Abstract
Much evidence indicates that women have a higher risk of developing Alzheimer's disease (AD) than do men. The reason for this gender difference is unclear. We hypothesize that estrogen deficiency in the brains of women with AD may be a key risk factor. In rapidly acquired postmortem brains from women with AD, we found greatly reduced estrogen levels compared with those from age- and gender-matched normal control subjects; AD and control subjects had comparably low levels of serum estrogen. We examined the onset and severity of AD pathology associated with estrogen depletion by using a gene-based approach, by crossing the estrogen-synthesizing enzyme aromatase gene knockout mice with APP23 transgenic mice, a mouse model of AD, to produce estrogen-deficient APP23 mice. Compared with APP23 transgenic control mice, estrogen-deficient APP23 mice exhibited greatly reduced brain estrogen and early-onset and increased β amyloid peptide (Aβ) deposition. These mice also exhibited increased Aβ production, and microglia cultures prepared from the brains of these mice were impaired in Aβ clearance/degradation. In contrast, ovariectomized APP23 mice exhibited plaque pathology similar to that observed in the APP23 transgenic control mice. Our results indicate that estrogen depletion in the brain may be a significant risk factor for developing AD neuropathology.
Keywords: amyloid deposition, aromatase, transgenic animal
Neuropathological hallmarks of Alzheimer's disease (AD) include significant deposition of extracellular β amyloid peptide (Aβ) and presence of neurofibrillary tangles in the brain (1). Aβ is derived from the two-step enzymatic processing of amyloid precursor protein (APP) in which β-secretase (BACE) cleaves the β-site of APP to release the N terminus of Aβ, and the γ-secretase protein complex cleaves the γ-site of APP to release the C terminus of Aβ (2, 3). Overproduction and progressive deposition of Aβ are known to underlie, in part, Aβ plaque formation, a key pathologic feature of AD. The initial cleavage of APP by BACE is critical for Aβ associated with AD neuropathology (4). Recent studies have shown that BACE activity increases with age and is elevated in AD brains (5, 6).
Impaired Aβ clearance and/or degradation may also contribute to Aβ plaque formation. Our previous findings support this hypothesis: Microglia isolated from AD brains have impaired phagocytic activity, leading to reduced Aβ clearance (7). Other groups have found that cytoplasmic Aβ granules in the plaque-associated glia and microglia participate in the clearance of Aβ in Aβ-immunized AD patients and APP transgenic mice (8, 9).
Two enzymes are involved in Aβ degradation and clearance: insulin-degrading enzyme (IDE) and neprilysin (NEP). IDE is expressed in high concentrations in the brain. Besides degrading insulin and several regulatory peptides, IDE also degrades the intracellular domain of APP and is responsible for degrading and clearing Aβ from the brain (10, 11). Indeed, genetic linkage studies have shown that late-onset AD loci on chromosome 10q, specifically eight disease-associated single-nucleotide polymorphisms, are linked to certain 5′-flanking sequences of the IDE gene (12, 13). Additionally, a recent study has demonstrated that Aβ levels and Aβ plaque density are reduced in double transgenic mice overexpressing IDE and APP mutations (14).
NEP, a plasma membrane glycoprotein, appears to be the principal Aβ-degrading enzyme (15-17). In the brain, NEP is expressed on the membranes of neurons. NEP mRNA and protein are significantly decreased in AD brains compared with age-matched normal brains (18, 19). An age-related decline of NEP has also been reported in mouse hippocampus (20). Interestingly, transcription of the gene that encodes NEP is regulated by various steroids, including progesterone and androgen (21). A study in rat showed that ovariectom decreases NEP activity in the brain by 30%, and the decreased NEP activity can be ameliorated by estrogen-replacement therapy (ERT) (22).
It is generally recognized that the prevalence of AD is higher in women (23). Women tend to live longer than men, and the longevity effect might be a large factor in the preponderance of women with AD. In addition, lack of education is found to be negatively associated with AD risk. However, the female prevalence in AD remains after adjusting for age and education (24-26) and female hormone may, in part, also contribute to gender-related differences in AD. After menopause, the decline of estrogen levels in the brain may make neurons more susceptible to age-related neurodegenerative processes (27). This hypothesis is supported by both clinical and basic studies. In vitro findings from cultured neurons suggest that estrogen may prevent AD pathogenesis by reducing Aβ production, protecting against Aβ-induced cell death, and enhancing Aβ clearance (7, 28). These findings are consistent with other clinical studies that suggest that ERT may reduce the risk or delay the onset of AD (29, 30). However, recent clinical trials have found that estrogen-progesterone combination therapy provides no apparent benefits or even an increased risk for AD or dementia (31). The reasons for these conflicting findings remain unclear.
Estrogens are synthesized in a number of human tissues. In the brain, estrogen is formed locally in neural tissue from the conversion of precursor androgens by aromatase, and estrogen may act only in a paracrine or intracrine fashion (32). Although neurons and astrocytes both express aromatase, studies found that astrocytes are the major producer of dehydroepiandrosterone and androgens, whereas neurons are the major producer of estrogens (33, 34).
Brain aromatase activity may be neuroprotective. Animal studies showed that inhibition of aromatase enhances kainic acid-induced neuronal cell death, whereas the enhancement could be prevented by estrogen treatment. In addition, aromatase gene knockout mice showed enhanced hippocampal neuronal loss in response to neurotoxins compared with WT mice (33). The neuroprotective action of brain aromatase may be mediated by increasing local estrogen levels of injured neurons.
In the present study, we studied estrogen levels and aromatase expression in postmortem brains of female AD patients and examined the role of brain estrogen deficiency in AD-like neuropathology of transgenic mice. Our data suggest that brain estrogen deficiency may be a risk factor for developing AD pathology.
Materials and Methods
Human Brains. Human brain tissues were obtained from routine brain autopsies of subjects enrolled in the Sun Health Research Institute Brain Donation Program. AD was diagnosed by using criteria of the National Institute of Neurological Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (NINCDS-ARDRDA) and was subsequently confirmed postmortem by a trained neuropathologist. Postmortem intervals for brain samples averaged 2.8 h (range, 2-3.5 h). Both AD subjects and non-AD control subjects were Caucasian females (mean age, 84 ± 1.9 y and 84 ± 2.0 y, respectively). We selected frontal cortex and cerebellum samples according to NINCDS-ARDRDA criteria. Serum samples were also collected antemortem from the same individuals for estrogen RIAs. None of the participants in our study had a history of ERT.
Experimental Mice. Mice were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Generation of the B6, D2-TgN (Thy1-APP23Swe) line of transgenic mice (APP23) and the aromatase gene knockout line of mice on a C57BL/6 background were described (35, 36). Heterozygous APP23 transgenic mice (APP23+/-) were crossed with homozygous aromatase knockout mice (Ar-/-), and the resulting APP23/Ar+/- offspring were intercrossed through brother-sister mating to obtain litter-matched genotypes (WT, APP23, and APP23/Ar+/-) for the present study. Age-matched littermates having APP23+/-Ar+/+ (APP23) and APP23-/-Ar+/+ (WT) genotypes were used as controls. Fourteen and ten 3-month-old APP23 mice were under bilateral ovariectomies [ovariectomized (OVX) APP] or sham surgery (SHAM APP), respectively. For ovariectomy, in brief, a longitudinal incision was made on the lower back in the midline at the level of the last rib. The uterine horns were tied with surgical silk, and the ovaries were cut and removed. The skin incision was closed by interrupted sutures.
Tissue Preparation and Immunohistochemistry. Female mice of each genotype were anesthetized, and their brains were quickly removed and bisected. For immunohistochemistry, tissue were either fixed with 4% paraformaldehyde or frozen at -80°C, then serially sectioned (15-30 μm in thickness) in the sagittal plane with a Leica CA 1900 cryostat. Eight to 10 sections (≈120 μm apart) were immunostained for Aβ (rabbit anti-Aβ-peptide; 1:250; Zymed) or for a microglia marker (monoclonal rat anti-mouse CD11b; 1:1,000; Serotec). Immunoreactivity was visualized by using 3,3′-diaminobenzidine as the chromogen (DAB Substrate kit, Vector Laboratories). Single and double immunostaining were documented by digital imaging and then processed with a Leica complementary software package (magnafire sp).
Western Blot Analysis. To extract total protein, brain samples were homogenized in buffer containing 1% Nonidet P-40, 0.1% SDS, 50 mM Tris (pH 8.0), 50 mM NaCl, 0.05% deoxycholate, and protease inhibitors (Roche Molecular Biochemicals, Indianapolis). Extracts (40 μg of protein) were subjected to electrophoresis, and separated proteins were transferred onto nitrocellulose membranes, which were then immunostained with the following primary antibodies: monoclonal anti-BACE (1:100; Calbiochem), anti-APP C-terminal fragment C99 [APPC8; 1:500; a gift from Dennis J. Selkoe (Harvard University School of Medicine, Boston)], polyclonal anti-IDE (1:1,000; Oncogene Research, San Diego), and anti-NEP (1:1,000; Chemicon). The membranes were incubated with peroxidase-conjugated secondary antibodies (1:1,000; Santa Cruz Biotechnology), and immunoreactive bands were visualized with an ECL system.
Primary Neonatal Mouse Microglia Cultures. We prepared primary microglia cultures from the whole brains of neonatal Ar+/- and WT mice (postnatal days 0-3) using previously described methods (7, 37), with slight modifications. Briefly, brain tissue was mechanically dissociated and plated in 75-mm flasks coated with poly-l-lysine (0.1 mg/ml) at a density of 5 × 107 cells per flask. The mixture of cell types was grown for 10 days before harvesting microglia. The purity of the cultures was determined immunocytochemically with antibodies against the microglial marker CD45 (1:500; Santa Cruz Biotechnology).
Aβ Clearance and Phagocytosis Assay. We quantified microglial Aβ clearance and phagocytotic activity by measuring the fluorescence of internalized Fluo-Aβ. Microglia isolated from Ar+/- and WT animals were cultured in 96-well plates and treated with Fluo-Aβ as described in ref. 7. Briefly, microglia pretreated with either 17β-estradiol or vehicle for 24 or 48 h were incubated in the aggregated Fluo-Aβ solution for 24 h. Fluorescence in the conditioned medium and cell lysates was quantified with a Multilabel Counter (Wallac, Gaithersburg, MD; excitation, 480 nm; emission, 520 nm). Data were analyzed with explore software (Wallac).
Quantitative Analysis of Aromatase mRNA. Aromatase mRNA was quantified according to the methods of Honda et al. (37). The total RNA fraction and aromatase mRNA in these samples was quantified by competitive RT-PCR. An internal standard RNA using modified aromatase human cDNA was mixed with the total RNA from our samples and amplified by PCR for 26 cycles with the following primers: a FAM-labeled sense primer (5′-TACTACAACCGGGTATATGG-3′) and an antisense primer (5′-TGTTAGAGGTGTCCAGCATG-3′). The FAM-labeled PCR products displayed two peaks, one corresponding to aromatase mRNA and the other corresponding to the internal standard RNA (≈378 and ≈399 bp, respectively).
Estrogen RIA. Estrogen levels were determined with a competitive RIA method. We mixed 200 μl of either serum or brain homogenate with 100 μl of antiestradiol serum. After an incubation of 4 h at 4°C, we added [125I]estradiol to this mixture, which was incubated for an additional 24 h at 4°C. Proteins were precipitated and then removed after centrifugation, and the radioactivity of the remaining supernatant was counted with a γ counter. The sensitivity of the estradiol RIA was 3.0 pg/ml.
BACE ELISA. The brain homogenate samples, including those used for Western blot analyses, were assayed in a double-blind manner. The BACE capture antibody used was affinity-purified SECB1 (1:1,000), and the detection antibody was biotin-labeled SECB2 (1:1,000). SECB1 and SECB2 are antibodies against two different N-terminal regions of human BACE (6). BACE-transfected cell lysates were used as positive controls, and nontransfected and empty-vector-transfected cell lysates were used as negative controls. The sensitivity of the BACE ELISA was 8 pg/ml.
BACE Enzymatic Activity Assays. Activity assays for BACE were performed by using synthetic peptide substrates containing either the APPwt BACE site [MCA-Glu-Val-Lys-Met-Asp-Ala-Glu-Phe-(Lys-DNP)-OH] or APPsw, in which Lys-Met are substituted with NL. In the MV mutant, M is substituted with V. Substrates were used at 50 mM, and reactions were performed in 50 mM [2-(N-morpholino)ethanesulfonic acid] with 50 mM acetic acid (pH 5.5). Enzymatic crude extracts and fluorescent-labeled peptides were incubated for various times at 37°C. The reaction mixtures were quenched and absorbed at 383 nm with a fluorescent plate reader.
Aβ ELISA. To quantify the levels of soluble Aβ, mouse brains were homogenized in 0.1 M carbonate/50 mM NaCl buffer, pH 11.5, containing 10 μg/ml leupeptin and 20 μg/ml aprotinin. Lysates were centrifuged at 16,000 × g and 4°C for 20 min, and the supernatants were used for measurement of soluble Aβ with an ELISA kit for human Aβ40 and Aβ42 (BioSource International, Camarillo, CA). The pellets were dissolved in 5 M guanidine (in 50 mM Tris·HCl, pH 8.0) for 4 h at room temperature and used for measurement of insoluble Aβ40 and Aβ42 with an ELISA kit, as above. The final values were normalized to the amount of loaded wet tissue and analyzed for significance by using the Student t test.
Statistics. Data were expressed as means ± SEM. Statistical analyses were performed with ANOVA followed by least significant difference post hoc analysis (multiple comparisons) or with an unpaired t test (pairwise comparisons). Pearson's correlation co-efficients were used for correlational analyses. The level of significance was set at P < 0.05.
Results
AD patients had lower Mini-Mental State Examination (Psychological Assessment Resources, Lutz, FL) scores than did age-matched, non-AD subjects (Table 1). Postmortem neuropathological examination also revealed that the brains of the AD patients displayed a significantly higher density of plaques than did those of the non-AD control subjects.
Table 1. Clinical and biochemical characteristics of the study subjects.
| Brain estradiol, pg/mg of protein
|
Serum estradiol, pg/ml
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | No. | Age, y | Education, y | MMSE score | Aβ density | Total | Free | Free index | Total | Free | Free index | Aromatase: β-actin ratio (× 10–3) |
| Non-AD | 10 | 84 ± 2.0 | 10.7 | 27.4 | 1.63 | 150.6 ± 14.3 | 44.4 ± 10.3 | 28.3 ± 4.8 | 8.3 ± 7.1 | 3.1 ± 2.5 | 13.5 ± 6.2 | 13.1 ± 3.5 |
| AD | 10 | 84 ± 1.89 | 10.6 | 5.7 | 13.25 | 60.0 ± 13.4 | 6.8 ± 1.6 | 11.9 ± 1.8 | 7.0 ± 3.8 | 2.1 ± 1.4 | 11.5 ± 5.4 | 4.5 ± 1.6 |
| P value | NS | NS | NS | 0.001 | 0.002 | 0.03 | 0.002 | 0.02 | NS | NS | NS | 0.019 |
MMSE, Mini-Mental State Examination; NS, not significant.
Reduced Brain Estrogen Synthesis in Female AD Patients. To determine whether local estrogen synthesis in the brain is a critical risk factor for AD, we measured 17β-estradiol levels in postmortem brain samples from 10 female AD patients and from 10 non-AD control subjects. We measured estrogen levels in frontal cortex and cerebellum lysates, because the frontal cortex is one brain region characteristically affected in AD, whereas the cerebellum is not. We also detected antemortem 17β-estradiol serum levels in AD patients and non-AD subjects. Both brain and serum 17β-estradiol were measured by RIA.
AD patients had significantly reduced levels of total and free brain estrogen (60% and 85%, respectively) compared with non-AD subjects (Table 1). These findings were consistent with our RT-PCR analysis of aromatase mRNA in the frontal cortex and cerebellum, which revealed a significant reduction in the expression of aromatase in AD brains compared with that in brains from non-AD subjects. Interestingly, serum estrogen levels of the AD patients were not significantly different from those of the non-AD subjects. We also found that estrogen levels in frontal cortex and cerebellum samples from AD patients did not differ significantly from each other (data not shown).
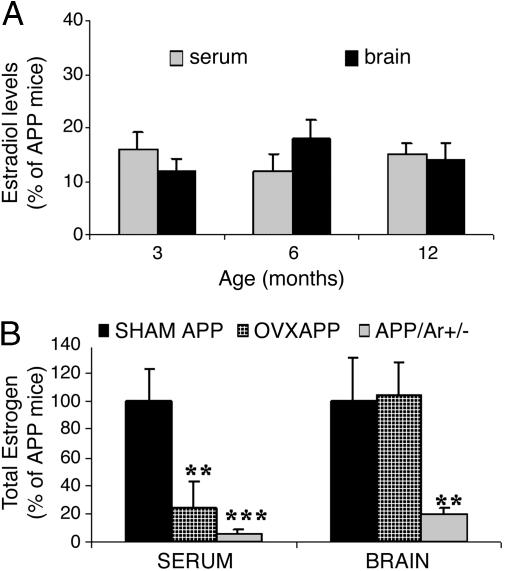
APP23 Mice with an Aromatase Gene Knockout (APP23/Ar+/-) Have Significantly Reduced Brain Estrogen. To further test the hypothesis that estrogen deficiency in the brain impacts AD pathogenesis, we examined estrogen levels in the brains and serum of estrogen-deficient transgenic mice harboring AD-like neuropathology. These APP23/Ar+/- mice were generated by crossbreeding aromatase knockout mice with APP23 transgenic mice (AD mouse model). Control groups consisted of WT mice and OVX, sham-operated (SHAM), and naive APP23 mice, the latter conditions allowing us to compare brain versus peripheral estrogen levels. The uteri of 6-month-old APP23/Ar+/- females were partially underdeveloped; otherwise, there were no significant phenotypic alterations observed. RIA revealed that brain and serum estrogen levels of APP23/Ar+/- mice were very low, ranging from 8% to 22% of those measured in the APP23 or SHAM APP control mice. Reduced estrogen levels were detected in APP23/Ar+/- mice as young as 3 months of age and as old as 12 months of age (Fig. 1A). There was no difference in the estrogen levels between APP23 and SHAM APP23 mice (Fig. 1B). In contrast to APP23/Ar+/- mice, estrogen levels in brain homogenates from OVX APP23 mice were not decreased, being comparable with those from SHAM APP23 mice (Fig. 1B). As expected, the serum estrogen levels of OVX APP23 mice were decreased, showing a 70-80% reduction of the levels of SHAM APP23 mice (Fig. 1B). There was no difference in estrogen levels (serum and brain) between APP23 and WT mice, regardless of age. These results indicate that serum estrogen levels of APP23/Ar+/- mice mirror those of OVX APP23 mice, but, unlike the OVX mice, APP23/Ar+/- mice have dramatically reduced brain estrogen levels.
Fig. 1.
Estrogen levels in mice. (A) Total 17β-estradiol was detected by RIA in the serum and brain from APP23/Ar+/- (n = 10) and APP23 (n = 10) mice at various ages. (B) Twelve APP23 mice were OVX or sham-operated at 3 months of age. By 6 months of age, levels of total 17β-estradiol were measured, in both serum and brain from APP23/Ar+/- (n = 10), OVX APP (n = 7), SHAM APP23 (n = 5), or naïve APP23 (n = 10) mice. *, P < 0.01; **, P < 0.001 (compared with naïve APP23 control mice).
Premature Amyloid Plaque Formation in APP23/Ar+/- Mice Is Associated with Brain Estrogen Deficiency. To examine whether brain estrogen deficiency affects onset and severity of amyloid-related pathogenesis, we examined amyloid plaques in different ages of APP23/Ar+/- mice. By 6 months of age, 30-40% of APP23/Ar+/- female mice had developed Aβ plaques in cerebral cortex and hippocampus (Fig. 2C). However, at this age, neither APP23 nor OVX APP23 mice had any sign of plaque formation (Fig. 2 A and B). By 12 months, APP23 and OVX APP23 mice had developed plaques, but plaque pathology was not as advanced as that of APP23/Ar+/- mice (Fig. 2 D-F). It is our impression that the size and density of plaques were qualitatively much greater in the APP23/Ar+/- mice. We observed no qualitative difference in plaque size and density between OVX and APP23 mice (Fig. 2 E and D), which is consistent with the findings of other studies (38, 39). Widely distributed Aβ plaques with or without a distinct core were observed in the hippocampus and cortex of APP23/Ar+/- mice (Fig. 2F).
Fig. 2.
The plaque formations in the brains of 6- and 12-month-old mice. Fourteen APP23 mice were OVX at 3 months of age. At age of 6 or 12 months, animals were killed and brains were prepared for immunohistochemistry. Shown is anti-Aβ (1-17) (6E10 clone antibody) immunostaining of sagittal brain sections from APP23 (n = 10; A and D), OVX APP23 (n = 7; B and E), and APP23/Ar+/- (n = 10; C and F) mice aged 6 (A-C) and 12 (D-F) months.
Together, our data suggest that brain estrogen deficiency accelerates Aβ plaque formation in a transgenic mouse model of AD. To our knowledge, this is first study to report that estrogen deficiency may cause premature plaque formation in brains susceptible to AD neuropathology.
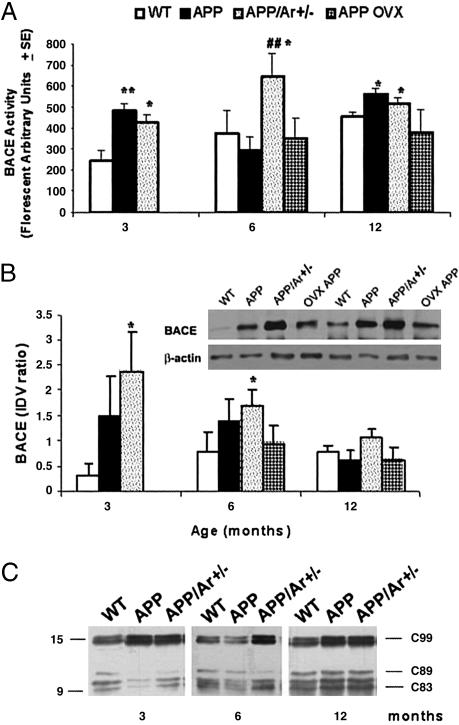
BACE Activity Is Elevated in the Brains of APP23/Ar+/- Mice. To determine whether estrogen depletion affects APP processing in vivo, we measured BACE protein levels, BACE enzyme activity, and Aβ levels in the frontal cortices of female WT, APP23, APP23/Ar+/-, and OVX APP23 mice. We found BACE activity to be elevated in APP23 mice compared with that in WT mice (Fig. 3A). BACE protein levels in APP23 mice, however, were not elevated. By contrast, in APP23/Ar+/- mice (aged 3 and 6 months), both BACE activity and protein levels were elevated, being much higher than those observed in APP23 and WT mice (Fig. 3 A and B). BACE activity remained elevated in APP23/Ar+/- mice as old as 12 months; however, BACE protein in these mice decreased to levels comparable with those measured in WT and APP23 mice (Fig. 3 A and B). No difference was observed in BACE protein levels of APP23 and APP23/Ar+/- mice, regardless of age (Fig. 3B). The estrogen-depletion-related increase in BACE activity we observed in APP23/Ar+/- mice was confirmed by Western blot analysis of frontal cortex lysates for C99, a fragment produced from the BACE-catalyzed cleavage of APP (Fig. 3C). As expected, we also observed increased steady-state levels of C99 in APP23/Ar+/- mice. In contrast, OVX APP animals (n = 7 per age group) showed no significant difference in BACE protein level and activity in the brain compared with APP mice at any age groups (Fig. 3 A and B).
Fig. 3.
Expression of BACE activity and protein in mice with age. At 3, 6, or 12 months of age, brain from APP23 (n = 10) and APP23/Ar+/- (n = 13) mice were prepared for enzyme activity and Western blot. The OVX APP mice (n = 14) were OVX at 3 months, and brains were harvested only at 6 or 12 months of age. (A) BACE activity was detected by detection of fluorescent-labeled peptides. (B) BACE protein levels were measured by Western blot by using monoclonal anti-BACE and were normalized according to those of a β-actin control. Bars indicate the mean ratio of BACE/β-actin integrated optical density values (IDV). (Inset) Western blot of 6-month-old mice. (C)Representative immunoblots were immunostained with APPC8, an antibody against the APP C-terminal fragment C99 that results from BACE cleavage of APP, in WT, APP23, and APP23/Ar+/- mice at various ages. *, P < 0.05; **, P < 0.01 (compared with WT mice). ##, P < 0.05 (compared with APP23 mice).
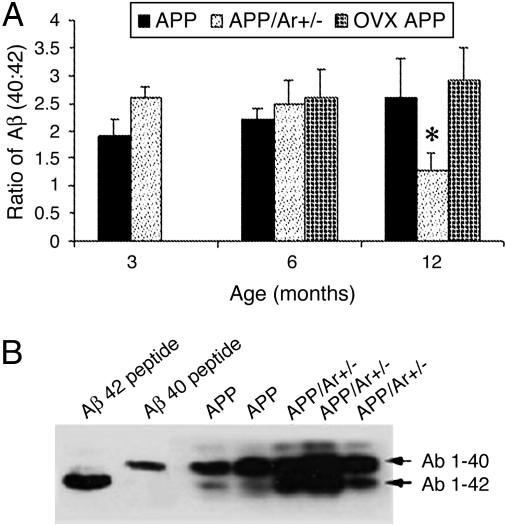
Because the deposition of Aβ42 is believed to play an important role in the formation of plaques in AD pathogenesis, we examined whether there was a specific relationship between BACE activity and protein levels and the deposition of different Aβ species in the brains of APP23/Ar+/- mice. We measured soluble and insoluble Aβ40 and Aβ42 using a newly developed Aβ ELISA (6). Combination urea gel/Western blot analyses revealed that the brains of both APP23 and APP23/Ar+/- mice contained significantly higher levels of formic acid-extracted Aβ than did those of WT mice (Fig. 4B). The brains of APP23/Ar+/- mice had higher levels of Aβ40 and Aβ42 than did APP23 mice, but this difference was not statistically significant. The ratio of Aβ40:Aβ42 calculated from APP23/Ar+/- mice decreased with age, indicating that relatively more Aβ42 was generated in older mice (Fig. 4A). These data suggest that the estrogen-deficiency-induced acceleration of plaque formation may be due to increased BACE activity, Aβ42 production, and Aβ aggregation. In OVX APP mice (n = 7 for each age group), there is an increase in Aβ 40 concentration, not in Aβ 42, and a slight increase in Aβ40:Aβ42 ratio at 12 months of age compared with APP mice (Fig. 4A).
Fig. 4.
Effect of genotypes on Aβ levels in the brain. (A) Ratio of Aβ40:Aβ42. Formic acid-extractable Aβ was measured in the mice. ELISA was used for detection of Aβ40:Aβ42 ratio in APP23 and APP23/Ar+/- mice. At various ages, brain tissues were harvested from APP23 (n = 10), APP23/Ar+/- (n = 13), and OVX APP (n = 7) mice and homogenized and prepared for ELISA as suggested by the manufacturer. Bars indicate the mean value for the experimental group. All values represent the average of four sets of duplicate determinations. *, P < 0.05 compared with APP23 mice. (B) Urea gel/Western blot analysis of the levels of Aβ40 and Aβ42 in APP23 (n = 8) and APP23/Ar+/- (n = 8) mice at 12 months of age. Synthetic peptide Aβ40 and Aβ42 were used as positive controls.
APP23/Ar+/- Mice Have Impaired Aβ Clearance/Degradation. In addition to examining Aβ production, we also investigated the effect of estrogen depletion on Aβ clearance by measuring microglia activation and phagocytotic activity. Table 2, which is published as supporting information on the PNAS web site, indicates estrogen levels in WT and Ar+/- mice at 6 months of age. Microglia isolated from Ar+/- and WT mice were incubated with fluorescent-labeled Aβ (Fluo-Aβ) for 3, 6, or 24 h. We found a decrease in Aβ clearance in microglia cultures from the Ar+/- mice after incubation with Fluo-Aβ for 6 or 24 h compared with cultures from WT mice (Fig. 5B, which is published as supporting information on the PNAS web site). However, the impaired Aβ clearance of Ar+/--derived microglia was reversed by adding 17β-estradiol (100 nM) into the culture medium and incubating for 24 h. An estrogen receptor antagonist, ICI 182,780 (10 μM), only partially blocked the action of estrogen (Fig. 5B). The estrogen-deficiency-induced impairment of Aβ clearance was further confirmed immunohistochemically. We observed fewer activated microglia surrounding plaques in cortex from APP23/Ar+/- mice than that from APP23 mice (Fig. 5A). Some Aβ fibrils appeared to be inside the microglia, but, because the cell membrane was not always visible, they may have been surrounded by the activated microglia. Taken together with our other results, it seems that the premature or accelerated Aβ plaque formation associated with estrogen deficiency might be related to an increase in Aβ production combined with decreased Aβ clearance.
To determine whether Aβ degradation is impaired in APP23/Ar+/- mice, we measured the expression of NEP and IDE, two Aβ-degrading enzymes, in the brains of these mice (n = 10 for each genotype). Western blot analyses revealed decreased IDE and NEP expression in 6-month-old APP23/Ar+/- mice as well as in 12-month-old ArKO mice (Fig. 5 C and D). IDE and NEP expression in WT and APP23 mice was not significantly different.
Discussion
The results presented here suggest that reduced brain estrogen production might be a risk factor for developing AD neuropathology. To our knowledge, this is the first report with direct experimental evidence establishing such an association. In support of this conclusion, we first demonstrated a robust reduction of brain 17β-estradiol levels in frontal cortex homogenates prepared from rapidly acquired postmortem brain tissue of women with AD. Examination of total 17β-estradiol levels also revealed that female AD patients had significantly reduced levels of free or biologically active estradiol. In the same AD and non-AD individuals, we found no difference in antemortem serum estrogen levels. Our data suggest the possibility of a brain-specific estrogen deficiency in AD patients. Because both total and free estrogen levels were low in brains from female AD patients, it is likely that local estrogen synthesis is impaired in the AD brain.
Although a recent clinical trial of estrogen-plus-progestin therapy failed to demonstrate any beneficial effect on treating AD symptoms (31), much evidence indicates that estrogen plays neuroprotective roles in the brain (29, 30). It has been suggested that postmenopausal women have a higher risk of developing AD and that the decline of circulating estrogen after menopause might underlie this increased risk. However, findings from previous studies of serum estrogen levels in AD have been controversial. Because of the great decline in the ovarian production of estrogen in menopausal women, other sites of estrogen biosynthesis become major sources of estrogen; these include adipose, skin, bone, and brain. Thus, circulating levels of estrogen in postmenopausal women might not represent estrogen action, because this estrogen originates in extragonadal sites where it acts locally, rather than directly by entering the circulation (40). Furthermore, because AD manifests itself primarily as a brain disease, local brain estrogen synthesis and brain estrogen levels might be more relevant in the search for the role of estrogen in disease. Thus, our demonstration of a brain estrogen deficiency in female AD patients, and the previously demonstrated neuroprotective effects of estrogen, suggest that further clinical trials of experimentally administered ERT are warranted as a possible AD treatment.
In the present study, we also investigated estrogen biosynthesis in cerebral cortex of AD and non-AD patients by measuring mRNA expression of aromatase, a key enzyme responsible for estrogen synthesis in the brain. We found a large reduction of aromatase mRNA levels in the brains of female AD patients compared with those of non-AD subjects. Moreover, there was a significant negative correlation between aromatase mRNA levels and amyloid plaque density, further strengthening the notion that local estrogen synthesis, and thus presumably estrogen activity, may be neuroprotective in the AD brain by interfering with plaque formation. This association remained after controlling for age and education level.
Extensive evidence indicates that aromatase may be critical for protecting neurons from death in various brain injuries through its increased induction, which results in the subsequent local conversion of androgen to estrogen at injured sites (33). Aromatase deficiency in effect may decrease the threshold for neurodegeneration (33). Furthermore, depletion or inhibition of aromatase can enhance various kinds of neurotoxin-induced damage to neurons (41). The present study investigates brain estrogen and aromatase mRNA expression in AD. Our mRNA expression data indicate that the low levels of brain estrogen in female AD patients may be due to impairment of aromatase expression. This finding is consistent with recent genetic studies suggesting that genetic variation in the brain aromatase gene may modify the risk for several diseases, including AD (41, 42). These, together with our present findings of low aromatase mRNA expression in AD brains, suggest that brain estrogen deficiency in postmenopausal women may disproportionately expose older women to the neurodegenerative processes underlying AD. Further genetic and molecular studies on estrogen synthesis in the AD brain will be needed to determine whether this is a direct link.
Seeking to understand this hypothesized link in more detail, we developed a transgenic animal model that overexpresses APP and lacks the estrogen synthesis enzyme aromatase. APP23/Ar+/- mice uniquely display significantly reduced levels of 17β-estradiol in both serum and brain compared with OVX APP23 mice, which display only reduced serum 17β-estradiol levels. In the present study, we showed that systemic, lifelong estrogen deficiency in APP23/Ar+/- mice is associated with early-onset plaque formation in the brains of these mice. APP23/Ar+/- mice as young as 6 months displayed distinct amyloid plaques in frontal cortex. Plaques eventually developed in the hippocampus at a later age. In contrast, OVX APP23 mice did not display plaques until they reached 9 months of age or older, which is consistent with other reports (38, 39). Taken together, these results suggest that brain estrogen deficiency accelerates Aβ plaque formation. These results also show that APP23/Ar+/- mice provide a unique in vivo system for examining the effects of brain estrogen depletion on amyloid neuropathology.
Recent findings have suggested that BACE plays a critical role in AD neuropathogenesis (43). Consistent with these are our observations that BACE protein expression and enzyme activity, as well as Aβ40 and Aβ42 levels, are elevated in the brains of APP23/Ar+/- mice. APP23/Ar+/- mice as young as 6 months displayed a significant increase in BACE activity; this increase was correlated with increased Aβ levels and the early appearance of plaques in the brains of these mice. These results suggest that the early-onset AD neuropathology in APP23/Ar+/- mice associated with brain estrogen deficiency may be mediated by increased BACE activity and accelerated Aβ production.
A number of studies have demonstrated that aggregated Aβ is removed from extracellular compartments by microglia (7, 44). In vivo multiphoton imaging has demonstrated that activated microglia are involved in the clearance of Aβ plaques (44), which is consistent with a growing body of literature that associates the activation of microglia with a reduction in Aβ deposition in transgenic mouse models (45). Activated microglia play an important role in local cell-mediated immunity (e.g., phagocytosis) and are also potent sources of reactive oxygen and other molecules that may be responsible for damaging neurons in various neurodegenerative diseases (46). Indeed, we observed that microglia-associated clearance of Aβ was greatly reduced in estrogen-deficient aromatase knockout mice, further supporting our hypothesis that the accelerated amyloid plaque formation we observed in the present study may, in part, be due to the estrogen-depletion-associated accumulation of Aβ.
Extracellular Aβ aggregates have also been shown to be degraded by IDE and NEP in vivo (10, 11). In the present study, we found decreased IDE and NEP levels in the brains of APP23/Ar+/- mice. The reduction of IDE at the age of 6 months is correlated with the early plaque formation in the APP/Ar+/- mice. Although there is no clear explanation for the reduction of IDE and NEP in these mice, our observation is consistent with the findings that NEP and IDE levels are reduced in AD brains (18, 19). The formation of plaques in our APP23/Ar+/- mice is also consistent with the finding that IDE and NEP protein levels and activity are reversely correlated with amyloid plaque burden (14, 18, 19). In addition, recent studies found that ERT reduced NEP activity in rat brain (22) and that the quantity and activity of IDE were decreased in rat uterus when OVX and increased after treated with estradiol (47). These studies suggest that estradiol may be important factor for the expression and regulation of NEP and IDE in the brain and uterus.
Taken together, our findings indicate that the combination of overproduction of Aβ and the impairment of Aβ clearance at an early age might be critical for the AD-like amyloid neuropathology in the APP/Ar+/- mice.
Supplementary Material
Acknowledgments
We thank Dr. Tom Beach and Mrs. Lucia Sue for their help with the human brain bank samples and information. We are also very grateful to Dr. Dennis Selkoe for the APPC8 antibody and to Drs. Harry Orr, Joseph Rogers, and Sangram S. Sisadia for helpful comments on the manuscript. This work was funded in part by the Alzheimer's Association, the National Alliance for Research On Schizophrenia and Depression, and the Arizona Disease Control Research Commission.
Author contributions: Y.S. and R.L. designed research; X.Y., M.L., T.L., P.C., Z.Z., and R.L. performed research; S.-I.H., N.H., and M.S. contributed new reagents/analytic tools; X.Y., M.L., and R.L. analyzed data; and R.L. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AD, Alzheimer's disease; APP, amyloid precursor protein; BACE, β-secretase; NEP, neprilysin; IDE, insulin-degrading enzyme; OVX, ovariectomized; Aβ, β amyloid peptide; ERT, estrogen-replacement therapy.
References
- 1.Evans, D. A., Funkenstein, H. H., Albert, M. S., Scherr, P. A., Cook, N. R., Chown, M. J., Hebert, L. E., Hennekens, C. H. & Taylor, J. O. (1989) J. Am. Med. Assoc. 262, 2551-2556. [PubMed] [Google Scholar]
- 2.Haass, C. & De Strooper, B. (1999) Science 286, 916-919. [DOI] [PubMed] [Google Scholar]
- 3.Vassar, R., Bennett, B. D., Babu-Khan, S., Kahn, S., Mendiaz, E. A., Denis, P., Teplow, D. B., Ross, S., Amarante, P., Loeloff, R., et al. (1999) Science 286, 735-741. [DOI] [PubMed] [Google Scholar]
- 4.Dewachter, I., van Dorpe, J., Spittaels, K., Tesseur, I., Van Den Haute, C., Moechars, D. & Van Leuven, F. (2000) Exp. Gerontol. 35, 831-841. [DOI] [PubMed] [Google Scholar]
- 5.Holsinger, R. M., McLean, C. A., Beyreuther, K., Masters, C. L. & Evin, G. (2002) Ann. Neurol. 51, 783-786. [DOI] [PubMed] [Google Scholar]
- 6.Li, R., Lindholm, K., Yang, L. B., Yue, X., Citron, M., Yan, R., Beach, T., Sue, L., Sabbagh, M., Cai, H., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 3632-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li, R., Shen, Y., Yang, L. B., Lue, L. F., Finch, C. & Rogers, J. (2000) J. Neurochem. 75, 1447-1454. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi, H., Sugihara, S., Ogawa, A., Saido, T. C. & Ihara, Y. (1998) Acta Neuropathol. 95, 217-222. [DOI] [PubMed] [Google Scholar]
- 9.Wyss-Coray, T., Loike, J. D., Brionne, T. C., Lu, E., Anankov, R., Yan, F., Silverstein, S. C. & Husemann, J. (2003) Nat. Med. 9, 453-457. [DOI] [PubMed] [Google Scholar]
- 10.Qiu, W. Q., Walsh, D. M., Ye, Z., Vekrellis, K., Zhang, J., Podlisny, M. B., Rosner, M. R., Safavi, A., Hersh, L. B. & Selkoe, D. J. (1998) J. Biol. Chem. 273, 32730-32738. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee, A., Song, E., Kihiko-Ehmann, M., Goodman, J. P., Jr., Pyrek, J. S., Estus, S. & Hersh, L. B. (2000) J. Neurosci. 20, 8745-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertram, L., Blacker, D., Mullin, K., Keeney, D., Jones, J., Basu, S., Yhu, S., McInnis, M. G., Go, R. C., Vekrellis, K., et al. (2000) Science 290, 2302-2303. [DOI] [PubMed] [Google Scholar]
- 13.Ertekin-Taner, N., Graff-Radford, N., Younkin, L. H., Eckman, C., Baker, M., Adamson, J., Ronald, J., Blangero, J., Hutton, M. & Younkin, S. G. (2000) Science 290, 2303-2304. [DOI] [PubMed] [Google Scholar]
- 14.Leissring, M. A., Farris, W., Chang, A. Y., Walsh, D. M., Wu, X., Sun, X., Frosch, M. P. & Selkoe, D. J. (2003) Neuron 40, 1087-1093. [DOI] [PubMed] [Google Scholar]
- 15.Iwata, N., Tsubuki, S., Takaki, Y., Watanabe, K., Sekiguchi, M., Hosoki, E., Kawashima-Morishima, M., Lee, H. J., Hama, E., Sekine-Aizawa, Y. & Saido, T. C. (2000) Nat. Med. 6, 143-150. [DOI] [PubMed] [Google Scholar]
- 16.Iwata, N., Tsubuki, S., Takaki, Y., Shirotani, K., Lu, B., Gerard, N. P., Gerard, C., Hama, E., Lee, H. J. & Saido, T. C. (2001) Science 292, 1550-1552. [DOI] [PubMed] [Google Scholar]
- 17.Marr, R. A., Rockenstein, E., Mukherjee, A., Kindy, M. S., Hersh, L. B., Gage, F. H., Verma, I. M. & Masliah, E. (2003) J. Neurosci. 23, 1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasojima, K., Akiyama, H., McGeer, E. G. & McGeer, P. L. (2001) Neurosci. Lett. 297, 97-100. [DOI] [PubMed] [Google Scholar]
- 19.Yasojima, K., McGeer, E. G. & McGeer, P. L. (2001) Brain. Res. 919, 115-121. [DOI] [PubMed] [Google Scholar]
- 20.Iwata, N., Takaki, Y., Fukami, S., Tsubuki, S. & Saido, T. C. (2002) J. Neurosci. Res. 70, 493-500. [DOI] [PubMed] [Google Scholar]
- 21.Shen, R., Sumitomo, M., Dai, J., Hardy, D. O., Navarro, D., Usmani, B., Papandreou, C. N., Hersh, L. B., Shipp, M. A., Freedman, L. P. & Nanus, D. M. (2000) Mol. Cell. Endocrinol. 170, 131-142. [DOI] [PubMed] [Google Scholar]
- 22.Huang, J., Guan, H., Booze, R. M., Eckman, C. B. & Hersh, L. B. (2004) Neurosci. Lett. 367, 85-87. [DOI] [PubMed] [Google Scholar]
- 23.Baum, L. W. (2005) J. Gerontol. A Biol. Sci. Med. Sci. 60, 736-743. [DOI] [PubMed] [Google Scholar]
- 24.Canadian Study of Health and Aging Work Group. (1994) Can. Med. Assoc. J. 150, 899-913. [PMC free article] [PubMed] [Google Scholar]
- 25.Hy, L. X. & Keller, D. M. (2000) Neurology 55, 198-204. [DOI] [PubMed] [Google Scholar]
- 26.Andersen, K., Launer, L. J., Dewey, M. E., Letenneur, L., Ott, A., Copeland, J. R., Dartigues, J. F., Kragh-Sorensen, P., Baldereschi, M., Brayne, C., et al. (1999) Neurology 53, 1992-1997. [DOI] [PubMed] [Google Scholar]
- 27.Coffey, C. E., Lucke, J. F., Saxton, J. A., Ratcliff, G., Unitas, L. J., Billig, B. & Bryan, R. N. (1998) Arch. Neurol. 55, 169-179. [DOI] [PubMed] [Google Scholar]
- 28.Atwood, C. S., Meethal, S. V., Liu, T., Wilson, A. C., Gallego, M., Smith, M. A. & Bowen, R. L. (2005) J. Neuropathol. Exp. Neurol. 64, 93-103. [DOI] [PubMed] [Google Scholar]
- 29.Xu, H., Gouras, G. K., Greenfield, J. P., Vincent, B., Naslund, J., Mazzarelli, L., Fried, G., Jovanovic, J. N., Seeger, M., Relkin, N. R., et al. (1998) Nat. Med. 4, 447-451. [DOI] [PubMed] [Google Scholar]
- 30.Paganini-Hill, A. & Henderson, V. W. (1996) Arch. Intern. Med. 156, 2213-2217. [PubMed] [Google Scholar]
- 31.Henderson, V. W. (1997) Neurology 48, S27-S35. [DOI] [PubMed] [Google Scholar]
- 32.Shumaker, S. A., Legault, C., Rapp, S. R., Thal, L., Wallace, R. B., Ockene, J. K., Hendrix, S. L., Jones, B. N., III, Assaf, A. R., Jackson, R. D., et al. (2003) J. Am. Med. Assoc. 289, 2651-2662. [DOI] [PubMed] [Google Scholar]
- 33.Labrie, F., Belanger, A., Cusan, L., Gomez, J. L. & Candas, B. (1997) J. Clin. Endocrinol. Metab. 82, 2396-2402. [DOI] [PubMed] [Google Scholar]
- 34.Zwain, I. H. & Yen, S. S. (1999) Endocrinology 140, 3843-3852. [DOI] [PubMed] [Google Scholar]
- 35.Azcoitia, I., Sierra, A., Veiga, S., Honda, S., Harada, N. & Garcia-Segura, L. M. (2001) J. Neurobiol. 47, 318-329. [DOI] [PubMed] [Google Scholar]
- 36.Sturchler-Pierrat, C., Abramowski, D., Duke, M., Wiederhold, K. H., Mistl, C., Rothacher, S., Ledermann, B., Burki, K., Frey, P., Paganetti, P. A., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 13287-13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honda, S., Harada, N., Ito, S., Takagi, Y. & Maeda, S. (1998) Biochem. Biophys. Res. Commun. 252, 445-449. [DOI] [PubMed] [Google Scholar]
- 38.Cheepsunthorn, P., Radov, L., Menzies, S., Reid, J. & Connor, J. R. (2001) Glia 35, 53-62. [DOI] [PubMed] [Google Scholar]
- 39.Zheng, H., Xu, H., Uljon, S. N., Gross, R., Hardy, K., Gaynor, J., Lafrancois, J., Simpkins, J., Refolo, L. M., Petanceska, S., et al. (2002) J. Neurochem. 80, 191-196. [DOI] [PubMed] [Google Scholar]
- 40.Green, P. S., Bales, K., Paul, S. & Bu, G. (2005) Endocrinology 146, 2774-2781. [DOI] [PubMed] [Google Scholar]
- 41.Simpson, E. R. (2003) J. Steroid Biochem. Mol. Biol. 86, 225-230. [DOI] [PubMed] [Google Scholar]
- 42.McCullough, L. D., Blizzard, K., Simpson, E. R., Oz, O. K. & Hurn, P. D. (2003) J. Neurosci. 23, 8701-8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iivonen, S., Corder, E., Lehtovirta, M., Helisalmi, S., Mannermaa, A., Vepsalainen, S., Hanninen, T., Soininen, H. & Hiltunen, M. (2004) Neurology 62, 1170-1176. [DOI] [PubMed] [Google Scholar]
- 44.Yang, L. B., Lindholm, K., Yan, R., Citron, M., Xia, W., Yang, X. L., Beach, T., Sue, L., Wong, P., Price, D., et al. (2003) Nat. Med. 9, 3-4. [DOI] [PubMed] [Google Scholar]
- 45.Bacskai, B. J., Kajdasz, S. T., McLellan, M. E., Games, D., Seubert, P., Schenk, D. & Hyman, B. T. (2002) J. Neurosci. 22, 7873-7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyss-Coray, T., Lin, C., Yan, F., Yu, G. Q., Rohde, M., McConlogue, L., Masliah, E. & Mucke, L. (2001) Nat. Med. 7, 612-618. [DOI] [PubMed] [Google Scholar]
- 47.Udrisar, D. P., Wanderley, M. I., Porto, R. C., Cardoso, C. L., Barbosa, M. C., Camberos, M. C. & Cresto, J. C. (2005) Exp. Biol. Med. 230, 479-486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.