Abstract
We find that CD11c+ cells with many markers of dendritic cells (DCs) are a major cell type in the skin lesions of psoriasis. These CD11c+ cells, which are evident in both epidermis and dermis, are the sites for the expression of two mediators of inflammation, inducible nitric oxide synthase (iNOS) and TNF-α in diseased skin. These cells express HLA-DR, CD40, and CD86, lack the Langerin and CD14 markers of Langerhans cells and monocytes, respectively, and to a significant extent express the DC maturation markers DC-LAMP and CD83. Treatment of psoriasis with efalizumab (anti-CD11a, Raptiva) strongly reduces infiltration by these DCs in patients responding to this agent. Disease activity after therapy was more related to DC infiltrates and iNOS mRNA levels than T cell infiltrates, and CD11c+ cells responded more quickly to therapy than epidermal keratinocytes. Our results suggest that a type of DC, which resembles murine “Tip-DCs” that can accumulate during infection, has proinflammatory effects in psoriasis through nitric oxide and TNF-α production, and can be an important target for suppressive therapies.
Keywords: autoimmune disease, CD11c, Tip-DC
The common skin disease psoriasis serves as an accessible, model type 1 autoimmune disease (1-3). Evidence for this view includes (i) a marked increase in the number of T cells in diseased skin, (ii) the differentiation characteristics of these T cells, including a skin-homing memory phenotype, a T helper 1/Tc1 predominance, and oligoclonality, and (iii) the disease improvements brought about by a variety of T cell-targeted immunosuppressive agents (4-6). T cell functions are often controlled by dendritic cells (DCs), and interestingly, psoriasis is characterized by the production of IL-23 by CD11c+ DCs in lesional skin (7). IL-23 is known to be a pivotal cytokine for inflammation during experimental autoimmune encephalomyelitis in mice (8, 9).
To study the contribution and source of inflammatory molecules in the pathogenesis of psoriasis (2), we have engaged in detailed studies of disease resolution induced by specific biologic antagonists. We have emphasized inflammatory mediators associated with CD11c+ cells, where high-level expression of this integrin is a marker for many types of DCs (10, 11). We now report that CD11c+ cells lacking the monocyte marker CD14 are greatly increased in the dermis and epidermis of psoriasis lesions, such that CD11c+ cells exceed T cells. Two critical mediators of inflammation, TNF and inducible nitric oxide synthase (iNOS), are simultaneously and primarily found in these CD11c+ cells, which express HLA-DR, CD40 CD86, and to some extent, DC maturation markers DC-LAMP and CD83. Successful treatment of psoriasis with efalizumab (5), which targets CD11a on leukocytes, strongly reduces infiltration by these inflammatory DCs, which are similar to a cell type recently described in the mouse: the TNF- and iNOS-producing DC or “Tip-DC” (12-14). Given recent evidence that TNF inhibitors are able to reverse disease activity in psoriasis (15, 16), our data suggest that Tip-DCs are a major inflammatory and effector cell in psoriasis.
Materials and Methods
Skin Samples. Psoriasis lesions, nonlesional skin, and normal skin were obtained from patients and normal volunteers under an approved protocol (The Rockefeller University). Experiments were conducted on samples obtained from a previously published placebo-controlled clinical trial with efalizumab (n = 65) (Genentech and Xoma) (5). Patients treated with efalizumab showed greater clinical improvement and reduction of epidermal hyperplasia in skin lesions compared to placebo treatment. We consider that thin epidermis without keratin 16 (K16) expression represents histopathologic remission of psoriasis. None of the patients in the placebo group were K16- at day 56, whereas 29 patients (37%) in the treatment arm were K16- at the end of the study. Samples from other clinical trials with efalizumab (weekly 1 mg/kg s.c. for 12 weeks) were used to measure iNOS mRNA (n = 13, see Fig. 4B), and a time-course of CD11c+ cell counts (n = 18, see Fig. 4D).
Fig. 4.
Changes in number of CD11c and CD83 positive cells, as well as iNOS mRNA expression, correlate highly with disease improvement. (A) Correlation between response score and changes in DCs (CD11c+ and CD83+) and T cells (CD3+)(u score) between day 0 and 56 for patients in the efalizumab-treated group. Correlation coefficients (r) are shown. (B) Correlation between response score and change in iNOS mRNA in lesional tissue between day 0 and 56 (r = 0.83). (C) In vitro monocyte-derived DCs produce iNOS mRNA. INOS/HARP mRNA was measured by RT-PCR in lymphocytes (L), T cells activated by CD3/CD28 (ActT), monocytes (M), adherent monocytes (AdM), immature DCs (iDC), mature dendritic cells (mDC), and HaCaT cells (HC). (D) Dermal CD11c+ cell counts/low power field (straight black line) and epidermal thickness (dotted black line) during therapy. During the first 2 weeks of treatment, there is 50% reduction in dermal CD11c+ cells (*, P = 0.006) and a 14% reduction in epidermal thickness (not significant). **, CD11c+ cell counts and epidermal thickness at weeks 6 and 12 compared to baseline, P < 0.0001.
Antibodies. For immunohistochemistry, we used mouse anti-human monoclonal antibodies to K16 (Sigma), CD3 (Becton Dickinson), CD8 (BD PharMingen), CD83 (Becton Dickinson), CD1a (Becton Dickinson), CD11c (BD PharMingen), iNOS (R & D Systems) and CD14 (BD PharMingen). For immunofluorescence, we localized CD11c (BD PharMingen), DC-LAMP (Immunotech, Westbrook, ME), CD83 (Immunotech) (1:50-1:100) with appropriate IgG goat anti-mouse Ig conjugated to Alexa Fluor 488 or 546 (1:250) (Molecular Probes). The second primary antibodies for two-color labeling were conjugated to the fluorochrome or labeled by using the appropriate labeling kit (Molecular Probes) (1:100-1:500): iNOS (R & D Systems) Alexa Fluor 546, TNF-α FITC (Becton Dickinson), HLA-DR FITC (Becton Dickinson), HLA-DR phycoerythrin (PE) (Becton Dickinson), Langerin (Immunotech) Alexa Fluor 488. The TNF signal was amplified with a second secondary goat anti-FITC antibody (Molecular Probes) for 30 min at 1 μg/ml. Antibodies for FACS include the following: mouse IgG1 FITC (Becton Dickinson), mouse IgG1 PE (Becton Dickinson), mouse IgG1 peridinin-chlorophyll-protein (PerCP) (Becton Dickinson), HLA-DR allophycocyanin (Becton Dickinson), CD3 PerCP (Becton Dickinson), CD83 FITC (Immunotech), CD86 FITC (BD PharMingen), CD40 FITC (BD PharMingen), CD14 FITC (Becton Dickinson), and CD11c PE (Becton Dickinson).
Tissue Sections. Skin biopsies were frozen in optimal cutting temperature compound (Sakura Finetek, Tokyo), stored at -80°C, stained with hematoxylin (Fisher Scientific, Fair Lawn, NJ) and eosin (Shandon, Pittsburgh), or with mouse anti-human monoclonal antibodies as above using a published technique (17). Data for epidermal thickness and K16 staining are taken from all patients, whereas the rest of the antibodies in the panel (CD1a, CD11c, CD83, CD3, and CD8) were applied to 42 subjects in the active drug group and 26 subjects in the placebo group because of a limited number of tissue sections. For immunofluorescence, frozen lesional tissue sections from psoriasis patients (n = 8) were fixed in acetone and treated with 10% normal horse serum. Primary antibodies were incubated overnight at 4°C, the secondary antibody for 30 min, and the second primary antibody for 2 h. Images were acquired by using appropriate filters of a Zeiss Axioplan 2I microscope with Plan Apochromat 20 × 0.7 numerical aperture lens and a Hagamatsu orca ER-cooled charge-coupled device camera, controlled by metavue software (Universal Imaging). Alternatively, images were acquired by using appropriate filters of an upright confocal microscope with attached Zeiss 5 Fluar/0.25 and 10 Fluar/0.50 lenses controlled by least squares means analysis.
FACS. Skin shave biopsies were obtained from two psoriasis patients, and dermal cells allowed to emigrate in culture according to a published protocol (18). Shave biopsies are small superficial (split thickness) skin biopsies that give the largest surface area for efficient emigration of DCs. Briefly, the skin was cultured in dispase overnight, epidermis and dermis were separated, then dermis was cultured for 3 days, and the dermal supernatant was treated with collagenase for 1 h. FACS was performed with antibodies listed above, as described (19). A provisional DC gate (R1) was selected based on large cell size (FSC) and high side scatter (SCC) (see Fig. 2E) (20), and cells in this gate were further selected for CD11c expression (R2).
Fig. 2.
CD11c identifies a type of DC. (A-D) Double-label immunofluorescence. (Insets) Single-color staining controls. (A) CD11c+ cells are all HLA-DR+.(B and C) Some iNOS+ cells coproduce DC-LAMP and CD83. DC-LAMP and iNOS+ cells are mature dermal DCs. (D) TNF+ cells are predominantly HLA-DR+. (E-G) FACS of dermal emigrants from a psoriatic patient. (E) R1, cells gated based on large cell size (FSC) and high side scatter (SCC); R2, CD11c-positive cells. (F) HLA-DR versus IgG1, CD86, CD40, CD83 and CD14 expression (R1+R2). Percent double-positive cells is shown in the upper right quadrant. (G) A lymphocyte gate (R3) indicates CD3-positive cells that have low CD11c and CD83 staining.
Analysis of iNOS Gene Expression. DCs were prepared and analyzed by quantitative PCR as described (6, 21). Monocytes or T cells were isolated by negative selection (Dynal, Oslo), and activated with CD3/CD28 beads (Dynal). HaCaT cells were grown in Dulbecco's modified eagle medium (R & D Systems).
Statistical Analysis. Significance was defined as P < 0.05 using a Student's t test. U scores and correlation coefficients were computed based on a recently published technique to analyze multivariate data (22).
Results
INOS and TNF Double-Positive CD11c+ Cells Are Greatly Increased in Psoriasis Lesions. Expression of iNOS mRNA is typically elevated by >10-fold in psoriasis lesions (4-6), and iNOS has been detected in psoriasis lesions (23). Because a product of iNOS, nitric oxide, is a key inflammatory mediator (24), we first set out to localize iNOS-producing cells in normal skin versus psoriasis lesions. Normal skin contained only a small number of iNOS+ cells in the dermis (Fig. 1A), but abundant iNOS+ cells were noted in both the epidermis and dermis of psoriasis lesions (Fig. 1B). In parallel, CD11c+ cells had a similar distribution in both normal and diseased skin (Fig. 1 C and D). The iNOS+ cells were large and stellate, with numerous dendritic processes (Fig. 5A, which is published as supporting information on the PNAS web site). Two-color immunofluorescence verified that iNOS was primarily localized to the CD11c+ cells (Fig. 1E). Moreover, TNF was also expressed almost exclusively in these CD11c+ cells (Fig. 1F), which failed to express the Langerhans cell markers, Langerin, (CD207) (Fig. 1D) or CD1a (data not shown), or the monocyte marker CD14 (Fig. 5B). These data indicate that the lesions of psoriasis contain numerous CD11c+ cells that coexpress two key inflammatory mediators, iNOS and TNF, but are not typical Langerhans cells or monocytes.
Fig. 1.
iNOS and TNF double-positive CD11c+ cells are greatly increased in psoriasis lesions. Single- and double-label immunofluorescence are shown. (Scale bar, 200 μm.) (Insets) Single color staining controls. (A) iNOS+ cells in the dermis of normal skin. (B) iNOS+ cells in the epidermis and dermis of psoriasis skin. (C) CD11c+ cells in the dermis of normal skin. (D) CD11c+ cells in the epidermis and dermis of psoriasis skin. No overlap with Langerin positive cells in the epidermis in C and D.(E and F) There is nearly complete overlap between CD11c and iNOS, and CD11c and TNF.
CD11c Identifies a Type of Dendritic Cell. We carried out additional two-color immunofluorescence studies to characterize the CD11c+ cells. They were larger cells and all strongly HLA-DR positive (Fig. 2A). Some of the iNOS producers also expressed markers of maturing DCs, DC-LAMP and CD83 (Fig. 2 B and C), and TNF+ cells were predominantly HLA-DR+ (Fig. 2D). To confirm that CD11c was primarily marking DCs, we performed more sensitive flow-cytometric analyses of cells from psoriasis lesions. The large, CD11c+ cells could be classified mainly as maturing DCs, because 95% were CD86+, 86% were CD40+, and 63% were CD83+ (Fig. 2F). Only a small percentage of CD11c+ cells were CD14+ (12%), and these cells were HLA-DRmid-low and probably best classified as monocytes. In contrast, the CD3+ cells in the lesions were primarily found in the small cell fraction (R3), and these cells failed to express CD11c and CD83 (Fig. 2G). Because DCs mature when emigrating from skin in vitro, the FACS experiments are likely to indicate that a high fraction of mature CD11c+ DCs are in the emigrating population. Ongoing functional studies indicate that DCs emigrating from the dermis of psoriatic skin are strongly stimulatory for proliferative responses in autologous blood T cells, a functional property of DCs (25).
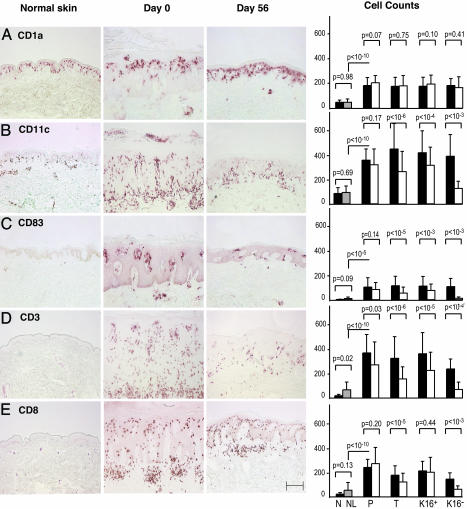
CD11c+ DCs Are the Major Type of Leukocyte in Psoriatic Skin. Different types of leukocytes were then quantified by immunohistochemical staining, including the mean number of cells/low power field expressing CD1a, CD11c, CD83, CD3, and CD8. We compared normal human skin and psoriatic lesions before and during efalizumab therapy (anti-CD11a) (Fig. 3) (5). Perhaps the most surprising alterations in the leukocytes of psoriatic skin lesions were the CD11c+ and CD83+ DCs (Fig. 3 B and C). Normal human skin or uninvolved psoriatic skin contained similar numbers of CD11c+ DCs (83 and 91 cells per field, respectively), which were exclusively localized in the dermis. In contrast in psoriasis, there was a large increase in CD11c+ cells (411 cells per field, P < 10-10 compared to normal skin), and nearly half (44%) of these CD11c+ cells were found in the epidermis, mainly in the lower portions and along the dermo-epidermal junction. CD83+ DCs were infrequent in normal skin (zero to five CD83+ cells per field in normal skin), but increased to 108 CD83+ DCs in active psoriasis lesions (P < 10-5). The overall number of CD11c+ cells in psoriatic skin actually exceeded the number of CD3+ T cells.
Fig. 3.
CD11c+ DCs are significantly decreased after efalizumab (anti-CD11a) treatment. Histomicrographs and cell counts of skin biopsies from a normal control and a treatment group patient (responder, K16 negative), on days 0 and 56. (Scale bar, 200 μm.) Mean number of total cells per low power field (10×) for CD1a (A), CD11c (B), CD83 (C), CD3 (D), and CD8 (E) (±SD) in normal skin (N, black), nonlesional psoriasis skin (NL, gray), and lesional skin of patients in placebo (P), treatment (T; all patients), K16+ (non- and partial responders), and K16- (high responders) groups on days 0 (filled squares) and 56 (open squares). P values are shown for the indicated comparison.
CD11c+ DCs Are Significantly Decreased After Efalizumab (Anti-CD11a) Treatment. Efalizumab treatment had a major impact on skin infiltration by CD11c+ and CD83+ DC populations. Overall, a 41% mean reduction in CD11c+ cells was measured in patients treated with efalizumab (P < 10-6), whereas placebo treatment produced no significant reduction. The reductions observed in K16- (high responder) patients was even greater, averaging 68% for CD11c+ DCs, and 89% when the final number of DCs was corrected for numbers present in nonlesional skin. CD83+ DCs were impacted even more by efalizumab treatment, as a 90% reduction in mature DCs was observed in high responders, with a 98% reduction when corrected by nonlesional skin cell counts. There was a mean of 49 CD1a+ (Langerhans) cells per field in normal skin, 183 lesional CD1a+ cells per field, and no significant reduction in number of CD1a+ cells after efalizumab treatment. These cells were evenly distributed throughout the epidermis of normal and uninvolved skin, often concentrated in the upper spinous layer in lesional psoriatic epidermis, and restored to normal with therapy. Highly significant decreases in CD3+ and CD8+ cells were observed in efalizumab-treated patients (Fig. 3 D and E). Total CD3+ cells declined by 47% in the patients who received efalizumab (P < 10-6), and by 70% in K16- (high responders) (P < 10-4). These results indicate that CD11c+ cells, and not just T cells, are a major responsive element in successful CD11a-based therapy of psoriasis.
Changes in Number of CD11c+ and CD83+ Cells, as Well as iNOS mRNA Expression, Correlate Highly with Disease Improvement. We used efalizumab as a disease-modulating agent in these studies because it is a leukocyte-specific antibody, and both DCs and T cells express LFA-1. A recently developed statistical approach was used to compare disease improvement in individual patients to changes in specific leukocyte subsets (DCs and T cells) (22). The amount of psoriasis disease activity at the end of treatment was quantified as a “response score” (Fig. 4), a composite U score derived from measurement of epidermal thickness and K16 expression (positive or negative) on day 56. As shown in Fig. 4A, disease improvement (response score) could be related to changes in T cells or DCs. However, the CD11c+ population showed the highest correlation with the response score (r = 0.62), whereas the correlation with T cell changes (CD3+) was relatively poor (r = 0.32). Changes in CD83+ DCs were also better correlated with response (r = 0.52) than T cells. We also examined the degree to which disease improvement during efalizumab treatment was related to altered expression of iNOS mRNA in skin lesions. As shown in Fig. 4B, response score was highly related to reduction in iNOS mRNA in individual patients (r = 0.83).
Therefore, we confirmed that myeloid (ex vivo peripheral blood monocyte-derived) DCs can synthesize high levels of iNOS mRNA by using real-time RT-PCR (Fig. 4C). Immature DCs were able to express iNOS, but the highest level of iNOS was measured in mature (CD83+) DCs, which were differentiated in vitro by using a cytokine mixture (21). In comparison, resting T cells, activated T cells, blood monocytes, and a keratinocyte cell line (HaCaT cells) produced very little iNOS mRNA. We then assessed whether CD11c+ cells might be reduced before clinical improvement. As shown in Fig. 4D, during the first 2 weeks of efalizumab treatment, there was a 50% reduction in lesional dermal CD11c+ cells (P = 0.006), but only a 14% reduction in epidermal thickness (an indicator of clinical response). The early kinetics of reduction in CD11c+ cells show that these cells are decreased before thinning of the epidermis and clinical improvement occurs.
Discussion
Two important mediators of inflammation, iNOS and TNF, are known to participate in the lesions of psoriasis (23, 26), and TNF blockade is an emerging therapy for this disease (15, 16). We now find that the iNOS enzyme and the TNF cytokine are abundant in a CD11c+ cell in lesional dermis and epidermis. These CD11c+ cells are the principal reservoir for these two mediators of inflammation and actually outnumber T cells in psoriatic lesions.
Our tissue and FACS staining data suggest that the abundant CD11c+ iNOS- and TNF-producing cells in psoriasis are DCs, and similar to the Tip-DCs seen in mice (12-14), except that expression of CD11b is lacking on human cells (unpublished data). Murine Tip-DCs were defined as splenic CD11c+, CD11b+, MHC-II+, CD40+, and CD86+ cells producing iNOS and TNF. Tip-DCs appeared to originate from circulating cells expressing CCR2 (receptor for monocyte chemotactic protein-1). They were largely absent in normal spleens, but accumulated in response to Listeria infection. Mice unable to recruit splenic CCR2+ cells were severely compromised in their capacity to fight Listeria infection. The differentiation of Tip-DCs required MyD88, a central regulatory molecule in myeloid development activated by Toll-like receptors that is responsible for the induction of a variety of proinflammatory cytokines (27). Tip-DCs may be derived from monocytes, because cultured monocyte-derived DCs express iNOS (Fig. 4C) and TNF (unpublished data).
Previously, epidermal DCs in inflammatory skin lesions (atopic dermatitis and psoriasis) have been classified as Langerhans cells, inflammatory dendritic epidermal cells (IDECs), and plasmacytoid DCs, by FACS techniques (28-30). Tip-DCs might contain the IDEC population because both express the integrin CD11c (unlike Langerhans cells). However, IDECs express CD1a, suggesting that they might be a subset of activated Langerhans cells, and we were unable to detect CD1a or Langerin/CD207 expression on epidermal CD11c+ DCs. In previous work, dermal DCs in psoriasis were identified by expression of factor XIIIa (31). We are finding an increase in XIIIa+ cells in psoriasis lesions, but these cells are confined to the dermis, even in active psoriasis lesions, and are distinct from the Tip-DCs.
Efalizumab clearly blocks trafficking of LFA-1+ T cells into psoriasis skin lesions (19). The observed DC reduction with efalizumab therapy may be due to either a direct effect on DCs (which express CD11a; ref. 32) or a direct effect on T cells, impacting on the DC-T cell relationship. We suspect that the DCs are a principal target because of our observation that the decrease in CD11c+ DC numbers during therapy is faster than the reduction in epidermal thickness. Likewise, both T cells and CD11c+ DCs are decreased in psoriasis lesions treated with other immunosuppressive therapies such as CTLA4Ig or alefacept (4, 6). Further proof that TNF- and iNOS-producing DCs could be direct inflammatory effector cells in autoimmune disease awaits the development of more DC-specific antagonists that can be used in clinical studies.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants; General Clinical Research Center Grant M01-RR00102 from the National Center for Research Resources at the NIH, and NIH Grants R01 AI-49572 and AI-49832.
Author contributions: R.M.S. and J.G.K. designed research; F.C., M.V.A., J.F.-D., R.N., I.N., H.C., I.C., T.K., P.G., and M.S.-W. performed research; K.P., M.G., and W.D. contributed new reagents/analytic tools; M.A.L., F.C., S.-L.L., K.M.W., and J.G.K. analyzed data; M.A.L., F.C., R.M.S., and J.G.K. wrote the paper; and M.A.L., P.G., M.S.-W., and J.G.K. were clinicians.
Conflict of interest statement: M.G. was employed by Xoma and W.D. was employed by Genentec at the time of the study.
Abbreviations: DC, dendritic cell; iNOS, inducible nitric oxide synthase; K16, keratin 16.
References
- 1.Krueger, J. G. (2002) J. Am. Acad. Dermatol. 46, 1-23. [DOI] [PubMed] [Google Scholar]
- 2.Lew, W., Bowcock, A. M. & Krueger, J. G. (2004) Trends Immunol. 25, 295-305. [DOI] [PubMed] [Google Scholar]
- 3.Nickoloff, B. J. & Nestle, F. O. (2004) J. Clin. Invest. 113, 1664-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams, J. R., Kelley, S. L., Hayes, E., Kikuchi, T., Brown, M. J., Kang, S., Lebwohl, M. G., Guzzo, C. A., Jegasothy, B. V., Linsley, P. S. & Krueger, J. G. (2000) J. Exp. Med. 192, 681-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papp, K., Bissonnette, R., Krueger, J. G., Carey, W., Gratton, D., Gulliver, W. P., Lui, H., Lynde, C. W., Magee, A., Minier, D., et al. (2001) J. Am. Acad. Dermatol. 45, 665-674. [DOI] [PubMed] [Google Scholar]
- 6.Chamian, F., Lowes, M. A., Lin, S. L., Lee, E., Kikuchi, T., Gilleaudeau, P., Sullivan-Whalen, M., Cardinale, I., Khatcherian, A., Novitskaya, I., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 2075-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, E., Trepicchio, W. L., Oestreicher, J. L., Pittman, D., Wang, F., Chamian, F., Dhodapkar, M. & Krueger, J. G. (2004) J. Exp. Med. 199, 125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cua, D. J., Sherlock, J., Chen, Y., Murphy, C. A., Joyce, B., Seymour, B., Lucian, L., To, W., Kwan, S., Churakova, T., et al. (2003) Nature 421, 744-748. [DOI] [PubMed] [Google Scholar]
- 9.Murphy, C. A., Langrish, C. L., Chen, Y., Blumenschein, W., McClanahan, T., Kastelein, R. A., Sedgwick, J. D. & Cua, D. J. (2003) J. Exp. Med. 198, 1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, Y. J. (2001) Cell 106, 259-262. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald, K. P., Munster, D. J., Clark, G. J., Dzionek, A., Schmitz, J. & Hart, D. N. (2002) Blood 100, 4512-4520. [DOI] [PubMed] [Google Scholar]
- 12.Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. (2003) Immunity 19, 59-70. [DOI] [PubMed] [Google Scholar]
- 13.Serbina, N. V., Kuziel, W., Flavell, R., Akira, S., Rollins, B. & Pamer, E. G. (2003) Immunity 19, 891-901. [DOI] [PubMed] [Google Scholar]
- 14.Tam, M. A. & Wick, M. J. (2004) Trends Immunol. 25, 335-339. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb, A. B., Masud, S., Ramamurthi, R., SAbdulghani, A., Romano, P., Chaudhari, U., Dooley, L., Fasanmade, A. A. & Wagner, C. L. (2003) J. Am. Acad. Dermatol. 48, 68-75. [DOI] [PubMed] [Google Scholar]
- 16.Leonardi, C. L., Powers, J. L., Matheson, R. T., Goffe, B. S., Zitnik, R., Wang, A. & Gottlieb, A. B. (2003) N. Engl. J. Med. 349, 2014-2022. [DOI] [PubMed] [Google Scholar]
- 17.Vallat, V. P., Gilleaudeau, P., Battat, L., Wolfe, J., Nabeya, R., Heftler, N., Hodak, E., Gottlieb, A. B. & Krueger, J. G. (1994) J. Exp. Med. 180, 283-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferenczi, K., Burack, L., Pope, M., Krueger, J. G. & Austin, L. M. (2000) J. Autoimmun. 14, 63-78. [DOI] [PubMed] [Google Scholar]
- 19.Vugmeyster, Y., Kikuchi, T., Lowes, M. A., Howell, K., Chamian, F., Kagen, M. H., Gilleaudeau, P., Lee, E., Dummer, W., Pippig, S., et al. (2004) Clin. Immunol. 113, 38-46. [DOI] [PubMed] [Google Scholar]
- 20.Pope, M., Betjes, M. G., Hirmand, H., Hoffman, L. & Steinman, R. M. (1995) J. Invest. Dermatol. 104, 11-17. [DOI] [PubMed] [Google Scholar]
- 21.Lee, A. W., Truong, T., Bickham, K., Fonteneau, J. F., Larsson, M., Da Silva, I., Somersan, S., Thomas, E. K. & Bhardwaj, N. (2002) Vaccine 20, Suppl. 4, A8-A22. [DOI] [PubMed] [Google Scholar]
- 22.Wittkowski, K. M., Lee, E., Nussbaum, R., Chamian, F. N. & Krueger, J. G. (2004) Stat. Med. 23, 1579-1592. [DOI] [PubMed] [Google Scholar]
- 23.Ormerod, A. D., Weller, R., Copeland, P., Benjamin, N., Ralston, S. H., Grabowksi, P. & Herriot, R. (1998) Arch. Dermatol. Res. 290, 3-8. [DOI] [PubMed] [Google Scholar]
- 24.Nathan, C. & Shiloh, M. U. (2000) Proc. Natl. Acad. Sci. USA 97, 8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinman, R. M. & Nussenzweig, M. C. (1980) Immunol. Rev. 53, 127-147. [DOI] [PubMed] [Google Scholar]
- 26.Feldmann, M. & Maini, R. N. (2003) Nat. Med. 9, 1245-1250. [DOI] [PubMed] [Google Scholar]
- 27.Janssens, S. & Beyaert, R. (2002) Trends Biochem. Sci. 27, 474-482. [DOI] [PubMed] [Google Scholar]
- 28.Wollenberg, A., Kraft, S., Hanau, D. & Bieber, T. (1996) J. Invest. Dermatol. 106, 446-453. [DOI] [PubMed] [Google Scholar]
- 29.Wollenberg, A., Wagner, M., Gunther, S., Towarowski, A., Tuma, E., Moderer, M., Rothenfusser, S., Wetzel, S., Endres, S. & Hartmann, G. (2002) J. Invest. Dermatol. 119, 1096-1102. [DOI] [PubMed] [Google Scholar]
- 30.Schuller, E., Teichmann, B., Haberstok, J., Moderer, M., Bieber, T. & Wollenberg, A. (2001) Arch. Dermatol. Res. 293, 448-454. [DOI] [PubMed] [Google Scholar]
- 31.Nestle, F. O., Turka, L. A. & Nickoloff, B. J. (1994) J. Clin. Invest. 94, 202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen, V. A., Ebner, S., Furhapter, C., Romani, N., Kolle, D., Fritsch, P. & Sepp, N. (2002) Eur. J. Immunol. 32, 3638-3650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.