scholar]
- JENKINS W. O., PASCAL G. R., WALKER R. W., Jr Deprivation and generalization. J Exp Psychol. 1958 Sep;56(3):274–277. doi: 10.1037/h0043850. [DOI] [PubMed] [Google Scholar]
- KALISH H. I. The relationship between discriminability and generalization: a revaluation. J Exp Psychol. 1958 Jun;55(6):637–644. doi: 10.1037/h0048049. [DOI] [PubMed] [Google Scholar]
- MYERS R. D., MESKER D. C. Operant responding in a horse under several schedules of reinforcement. J Exp Anal Behav. 1960 Apr;3:161–164. doi: 10.1901/jeab.1960.3-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migler B., Millenson J. R. Analysis of response rates during stimulus generalization. J Exp Anal Behav. 1969 Jan;12(1):81–87. doi: 10.1901/jeab.1969.12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody D. B., Stebbins W. C., Iglauer C. Auditory generalization gradients for response latency in the monkey. J Exp Anal Behav. 1971 Jul;16(1):105–111. doi: 10.1901/jeab.1971.16-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERREL R., SHERMAN J. G. Generalization of auditory intensity following discrimination training. J Exp Anal Behav. 1960 Oct;3:313–322. doi: 10.1901/jeab.1960.3-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrel R. A generalization gradient for auditory intensity in the rat. J Exp Anal Behav. 1958 Oct;1(4):303–313. doi: 10.1901/jeab.1958.1-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBAUM G. Stimulus generalization as a function of level of experimentally induced anxiety. J Exp Psychol. 1953 Jan;45(1):35–43. doi: 10.1037/h0054120. [DOI] [PubMed] [Google Scholar]
- Riccio D. C., Urda M., Thomas D. R. Stimulus control in pigeons based on proprioceptive stimuli from floor inclination. Science. 1966 Jul 22;153(3734):434–436. doi: 10.1126/science.153.3734.434. [DOI] [PubMed] [Google Scholar]
- Rudolph R. L., Honig W. K. Effects of monochromatic rearing on spectral discrimination learning and the peak shift in chicks. J Exp Anal Behav. 1972 Jan;17(1):107–111. doi: 10.1901/jeab.1972.17-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLOANE H. N., Jr STIMULUS GENERALIZATION ALONG A LIGHT FLICKER RATE CONTINUUM AFTER DISCRIMINATION TRAINING WITH SEVERAL S-'S. J Exp Anal Behav. 1964 May;7:217–222. doi: 10.1901/jeab.1964.7-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS D. R. The effects of drive and discrimination training on stimulus generalization. J Exp Psychol. 1962 Jul;64:24–28. doi: 10.1037/h0047080. [DOI] [PubMed] [Google Scholar]
- Thomas D. R., Lyons J. Further evidence of a sensory-tonic interaction in pigeons. J Exp Anal Behav. 1968 Mar;11(2):167–171. doi: 10.1901/jeab.1968.11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy W. K. Wavelength generalization and preference in monochromatically reared ducklings. J Exp Anal Behav. 1970 Mar;13(2):163–178. doi: 10.1901/jeab.1970.13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]


